Thermodynamic Stability: Free Energy and ... - McGill University
Thermodynamic Stability: Free Energy and ... - McGill University
Thermodynamic Stability: Free Energy and ... - McGill University
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
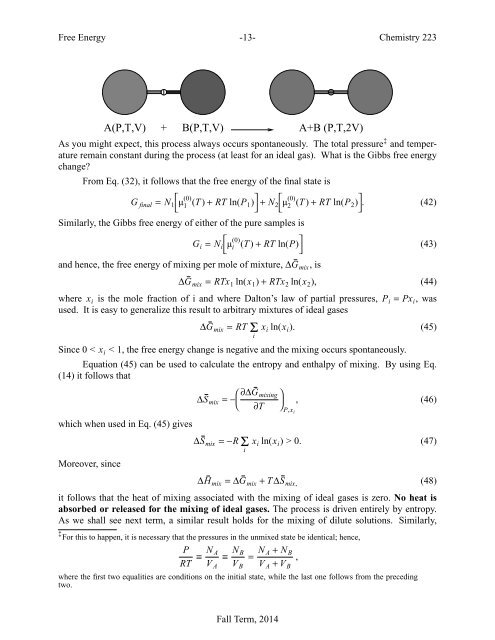
<strong>Free</strong> <strong>Energy</strong> -13- Chemistry 223A(P,T,V) +B(P,T,V)A+B (P,T,2V)As you might expect, this process always occurs spontaneously. The total pressure ‡ <strong>and</strong> temperatureremain constant during the process (at least for an ideal gas). What is the Gibbs free energychange?From Eq. (32), it follows that the free energy of the final state isG final = N 1⎡⎣µ (0)1 (T ) + RT ln(P 1) ⎤ ⎦ + N 2 ⎡ ⎣ µ(0) 2 (T ) + RT ln(P 2) ⎤ ⎦ . (42)Similarly, the Gibbs free energy of either of the pure samples isG i = N ⎡ i µ (0)⎣i (T ) + RT ln(P) ⎤ ⎦(43)<strong>and</strong> hence, the free energy of mixing per mole of mixture, ∆G mix ,is∆G mix = RTx 1 ln(x 1 ) + RTx 2 ln(x 2 ), (44)where x i is the mole fraction of i <strong>and</strong> where Dalton’s law of partial pressures, P i = Px i ,wasused. It is easy to generalize this result to arbitrary mixtures of ideal gases∆G mix = RTΣ x i ln(x i ). (45)iSince 0 < x i 0. (47)i∆H mix =∆G mix + T ∆S mix, (48)it follows that the heat of mixing associated with the mixing of ideal gases is zero. No heat isabsorbed or released for the mixing of ideal gases. The process is driven entirely by entropy.As we shall see next term, a similar result holds for the mixing of dilute solutions. Similarly,‡ For this to happen, it is necessary that the pressures in the unmixed state be identical; hence,PRT ≡ N A≡ N B= N A + N BV A + V B,V A V Bwhere the first two equalities are conditions on the initial state, while the last one follows from the precedingtwo.Fall Term, 2014