Material Improvement.indd - MWE - Medical Wire
Material Improvement.indd - MWE - Medical Wire
Material Improvement.indd - MWE - Medical Wire
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
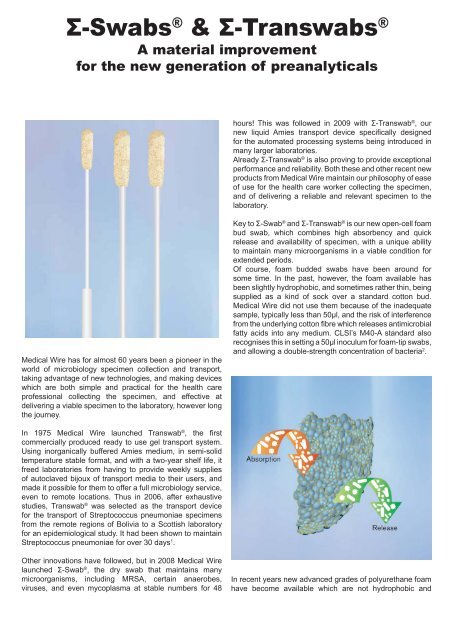
Σ-Swabs ® & Σ-Transwabs ®A material improvementfor the new generation of preanalyticalshours! This was followed in 2009 with Σ-Transwab ® , ournew liquid Amies transport device specifi cally designedfor the automated processing systems being introduced inmany larger laboratories.Already Σ-Transwab ® is also proving to provide exceptionalperformance and reliability. Both these and other recent newproducts from <strong>Medical</strong> <strong>Wire</strong> maintain our philosophy of easeof use for the health care worker collecting the specimen,and of delivering a reliable and relevant specimen to thelaboratory.<strong>Medical</strong> <strong>Wire</strong> has for almost 60 years been a pioneer in theworld of microbiology specimen collection and transport,taking advantage of new technologies, and making deviceswhich are both simple and practical for the health careprofessional collecting the specimen, and effective atdelivering a viable specimen to the laboratory, however longthe journey.Key to Σ-Swab ® and Σ-Transwab ® is our new open-cell foambud swab, which combines high absorbency and quickrelease and availability of specimen, with a unique abilityto maintain many microorganisms in a viable condition forextended periods.Of course, foam budded swabs have been around forsome time. In the past, however, the foam available hasbeen slightly hydrophobic, and sometimes rather thin, beingsupplied as a kind of sock over a standard cotton bud.<strong>Medical</strong> <strong>Wire</strong> did not use them because of the inadequatesample, typically less than 50μl, and the risk of interferencefrom the underlying cotton fi bre which releases antimicrobialfatty acids into any medium. CLSI’s M40-A standard alsorecognises this in setting a 50μl inoculum for foam-tip swabs,and allowing a double-strength concentration of bacteria 2 .In 1975 <strong>Medical</strong> <strong>Wire</strong> launched Transwab ® , the fi rstcommercially produced ready to use gel transport system.Using inorganically buffered Amies medium, in semi-solidtemperature stable format, and with a two-year shelf life, itfreed laboratories from having to provide weekly suppliesof autoclaved bijoux of transport media to their users, andmade it possible for them to offer a full microbiology service,even to remote locations. Thus in 2006, after exhaustivestudies, Transwab ® was selected as the transport devicefor the transport of Streptococcus pneumoniae specimensfrom the remote regions of Bolivia to a Scottish laboratoryfor an epidemiological study. It had been shown to maintainStreptococcus pneumoniae for over 30 days 1 .Other innovations have followed, but in 2008 <strong>Medical</strong> <strong>Wire</strong>launched Σ-Swab ® , the dry swab that maintains manymicroorganisms, including MRSA, certain anaerobes,viruses, and even mycoplasma at stable numbers for 48In recent years new advanced grades of polyurethane foamhave become available which are not hydrophobic and
which will absorb a much larger sample, typically 70-80μl,similar to a standard fi bre bud. Indeed the latest materialcan absorb more than 125μl, while retaining the ability tomaintain microorganisms in a viable condition for at least48 hours (and in practice much more). Our own, andindependent studies have shown these new materials tobe entirely suitable for specimen collection and transport,and it is on the basis of these developments that we havelaunched both Σ-Swabs® & Σ-Transwabs®.<strong>Medical</strong> <strong>Wire</strong> has also investigated fl ock swabs. We do haveaccess to fl ock, but feel there are issues which mean it isnot yet ready to use for specimen collection, not least ofwhich is the issue of patient and healthcare worker safety.Flocking technology has been around for many years.You may be familiar with it in as the lining of your carglove box, or on expensive wallpaper. The problem is thatmany of the microfi bers are not captured by the underlyinglayer of glue, and remain loose. These microfi bers are ofrespirable size, and can present a hazard, even on theirown 3 . The manufacturers take care to remove as many ofthese microfi bers as possible, but some remain. If you havesome fl ock swabs you can demonstrate this by rubbing orgently fl icking them over a glass surface. It is particularlynoticeable on the coated fl ock swabs supplied with sometransport swab devices. The risk of disease (Flock Worker’sLung) is known within the fl ocking industry, although it cantake some years to become manifest. But the combinationof loose, respirable microfi bers and particles, and infectiouspathogens creates a new potential hazard which has yet tobe accounted for.the coating apparent on the fl ock fi bers in certain transportdevices seems to contribute to overgrowth when samplesare carried at ambient temperatures.In a new study 4 , polyurethane foam tipped swabs (Σ-Swabs ® )recovered signifi cantly more bacteria of all types than nylonfibre fl ocked swabs (Copan).For now, advanced hydrophilic polyurethane foams, asused on Σ-Swabs ® & Σ-Transwabs ® , are the best materialfor the collection and integrity of the specimen, and optimumperformance in the laboratory, whether automated orconventional.Results (independent) for MRSA release & recovery usingadvanced polyurethane foam tipped swabs with standardsize buds (Sigma Dry Swab)Average swab absorption (microlitres of saline) 125.60h 24h 48hMRSA RT 3.86x10 6 cfu/ml 2.99x10 7 cfu/ml 4.21x10 7 cfu/mlMRSA 4 O C 3.86x10 6 cfu/ml 9.08x10 6 cfu/ml 7.86x10 6 cfu/mlResults are within the parameters of CLSI M40, with limitedloss or growth, even at ambient temperature.References1.2.3.4.Inverarity, D., M. Diggle, G. Edwards, & T. Mitchell, 2006, An Evaluationof Media Suitable for the Transportation by Air of Streptococcuspneumoniae. Federation of Infection Societies Conference, CardiffCLSI, 2003, Quality Control of Microbiological Transport Systems:Approved Standard CLSI Document M40-AKern, D.G. et al, 1998, Flock Worker’s Lung: Chronic Interstitial LungDisease in the Nylon Flocking Industry, Annals of Internal Medicine(American College of Physicians), 129, 261-272Turner, J., et al, 2010, The Characterization of the Absorption andRelease Properties of Various Clinical Swab Types. Poster T89, 26thClinical Virology Symposium, Daytona Beach, US.Flock swab surfacePerformance, too, has some questions. We have all heard themyth of the neatly arranged perpendicular fi bers. The realityis somewhat different, as can be seen in the photograph.There is no doubt that the fl ocking creates a large surfacearea, and that the swab picks up a large specimen. Butwww.mwe.co.ukDon’t just go with the flock!