download - Sandler Asthma Basic Research Center - University of ...
download - Sandler Asthma Basic Research Center - University of ...
download - Sandler Asthma Basic Research Center - University of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
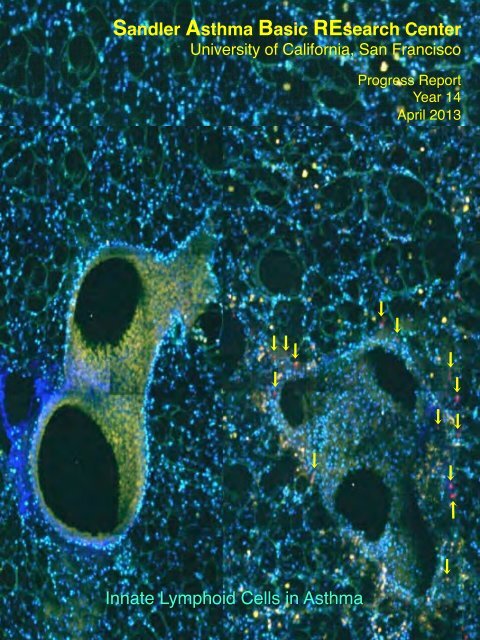
!<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>!<strong>University</strong> <strong>of</strong> California, San Francisco!!Progress Report!Year 14!April 2013!Innate Lymphoid Cells in <strong>Asthma</strong>!
Figure Legend: Lung slices from mice before and after allergen challenge were imagedusing three-channel multiphoton microscopy. Animals contain a red fluorescent markerintroduced into the interleukin-5 gene, which reveals IL-5-producing innate lymphoidcells organized at airway branchpoints that respond to provocation by activating cytokineexpression. IL-5 is critical for recruiting and sustaining eosinophils, a central cell typeseen in allergic asthma. Image courtesy <strong>of</strong> Emily Thornton, Steve Van Dyken, JesseNussbaum from the laboratories <strong>of</strong> Drs. Krummel and Locksley.
TABLE OF CONTENTSMission StatementOverviewSummary <strong>of</strong> AccomplishmentsExecutive Committee: Members and FunctionsScientific Advisory BoardMitchell Kronenberg, Ph.D.Philippa Marrack, Ph.DChristopher Wilson, M.D.SABRE <strong>Center</strong> InvestigatorsRichard Locksley, M.D., Ph.D.Christopher Allen, Ph.D.K. Mark Ansel, Ph.D.Limin Liu, Ph.D.Jeoung-Sook Shin, Ph.D.Core FacilitiesMouse Physiology and MorphologyFunctional GenomicsGeneticsCell Sorting and AnalysisMicroscopy<strong>Asthma</strong> Related <strong>Research</strong> ProjectsEvolving Microenvironments in Airway InflammationNIAID <strong>Asthma</strong> and Allergic Diseases Cooperative <strong>Research</strong> <strong>Center</strong>Contributions to Relevant Scientific ActivitiesPublications Supported by the <strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong><strong>Research</strong> <strong>Center</strong>Looking to the FutureBiographical Sketches
Mission StatementThe <strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong> (SABRE <strong>Center</strong>) at UCSF is an investigative unitdedicated to basic discovery in asthma research. The SABRE <strong>Center</strong> is nucleated by a smallgroup <strong>of</strong> five basic scientists who are supported by advanced technology cores and linked withthe larger scientific community through <strong>Center</strong> Grants and Program Project Grants focusedaround asthma research. Strong linkage with the Airway Clinical <strong>Research</strong> <strong>Center</strong> at UCSF hasenabled increasing focus on human studies. Regular seminars promote additional integration <strong>of</strong>the SABRE <strong>Center</strong> into the more extended UCSF research community to facilitate collaborationand to increase awareness for needs in fundamental discovery in asthma research. Founded in1999, the SABRE <strong>Center</strong> is made possible by the generous support <strong>of</strong> the <strong>Sandler</strong> Foundation.Summary <strong>of</strong> Accomplishments over the Past YearThe SABRE <strong>Center</strong> is evolving into a successful research enterprise within the greater UCSFscientific community. Notable benchmarks achieved this year include: (1) the award <strong>of</strong> an NIHDirector’s New Innovator Award to Chris Allen, the SABRE <strong>Center</strong> UCSF Fellow, who wasrecruited to stay at UCSF on the faculty as a member <strong>of</strong> the Cardiovascular <strong>Research</strong> Institute,where he will continue his work as a SABRE <strong>Center</strong> member; (2) the first full year <strong>of</strong> funding <strong>of</strong>the SABRE <strong>Center</strong> Program Project grant to study the role <strong>of</strong> innate lymphoid cells in humanasthma using patients accrued from the Airway Clinical <strong>Research</strong> <strong>Center</strong>; (3) the successfulrenewal <strong>of</strong> the NIH NIAID <strong>Asthma</strong> and Allergic Diseases Cooperative <strong>Research</strong> <strong>Center</strong> tocontinue investigations <strong>of</strong> cytokine regulation <strong>of</strong> airway smooth muscle contraction; (4) thesuccessful organization and planning <strong>of</strong> a Keystone Symposium in January 2013 devoted torecent advanced in Type 2 Immunity and Pathogenic Processes in <strong>Asthma</strong> in Santa Fe, NewMexico, that drew over 250 scientists together; and (5) continued successes in obtainingextramural funding and publishing high impact manuscripts in competitive journals.Increasingly, these studies have begun to work with and focus on human patients and materialsand the interactions with our clinical colleagues in the Pulmonary Division continue tostrengthen with the establishment <strong>of</strong> the Airway Clinical <strong>Research</strong> <strong>Center</strong>. We look forward toongoing successes in the coming year as we continue with our mission to conquer asthma.2
Overview – 2013Richard M. Locksley, M.D.The SABRE <strong>Center</strong> consists <strong>of</strong> the Director, Dr. Locksley, three full-time faculty, Drs. Ansel,Liu and Shin, and one UCSF Fellow, Dr. Allen, who has just completed his five yearappointment and has been recruited to join the faculty. In turn, these investigators now support10 postdoctoral trainees, 9 graduate students in Immunology and Biomedical Sciences, and 3pr<strong>of</strong>essional staff. Dr. Chapman, the former head <strong>of</strong> the Pulmonary Division at UCSF, works incontiguous space. His interests focus on lung inflammation and fibrosis, which overlap theinterests <strong>of</strong> the SABRE <strong>Center</strong>. The SABRE <strong>Center</strong> maintains close working relationships withthe Airway Clinical <strong>Research</strong> <strong>Center</strong> (ACRC) under the leadership <strong>of</strong> Dr. John Fahy, who hasbecome a member <strong>of</strong> the Executive Board. The fruits <strong>of</strong> this collaborative effort resulted in thefirst NIH Program Project Grant awarded to SABRE investigators beginning in 2012, with amajor focus centered on human patients and tissues as organized through the ACRC. TheSABRE <strong>Center</strong> has evolved into a self-standing research addition to the UCSF campus with thecapacity to contribute to our understanding <strong>of</strong> this important human disease. We will review theindividual investigators and their progress, followed by an overview <strong>of</strong> the constituents <strong>of</strong> the<strong>Center</strong>, a brief discussion <strong>of</strong> achievements and finally a look at the extramural grants that havebeen obtained to support these activities.InvestigatorsDr. Ansel is working to understand the gene expression pathways that mediate differentiationand regulation <strong>of</strong> cell fate and function in allergy, particularly asthma. His focus remains onmicroRNAs, transcription factors and epigenetics as critical executioners <strong>of</strong> these pathways. Hecontinues to push new technologies relevant to his studies, including a method for assessingmicroRNA targets by demonstrating their associations with the miRISC processing complex, ananalysis which could greatly spur the field. He published 4 manuscripts in 2012-13 as seniorauthor and contributed to a fifth; an additional manuscript is under revision at NatureImmunology and describes the role <strong>of</strong> a specific microRNA, miR-17~92, in follicular T celldifferentiation. He is building increasing excitement over his discoveries in the field asevidenced by recognition <strong>of</strong> his early achievements through solid publications and enhancedamounts <strong>of</strong> extramural grant support. He was asked to write a review on RNA regulation <strong>of</strong> theimmune system for Nature Reviews Immunology and to organize an issue <strong>of</strong> ImmunologicalReviews on the subject, attesting to his recognition by leaders in the field. Dr. Ansel participatedin several national and international scientific conferences related to his expertise, including talksin Japan and Korea.Based on studies completed with his 3-year Dana Foundation Award, Mark received his firstR01 funded grant from the National Heart, Lung and Blood Institute <strong>of</strong> the NIH to pursuecollaborations with Dr. Prescott Woodruff in the Airway Clinical <strong>Research</strong> <strong>Center</strong>, bringing in$1,796,403 in direct research dollars over a five year period. Dr. Ansel also was a participatingPrincipal Investigator as part <strong>of</strong> the first Program Project Grant among SABRE Investigatorsfrom the NIH. This grant began funding in July 2012 at a total level <strong>of</strong> approximately3
$1,575,000 per year over the next 5 years. In recognition <strong>of</strong> his outstanding accomplishments asa young investigator, Dr. Ansel was awarded a Leukemia & Lymphoma Society Scholar Award,resulting in approximately $525,000 in direct costs from 2012-2017. He has a U19 applicationunder consideration that involves a concerted effort towards understanding microRNAbiogenesis and regulation in immune cells using allergic immunity as a template.Personnel in the Ansel laboratory include two graduate students, with one supported byNational Science Foundation Predoctoral <strong>Research</strong> Fellowship and one a recipient <strong>of</strong> an HHMIGraduate Education in Medical Science (GEMS) Training Program Award to pursuetranslational studies in asthma patients in collaboration with Dr. Fahy in the Airway Clinical<strong>Research</strong> <strong>Center</strong>. The postdoctoral fellow is supported by a Swiss National Fellowship. Dr.Ansel’s first student graduated and is doing a postdoctoral fellowship. He is actively pursuingstudies using materials collected from patients with asthma in the Airway Clinical <strong>Research</strong><strong>Center</strong>. He is collaborating in studies with the Functional Genomics Core, the AnimalPhysiology Core and the Flow Cytometry Core. He collaborates with multiple otherinvestigators in the SABRE <strong>Center</strong>.In recognition <strong>of</strong> Dr. Ansel’s success to date, the Department <strong>of</strong> Microbiology & Immunologyhas submitted his materials for consideration <strong>of</strong> early advancement to Associate Pr<strong>of</strong>essor.Dr. Liu is pursuing the mechanisms that control the targeting and turnover <strong>of</strong> nitric oxide, animportant airway and blood vessel relaxant. Work in his postdoctoral training identified theenzyme GSNOR as an important molecule regulating nitrosylation and nitric oxide turnover intissues, and this molecule has become an important target in asthma, although it will be critical t<strong>of</strong>irst identify potential toxicities that might be associated with inhibiting this pathway. Duringhis studies at UCSF, Dr. Liu identified an important role for GSNOR in protecting liver cellsfrom genotoxic stress. He went on to identify the mechanism as linked to the nitrosativeinactivation <strong>of</strong> a DNA alkyltransferase involved in DNA repair. He continues to address the role<strong>of</strong> GSNOR in immune pathways related to asthma in a collaborative publication with Dr.Krummel in the Imaging Core and in on-going but incomplete studies addressing cell-specificdeletion <strong>of</strong> GSNOR in different lung cell populations in order to pinpoint the origin <strong>of</strong> GSNORamenable to therapeutic intervention in asthma. He is actively attempting to identify the targets<strong>of</strong> protein nitrosylation potentially impacted by this pathway. He had two manuscripts related toGSNOR in publication over the past year and two additional manuscripts submitted. He hasbeen working recently with Dr. Steven An to define direct roles for GSNOR in airway smoothmuscle cell contraction and relaxation pathways. Dr. Liu is working collaboratively withindividuals in the Functional Genomics, Genetics and Flow Cytometry Cores. He presented hiswork at both national and international scientific meetings.Dr. Liu is supported by an R01 and P01 on which he participates. These grants, whichcontribute over $475,000 direct costs per year, are from the National Cancer Institute <strong>of</strong> the NIHand study aspects <strong>of</strong> oncogenesis mediated by GSNOR deficiency, an important issue that mustbe addressed in order to sustain interest in this enzyme as a viable asthma therapeutic target. Hehas three postdocs in his laboratory. He continues to work to develop sufficient preliminary andpublished information on the role <strong>of</strong> GSNOR in smooth muscle contraction in order to pursuethis as an asthma-related source <strong>of</strong> NIH funding. Dr. Liu was promoted to Associate Pr<strong>of</strong>essor,4
effective July 2012, thus becoming the first SABRE <strong>Center</strong> recruit to achieve academic tenure atUCSF.Dr. Shin studies dendritic cell maturation and antigen processing, which represent anextension <strong>of</strong> her postdoctoral training where she described a novel pathway affecting theturnover <strong>of</strong> surface complexes <strong>of</strong> major histocompatibility-peptide complexes at the surface <strong>of</strong>the dendritic cells, a process which was regulated by ubiquitination. She is continuing studies <strong>of</strong>dendritic cells with an emphasis on cells collected from the human lung. She published amanuscript addressing the role <strong>of</strong> ubiquitination in controlling MHC class II surface distributionon antigen presenting cells. A manuscript describing the unexpected finding that IgE receptor ondendritic cells promotes serum IgE clearance, was reviewed positively at the Journal <strong>of</strong> ClinicalInvestigation and is under revision. A third manuscript describing the role <strong>of</strong> ubiquitination inthe process <strong>of</strong> dendritic cell <strong>of</strong> thymic regulatory T cells was reviewed positively and is beingrevised for the Journal <strong>of</strong> Experimental Medicine. Two additional manuscripts are inpreparation. She was invited to present her work at a Keystone Meeting on Dendritic CellBiology.Dr. Shin supports her laboratory with two young investigator awards from the Cancer<strong>Research</strong> Institute and from the American Heart Association. She is re-submitting an R01 to theNIH for the next deadline after closely missing the payline on her first submission. She receiveda UCSF School <strong>of</strong> Medicine Bridge Fund Award, which is awarded to individuals whose grantsare felt most likely to succeed if given support for re-submission, at the start <strong>of</strong> 2013.Dr. Shin has established close collaborations with pulmonary physicians in the AirwayClinical <strong>Research</strong> <strong>Center</strong> – Drs. Paul Wolters, Prescott Woodruff and John Fahy – to obtainfreshly dissected human lung tissues from transplant procedures and samples <strong>of</strong> biopsies and celllavages from asthma patients and individuals with idiopathic pulmonary fibrosis. She is alsousing humanized transgenic mice with human FcεRI expressed on mouse cells in a patternresembling its expression in humans (developed in the Kinet lab at Harvard). Dr. Shin worksclosely with the Airway Clinical <strong>Research</strong> <strong>Center</strong>, the Flow Cytometry Core and the Imaging<strong>Center</strong>. She currently has a graduate student, two postdoctoral trainees and a technician in herlaboratory.Dr. Allen began 5 years ago as a UCSF Fellow and is the first member <strong>of</strong> the UCSF FellowsProgram (http://biochemistry.ucsf.edu/~ucsffellows/current.html) who was selected to work on aspecific human disease, in this case, asthma. Dr. Allen combines skills in cellular and molecularimmunology with optical imaging capacities that have powered new insights in allergicinflammation. His primary research focuses on understanding the mechanisms by which highaffinityIgE-producing B cells and plasma cells are produced. Surprisingly, this remains a poorlyunderstood pathway <strong>of</strong> fundamental importance to the pathogenesis <strong>of</strong> allergy and asthma. Dr.Allen published some <strong>of</strong> his initial findings in a report in Immunity reporting his discovery thatIgE heavy chains inherently drive movement <strong>of</strong> B cells out <strong>of</strong> germinal centers, a process thatmay serve to limit somatic hypermutation and thus affinity. This finding will drive newhypotheses regarding mechanisms by which some allergic individuals develop high-affinity IgE,and constitute major efforts <strong>of</strong> his laboratory. His generation <strong>of</strong> an IgE reporter mouse thatpermits the efficient tracking <strong>of</strong> IgE-switched B cells constitutes an important technical advance5
for the field. He was invited to two Keystone meetings to present his findings. He continues towork closely with other investigators in the SABRE <strong>Center</strong> as he optimizes lung and immunecell imaging technologies that are applicable to broader use by other investigators on campus.Dr. Allen has attracted substantial new extramural funding to support his studies. Thisincludes an NIH R01 that will focus on imaging <strong>of</strong> basophils in tissues in situ and an NIHDirector’s New Innovator Award, fewer than 8% <strong>of</strong> which were funded in the last year.Together, these awards, both for 5 years duration, will bring in over $500K in direct costs tosupport his lab over the coming year.In recognition <strong>of</strong> these accomplishments, Dr. Allen was recruited to the Cardiovascular<strong>Research</strong> Institute (CVRI) at UCSF, where he joined the UCSF faculty as an Assistant Pr<strong>of</strong>essorin the Department <strong>of</strong> Anatomy in 2013. He remains committed to investigations into the basicpathogenesis <strong>of</strong> asthma. Dr. Allen will continue to be a member <strong>of</strong> SABRE, and will continue toparticipate in monthly and quarterly meetings with SABRE investigators on the Parnassus site.Core ActivitiesAn integral component <strong>of</strong> the SABRE <strong>Center</strong> has been support and guidance for advancedtechnology cores. The specific workings <strong>of</strong> these Cores and their achievements are detailedelsewhere in this report. The SABRE <strong>Center</strong> contributes to cores in Mouse Physiology (whichprovides both acute and chronic mouse models <strong>of</strong> allergic lung inflammation, includingchallenge with model antigens, fungal antigens and house dust mite antigens), FunctionalGenomics, Genetics, Flow Cytometry and Imaging, including video, two-photon, confocal andtotal internal reflection instruments. Our largest monetary support goes to the Mouse Physiologyand the Genetics Cores, which support small animal models and the collection <strong>of</strong> cohorts <strong>of</strong>carefully phenotyped families with asthma, respectively, in order to meet specialized needs <strong>of</strong>importance to asthma investigators. These cores continue to attract substantial use amonginvestigators across the campus as well as scientists in related fields, including inflammation,genetics and stem cell biology. The Animal Core contributed to 5 AAF studies from non-UCSFinvestigators over the past year and helped train investigators at the <strong>University</strong> <strong>of</strong> Arizona and atVirginia Commonwealth who have started to center such studies at those universities. TheGenetics Core has procured very large cohorts <strong>of</strong> Latino and African American families afflictedwith asthma for use genetic studies, and continues to participate in a number <strong>of</strong> multiinstitutionalstudies for genome-wide investigations and gene replicative studies. Dr. Burchard,the Core Director, has attained national prominence in recognition <strong>of</strong> this highly annotatedcohort. The remaining cores are supported by smaller amounts <strong>of</strong> funding, typically to coredirectors and operators or to help support maintenance contracts for equipment, costs that aredifficult to recover through recharge or other mechanisms. The Imaging Core has made largetechnical strides in providing optical support for live lung and lymph node imaging. SABREfunding is concentrated on developing novel technologies to enhance live imaging capacity, toestablish site-specific photo-ablation technology and to extend imaging capacity to sections <strong>of</strong>human lung. Participation in the Imaging <strong>Center</strong> has grown to support over 166 users involving66 Principle Investigators from 26 different Department or Organized <strong>Research</strong> Units at UCSF.Substantial training <strong>of</strong> new users and s<strong>of</strong>tware development to support these new technologies is6
an ongoing part <strong>of</strong> the mission. Thus, in a relatively short time, the Imaging Core has become anintegral and productive component <strong>of</strong> the scientific community at UCSF. The FunctionalGenomics Core, led by Dr. David Erle, has used SABRE support to enable improvedtechnologies for high throughput sequencing, CHIP-seq, microRNA pr<strong>of</strong>iling and RNAseq. Thishas proven particularly useful in Dr. Ansel’s studies in identifying important regulatory RNAsinvolved in T cell fate and effector function.SABRE support for these technology cores has been leveraged to attract NIH funding: theAnimal Physiology Core is additionally supported by technical core funds from NIAID <strong>Asthma</strong>and Allergic Disease Cooperative <strong>Research</strong> <strong>Center</strong> (Sheppard, Krummel, Woodruff, Erle) andthe Imaging Core is additionally supported by technical core funds supported by a PPG fromNHLBI to assess the microenvironments induced by lung inflammation (Caughey, Krummel,McDonald). In each case, funds from the SABRE Innovative Grants program initiated projectsthat led to the organization and support for these programmatic awards, indicative <strong>of</strong> the leverageenabled by a focused efforts from relatively small groups <strong>of</strong> investigators. Support for the FlowCytometry Core, which is largely self-supported and self-operated, is restricted to financial helpwith maintenance contracts, data base management and emergency laser failure, issues that areincompletely covered through recharge or grant-based mechanisms.These Cores achieve substantial leverage <strong>of</strong> resources, and over the past 2 years contributedto at least 12 successfully funded extramural grants, 7 postdoctoral fellowships and over 40 peerreviewedmanuscripts. Recharge systems have been set in place for the Functional GenomicsCore, the Mouse Physiology Core and the Flow Cytometry Core, and these are constantlyreassessed to ensure that fiscal accountability is maintained such that these services are not onlysustained, but enhanced by upgrading and enabling new technologies.Airway Clinical <strong>Research</strong> <strong>Center</strong>The Airway Clinical <strong>Research</strong> <strong>Center</strong> (ACRC) (see Figure) is a customized space <strong>of</strong> 3500 sqft. located on the 13 th floor <strong>of</strong> the UCSF Medical <strong>Center</strong>. The Airway <strong>Center</strong> comprises 5separate testing rooms for history and physical examination, phlebotomy, allergen skin tests,spirometry and methacholine challenge. This center also has a research bronchoscopy suite, asample processing lab, and administrative space for twelve research coordinators and sixresearch fellows. All <strong>of</strong> this space is dedicated to clinical research in airway disease; there is noclinical patient activity in this space. The Airway Clinical <strong>Research</strong> <strong>Center</strong> has fully equippedexam rooms for conducting pulmonary function testing, research bronchoscopy, participantinterviews and specimen collection and processing.7
The ACRC is equipped to see patients and collect tissue specimens quickly and efficiently.The following instruments are currently on site.Spirometers: Eight spirometers (Jaeger Masterscope (2), nSpire HDpft 1000 (1), SensormedicsVMax22 (1), Medgraphics CPFS/D Spirometer (2), nSpire KoKo PFT (2).Bronchoscopy equipment: Pentax Fiberoptic Bronchoscope Model #EB-1530T3 (2), PentaxProcessor Model #EPM-3500, Welch Allyn ProPaq CS vital signs monitor.Sputum Induction: Devilbiss UltraNeb 99 ultrasonic nebulizer (2), Nouvag UltraNeb ultrasonicnebulizer (2), NuAire NU-810-SPEC Biohood sputum induction booths (2).Biospecimen Processing room: Smith Kline Beecham VanGuard V6500 centrifuge, FisherScientific centrifuge Model #228, Thermo Shandon Cytospin 4 cytocentrifuge, Reicherthemocytometer (2), Eppendorf 5810R refrigerated centrifuge, Lab Companion B5-06 shakingwater bath, Fisher Scientific specimen refrigerator #97-915-1, Frigidaire refrigerator/freezer formedication storage, Sanyo -80 freezer MDF-U53VA, Sanyo -80 freezer MDF-U73VC, FormaClass II A2 Biological Safety Cabinet, TLS2200 Thermal Labeling System, barcode reader (2),Van Guard microscope.Other: Devilbiss PulmoAide compressor nebulizer (Rooms 1333, 1329A, 1329E), IsoTemp 205water bath, Fisher Scientific Stereomaster Zoom Microscope Model #12-562-1, Niox Mino nitricoxide (NO) monitor, ECG machines (2) HP Pagewriter Xli and Burdick Eclipse LE, Nellcorpulse oximeter (2), Welch Allyn Sure Temp Plus (2), SM DSM-2 micro-dosimeter, Salter LabsDosimeter (2), Tanita Scale, stadiometer, Bedfont Micro+ Smokerlyzer carbon monoxide (CO)monitors (2), Omron HEM-907XL blood pressure monitor.The ACRC has 12 research coordinators, a part time nurse, and a data manager. The modelfor these staff is that individual coordinators take ownership <strong>of</strong> specific research studies and8
manage that study in terms <strong>of</strong> recruitment, study visits, and biospecimen handling. Weeklymeeting <strong>of</strong> Airway <strong>Research</strong> <strong>Center</strong> staff and faculty involve presentations <strong>of</strong> specific projectsand administrative and quality assurance meeting focused on compliance with local, state, andfederal regulations governing research in human subjects.The ACRC enables approximately 1200 subject visits per year.The ACRC supports four large NIH research programs that involve multicenter networkcollaborations in asthma and COPD, thus supporting large numbers <strong>of</strong> patient visits per year:(i) <strong>Asthma</strong>Net - U10 HL098107 (Boushey, Lazarus, Cabana [Pediatrics])(ii) Severe <strong>Asthma</strong> <strong>Research</strong> Program (SARP) - 1U10HL109146-01 (Fahy).(iii) COPD Clinical <strong>Research</strong> Network (CCRN) (Lazarus)(iv) SPIROMICS (Woodruff) - N01-08-08, The goal <strong>of</strong> this study is to identify sub-populationsand intermediate outcome measures in COPD (chronic obstructive pulmonary disease) through alarge multi-center longitudinal study.The <strong>Center</strong> also supports the clinical components <strong>of</strong> multiple other NIH awards involved withvarious aspects <strong>of</strong> the SABRE <strong>Center</strong>, including:(i) 1P01HL107202 (Fahy) 8/1/12 - 6/30/17NIH/NHLBI. Program Project Grant. Innate and Adaptive Immune Responses in Th2-high<strong>Asthma</strong>. Fahy, Ansel, Woodruff, Locksley.(ii) 2U19AI077439-06 (Sheppard) 4/1/13 - 3/31/18NIH/NIAID. <strong>Asthma</strong> and Allergic Diseases Cooperative <strong>Research</strong> <strong>Center</strong>s (AADCRC). IL-13and IL-17 dynamics in the asthmatic airway - Sheppard, Woodruff, Krummel, Erle.(ii) 5R01 HL080414-05 (Fahy) 7/1/5-5/31/15NIH/NHLBI. Protein carbohydrate interactions in the pathophysiology <strong>of</strong> acute asthmaexacerbations.(iii) Inner City <strong>Asthma</strong> Consortium (Boushey) – a part <strong>of</strong> NIH <strong>Asthma</strong>-Net.(iv) 1P50HL107191-01 (Fahy) 4/1/11- 3/31/13NIH/NHLBI. Preventing fucose-dependent binding <strong>of</strong> aspergillus and pseudomonas to lungmucin(v) 1 R01 HL097591 (Woodruff, PG) 7/1/09 - 06/30/13NIH/NHLBI. Role <strong>of</strong> Th2 and non-Th2 Inflammation in Airway Smooth Muscle Remodeling in<strong>Asthma</strong>.In addition to NIH grants, the ACRC is a resource for industry supported clinical research in9
airway disease at UCSF. Recent industry sponsors have included Genentech, BoehringerIngelheim, and Roche and research alliances with Gilead and Pfizer are pending. The hope is toexpand this aspect <strong>of</strong> SABRE-industry interactions as a platform for successful movement <strong>of</strong>target identification and pathophysiology onwards to drug and therapeutic developmentpathways.SABRE <strong>Center</strong> core scientists and the Director meet quarterly with Dr. Fahy and colleaguesto further communication, planning and collaborative investigations <strong>of</strong> human asthma patients.Each <strong>of</strong> the core scientists is already involved in ongoing or planned investigations withtranslational physician scientists in the ACRC, confirming that this will serve as an importantintegrative unit for translational interests <strong>of</strong> the SABRE <strong>Center</strong>. There is also a monthly researchconference for SABRE/ACRC investigators at the Parnassus site to promote interactions andcollaborations.Successful competition for extramural supportEvidence-based metrics for success will be important in leveraging continuing support in thefuture, including from philanthropic entities. Fund-raising will require evidence for metrics <strong>of</strong>success, including our capacity to attract extramural research dollars to the community, tocontribute high-impact papers that establish novel paradigms in the asthma research arena, toattract new investigators into the field and, ultimately, to drive the discovery <strong>of</strong> new therapiesthat affect the disease. Although therapeutic discoveries will take time, we believe we can pointto successes in these evidence-based metric achievements over this past year.Since the initial recruitment <strong>of</strong> Dr. Liu and the additions <strong>of</strong> Drs. Ansel, Shin and Allen, wehave seen a steady climb in the amounts <strong>of</strong> external funds accrued by the core five SABREinvestigators in support <strong>of</strong> their research efforts. This has occurred despite the difficult fundingclimate, and attests to the capacity <strong>of</strong> the <strong>Center</strong> to serve as a nidus for successful asthma basicresearch. As demonstrated by our ability to obtain a Program Project this year by capitalizing onthe access and expertise <strong>of</strong> colleagues in the Airway Clinical <strong>Research</strong> <strong>Center</strong>, we believe thatbuilding multicomponent research teams to take on difficult problems associated with asthmawill prove a successful strategy for maintaining this funding momentum.10
Growth in accumulated extramural funds by the five core SABRE investigatorsIn addition, activities related to the SABRE <strong>Center</strong> resulted in publication <strong>of</strong> numerousmanuscripts and contributions to many successfully awarded grants and fellowships <strong>of</strong> varioustypes to investigators at UCSF. These are catalogued in the individual Core and ProgramReports.Highlighted SABRE <strong>Center</strong>-supported papers with impact on asthma-related research in 2012-13Cheng LE, K Hartman, A Roers, MF Krummel, RM Locksley. 2013. Perivascular mast celldynamically probe cutaneous blood vessels to capture IgE. Immunity 38:166-175.Although cross-linking <strong>of</strong> IgE bound to high-affinity receptors on mast cells and basophilshas been associated with the acute pathology <strong>of</strong> allergy and asthma, the mechanism by whichtissue mast cells acquire serum IgE remains unclear. Using molecularly designed reagents andlive tissue imaging, this manuscript demonstrates that mast cells positioned along smallcutaneous blood vessels can extend elongated cellular processes between endothelial capillarycells into the blood stream, where they can extract IgE directly upon binding to surface IgEreceptors. Efforts are underway to discover the molecular determinants <strong>of</strong> this probing behaviorin hopes <strong>of</strong> uncovering new strategies for blocking this process that is central to allergichypersensitivity.Seumois G, P Vijayanand, CJ Eisley, N Omran, L Kalinke, M North, AP Ganesan, LJ Simpson,N Hunkapiller, F Moltzahn, PG Woodruff, JV Fahy, DJ Erle, R Djukanovic, R Blelloch, KM11
Ansel. 2012. An integrated nano-scale approach to pr<strong>of</strong>ile miRNAs in limited clinical samples.Am J Clin Exp Immunol 1:70-89.Clinical research is limited by the amounts <strong>of</strong> material and the heterogeneity <strong>of</strong> specimensavailable for study. Standardized methods applicable to analysis <strong>of</strong> small amounts <strong>of</strong> materialare needed. The manuscript demonstrates a flow cytometric and nano-scale quantitative reversepolymerase chain reaction method that is capable <strong>of</strong> accurately and reproducibly measuringmicroRNAs from as few as 100 cells collected in bronchial biopsy specimens from asthmapatients obtained in the UCSF Airway Clinical <strong>Research</strong> <strong>Center</strong>. The study provides methodsthat should be applicable to other complex human diseases.Kudo M, AC Melton, C Chen, MB Engler, KE Huang, X Ren, Y Wang, X Berstein, JT Li, KAtabai, X Huang, D Sheppard. 2012. IL-17A produced by αβ T cells drives airway hyperresponsivenessin mice and enhances mouse and human airway smooth muscle contraction.Nature Medicine 18:547-54.The role <strong>of</strong> IL-17, a cytokine that serves to attract neutrophils to sites <strong>of</strong> inflammation, inasthma is <strong>of</strong> increasing interest as clinicians begin to understand non-allergic forms <strong>of</strong> asthma.Using both mouse and human tissues, the authors here show that IL-17A produced by CD4 Tcells in the lung can directly mediate airway smooth muscle hyper-contractility and expose amechanism related to smooth muscle myosin responsiveness. This study will drive much interestin understanding whether targeting this cytokine or these cells may comprise a therapeuticstrategy in some patients with non-allergic asthma.Thornton EE, Looney MR, O Bose, D Sen, D Sheppard, RM Locksley, X Huang, MF Krummel.2012. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airwaypresentation to T cells in the lung. J Exp Med 209:1183-1199.How allergens are sampled and processed in the airways is not currently understood. Usinggenetically altered mice with fluorescent cells and two-photon intravital imaging <strong>of</strong> the lung, thismanuscript demonstrates that inhaled antigens are collected by probing dendritic cells in distalairway spaces, but these cells then migrate proximally to present processed antigen to T cells atbranch points along the conducting airways. This finding contributes to our understanding <strong>of</strong>the pathologic processes that center the pathology <strong>of</strong> asthma on the conducting airways ratherthan the more distal air-exchange portions <strong>of</strong> the lung.Yang Z, BM Sullivan, CDC Allen. 2012. Fluorescent in vivo detection reveals that IgE+ B cellsare restrained by an intrinsic cell fate predisposition. Immunity 36:857-72.Despite the importance <strong>of</strong> IgE in mediating manifestations <strong>of</strong> atopy, methods for studying theB cells that produce IgE were lacking, thus precluding the examination <strong>of</strong> this pathway as apotential strategy for intervention. The authors devised a knockin reporter mouse strain thatreveals cells that have productively switched the immunoglobulin locus to produce IgE. Afterconfirming that they can now isolate and image IgE-producing B cells in vitro, the authors makethe unexpected observation that IgE B cells mature in germinal centers, in contrast to priordogma, but that expression <strong>of</strong> a successfully rearranged IgE BCR leads to immediateupregulation <strong>of</strong> Blimp-1 and egress from the germinal center to become a memory B cell orplasma cell. This study reveals a cell-intrinsic mechanism that serves to limit the extent <strong>of</strong>12
somatic hypermutation, and hence affinity maturation, which occurs once a B cell expresses IgE.The possibility that patients with severe atopy might dysregulate this highly controlled pathwayis being explored.Organization <strong>of</strong> the body <strong>of</strong> this Annual ReportAs in prior years, we will organize this report by reviewing the SABRE <strong>Center</strong> activities andupdating the core technologies that focus on asthma-related research. We will summarize ourinteractions with other campus asthma-oriented research projects and provide listings <strong>of</strong> theseminar speakers <strong>of</strong> conferences to which we lend support. We will follow this with a listing <strong>of</strong>the newly funded, pending or submitted grants and publications since the prior annual reportsthat reflect support from the many SABRE <strong>Center</strong> activities. We will summarize the FinancialReport for the Program. Finally, we will outline the strategies for the coming years and appendthe current biographical summaries <strong>of</strong> the members, awardees and participants in the SABRE<strong>Center</strong> at UCSF.We wish to thank the <strong>Sandler</strong> family for their vision and support in creating and sustainingthe SABRE <strong>Center</strong>. Support for high-risk, open-ended, basic science is difficult to procure in thecurrent funding and fiscal climate. As noted in the overview above, we can identify manyexamples where support from the SABRE <strong>Center</strong> has been leveraged greatly to achievesubstantial gains for the scientific and academic study <strong>of</strong> asthma at UCSF. We are most gratefulfor the <strong>Sandler</strong>s’ continued support.13
Executive CommitteeRichard M. Locksley, M.D.The goals <strong>of</strong> the SABRE <strong>Center</strong> are to drive innovation in basic asthma research. Weapproach this goal by establishing a core basic science group dedicated to the study <strong>of</strong>asthma, by promoting access to state-<strong>of</strong>-the-art technologies required to drive theresearch, by integrating their accomplishments across the greater UCSF campus, and bycreating opportunities for interactions with translational and clinical investigatorsstudying asthma patients. The Committee includes Dr. John Fahy, the Director <strong>of</strong> theAirway Clinical <strong>Research</strong> <strong>Center</strong> at UCSF, who continues to facilitate interactionsbetween SABRE members and the activities <strong>of</strong> the Clinical <strong>Research</strong> <strong>Center</strong>. TheExecutive Committee is constituted to provide the Director with counsel regarding issues<strong>of</strong> scope, direction and execution. The Executive Committee played a major role in therecruiting faculty to the SABRE <strong>Center</strong> and provides oversight in sustaining progresstowards the overall goals <strong>of</strong> the <strong>Center</strong>.SABRE <strong>Center</strong> Executive Committee MembersRichard Locksley, M.D., Pr<strong>of</strong>essorDirector, SABRE <strong>Center</strong>Departments <strong>of</strong> Medicine and Microbiology/ImmunologyHomer Boushey, M.D., Pr<strong>of</strong>essorDepartment <strong>of</strong> MedicineHal Chapman, M.D., Pr<strong>of</strong>essorDepartment <strong>of</strong> MedicineJohn V. Fahy, M.D., Pr<strong>of</strong>essorDepartment <strong>of</strong> MedicineWilliam Seaman, M.D., Pr<strong>of</strong>essorDepartment <strong>of</strong> MedicineDean Sheppard, M.D., Pr<strong>of</strong>essorDepartment <strong>of</strong> MedicineArt Weiss, M.D., Ph.D., Pr<strong>of</strong>essorDepartments <strong>of</strong> Medicine and Microbiology/ImmunologyZena Werb, Ph.D., Pr<strong>of</strong>essorDepartment <strong>of</strong> Anatomy
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Scientific Advisory BoardSCIENTIFIC ADVISORY BOARD15
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Philippa (Pippa) Marrack, Ph.D.Pr<strong>of</strong>essor <strong>of</strong> Molecular Biology and ImmunologyVice Chair, Department <strong>of</strong> ImmunologyNational Jewish Medical and <strong>Research</strong> <strong>Center</strong>, DenverPr<strong>of</strong>essor at the Health Sciences <strong>Center</strong>, <strong>University</strong> <strong>of</strong> Colorado<strong>Research</strong> Investigator at the Howard Hughes Medical Institute, USAAs one <strong>of</strong> the world’s leading research scientists investigating T cells, the family <strong>of</strong> cellsthat help the body fight <strong>of</strong>f disease, Dr. Marrack’s work has led to a greaterunderstanding <strong>of</strong> their role in the immune system.Born in the United Kingdom, Philippa Marrack earned her undergraduate and doctoraldegrees in biological sciences from the <strong>University</strong> <strong>of</strong> Cambridge. She left the UK in 1971to do postdoctoral work in the USA, where she has lived and worked ever since, initiallyat the <strong>University</strong> <strong>of</strong> California, and then at the <strong>University</strong> <strong>of</strong> Rochester. Since 1979, shehas been based in Denver, Colorado, where she is now a research investigator at theHoward Hughes Medical Institute, Vice Chair <strong>of</strong> the Department <strong>of</strong> Immunology andPr<strong>of</strong>essor at National Jewish Medical and <strong>Research</strong> <strong>Center</strong>, and Pr<strong>of</strong>essor at the<strong>University</strong> <strong>of</strong> Colorado’s Health Sciences <strong>Center</strong>.During her career, Philippa Marrack has published more than 300 peer-reviewed journalarticles and she has served on the editorial boards <strong>of</strong> numerous journals, including Cell,Science, and the Journal <strong>of</strong> Immunology. Amongst her many honors are the RoyalSociety’s Wellcome Foundation Prize (1990), the Paul Ehrlich and Ludwig DarmtsädterPrize (1993) and the Louisa Gross Horwitz Prize (1995). She has served on variouspanels and boards for the American Cancer Society, the U.S. National Institutes <strong>of</strong>Health, and the Burroughs Wellcome Fund. She was the President <strong>of</strong> the AmericanAssociation <strong>of</strong> Immunologists in 2000-2001, and is currently the President <strong>of</strong> theInternational Union <strong>of</strong> Immunological Societies.17
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Christopher Wilson, M.D.Director, Global Health Discovery Program, Gates FoundationDr. Chris Wilson, Director <strong>of</strong> the Global Health Discovery program, leads a team thattargets fundamental scientific and technological advances in global health that could leadto new ways to prevent, treat, and diagnose disease.Wilson joined the foundation in 2009 as Deputy Director, Vaccine Discovery and HumanBiology, Global Health Discovery.Wilson is a pediatrician and immunologist. He joined the faculty at the <strong>University</strong> <strong>of</strong>Washington in 1979 in the Infectious Diseases Division <strong>of</strong> the Department <strong>of</strong> Pediatricsand later served as head <strong>of</strong> the Division <strong>of</strong> Infectious Diseases, Immunology andRheumatology. In 1989, he became one <strong>of</strong> the founding faculty members in the newDepartment <strong>of</strong> Immunology, and served as Chairman <strong>of</strong> the Department <strong>of</strong> Immunologyand head <strong>of</strong> the graduate program in immunology from 1999-2009.He has also served on a number <strong>of</strong> national advisory panels, including the Institute <strong>of</strong>Medicine Vaccine Safety Review Committee (2001-2004) and the National AdvisoryCouncil on Child Health and Human Development, NICHD, NIH, and he co-chaired theNIAID US Immunodeficiency Network Pilot Grant Review Committee. He is an electedfellow <strong>of</strong> the American Association for the Advancement <strong>of</strong> Science.Wilson received a bachelor’s degree from the <strong>University</strong> <strong>of</strong> California, Irvine and amedical degree from UCLA. He trained in pediatrics at Boston Children’s Hospital/Harvard Medical School, served in the US Public Health Service, and then was a postdoctoralfellow in infectious diseases while performing immunology research at Stanford<strong>University</strong>.18
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>SABRE CENTER INVESTIGATORS19
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Richard M. Locksley, M.D.Pr<strong>of</strong>essor, Departments <strong>of</strong> Medicine andMicrobiology & ImmunologyInvestigator, Howard Hughes Medical InstituteUCSF513 Parnassus AvenueMedical Sciences, S-1032B, Box 0795San Francisco, CA 94143-0795Tel: 415-476-3087Fax: 415-502-5081Website: Cancer <strong>Center</strong>Immunology Graduate ProgramPulmonary & Critical Care DivisionHoward Hughes Medical InstituteVirology & Microbial PathogenesisDr. Locksley is the Director <strong>of</strong> the <strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong> (SABRE) anda Howard Hughes Medical Institute Investigator. He is a Pr<strong>of</strong>essor in the Departments <strong>of</strong>Medicine and Microbiology & Immunology. He received his undergraduate degree inbiochemistry from Harvard and his M.D. from the <strong>University</strong> <strong>of</strong> Rochester. Aftercompleting his residency at UCSF, he trained in infectious diseases at the <strong>University</strong> <strong>of</strong>Washington. Prior to his position as director <strong>of</strong> the SABRE <strong>Center</strong>, Dr. Locksley served18 years as the Chief <strong>of</strong> the Division <strong>of</strong> Infectious Diseases at UCSF Medical <strong>Center</strong>. Dr.Locksley is a fellow <strong>of</strong> the American Academy <strong>of</strong> Arts and Sciences.Dr. Locksley's laboratory focuses on mechanisms by which the immune system becomesorganized in stereotyped ways against discrete types <strong>of</strong> challenges. This involves thedifferentiation <strong>of</strong> naïve helper T cells to distinct types <strong>of</strong> subsets that produce differentkinds <strong>of</strong> cytokines, key effector molecules <strong>of</strong> the immune system. In turn, these differentT cells subsets work with different kinds <strong>of</strong> innate cells, including neutrophils,eosinophils, macrophages and others, to mediate immunity. Properly executed, suchresponses mediate protection against infectious organisms or repair <strong>of</strong> damaged tissues,but, when dysregulated, these immune responses lead to disease, including asthma.Dr. Locksley’s laboratory investigates immunity using mice genetically engineered toreport cytokines expressed during the different types <strong>of</strong> immune responses. Thisapproach reveals the shared expression <strong>of</strong> important cytokines by both innate andadaptive immune cells. Using these methods, the laboratory participated in recentdiscoveries <strong>of</strong> innate lymphoid type 2 cells, which represent a previously unstudied cellnow implicated in allergic immunity. The laboratory also revealed a novel mechanism bywhich cutaneous mast cells capture IgE from blood. The laboratory continues to studythe role <strong>of</strong> chitin, a structural component <strong>of</strong> many allergens – including dust mites,cockroaches, shellfish and molds – as well as helminthes, in inducing infiltration <strong>of</strong> cells20
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>involved in allergy into tissues, and to pursue recent finding connecting the cells <strong>of</strong>allergic immunity with metabolic pathways involved in vertebrate homeostasis.Representative Publications1. Reese TA, H-E Liang, AM Tager, AD Luster, N van Rooijen, D Voehringer, RMLocksley. 2007. Chitin induces accumulation in tissue <strong>of</strong> innate immune cellsassociated with allergy. Nature 447:92-6.2. Reinhardt RL, HE Liang, RM Locksley. 2009. Cytokine-secreting follicular Tcells shape the antibody repertoire. Nature Immunol 10:385-393.3. Sullivan BM, RM Locksley. 2009. Basophils: a nonredundant contributor tohost immunity. Immunity 30:12-20.4. Siebold MA, TA Reese, S Choudhry, MT Salam, K Beckman, C Egn, A Atakilit,K Meade, M Lenoir, HG Watson, S Thyne, R Kumar, KB Weiss, LC Grammar, PAvila, RP Schleimer, JV Fahy, J Rodriguez-Santana, W Rodriguez-Cintron, RGBoot, D Sheppard, FD Gilliland, RM Locksley, EG Burchard. 2009. Differentialenzymatic activity <strong>of</strong> common haplotypic versions <strong>of</strong> the human acidicmammalian chitinase protein. J Biol Chem 284:19650-8.5. Locksley RM. 2010. <strong>Asthma</strong> and allergic inflammation. Cell 140:777-83.6. Price AE, HE Liang, BM Sullivan, RL Reinhardt, CJ Eisley, EJ Erle, RMLocksley. 2010. Systemically dispersed innate IL-13-expressing cells in type 2immunity. Proc Natl Acad Sci USA 107:11489-94.7. Wu D, AB Mol<strong>of</strong>sky, HE Liang, RR Ricardo-Gonzalez, HA Jouihan, JK Bando,A Chawla, RM Locksley. 2011. Eosinophils sustain adipose alternativelyactivated macrophages associated with glucose homeostasis. Science 332:243-7.8. Sullivan BM, H-E Liang, JK Bando, D Wu, LE Cheng, JK McKerrow, CDCAllen, RM Locksley. 2011. Genetic analysis <strong>of</strong> basophil function in vivo.Nature Immunol 12:527-35.9. Nguyen KD, Y Qui, X Cui, YP Sharon Goh, J Mwangi, T David, L Mukundan, FBrombacher, RM Locksley, A Chawla. 2011. Alternatively activatedmacrophages produce catecholamines to sustain adaptive thermogenesis. Nature480:104-8.10. Liang H-E, RL Reinhardt, JK Bando, BM Sullivan, I-C Ho, RM Locksley. 2011.Divergent expression patterns <strong>of</strong> IL-4 and IL-13 define unique functions inallergic immunity. Nature Immunol 13:58-66.11. Cheng LE, K Hartmann, A Roers, MF Krummel, RM Locksley. 2013.Perivascular mast cells dynamically probe cutaneous blood vessels to capture IgE.Immunity 38:166-75.12. Van Dyken SJ, RM Locksley. 2013. Interleukin-4- and interleukin-13-mediatedalternatively activated macrophages: role in homeostasis and disease. Annu RevImmunol 31:317-43.13. Mol<strong>of</strong>sky AB, JC Nussbaum H-E Liang, SJ Van Dyken, LE Cheng, A Mohapatra,A Chawla, RM Locksley. 2013. Innate lymphoid type 2 cells (ILC2) sustainvisceral adipose tissue eosinophils and alternatively activated macrophages. JExp Med (in press).21
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Christopher D. C. Allen, Ph.D.Assistant Pr<strong>of</strong>essorCardiovascular <strong>Research</strong> InstituteDepartments <strong>of</strong> Anatomy and Microbiology and Immunology<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>513 Parnassus Ave. Box 0414, HSE-205ASan Francisco, CA 94143-0414Tel: 415-476-5178Fax: 415-502-4995Dr. Allen is an Investigator <strong>of</strong> the Cardiovascular <strong>Research</strong> Institute and an AssistantPr<strong>of</strong>essor in the Departments <strong>of</strong> Anatomy and Microbiology and Immunology at UCSF.He completed his B.S. in Biology at MIT, and then his Ph.D. at UCSF in the BiomedicalSciences Graduate Program in the laboratory <strong>of</strong> Jason Cyster, with the support <strong>of</strong> aHoward Hughes Medical Institute Predoctoral Fellowship. Dr. Allen was then selected asthe first <strong>Sandler</strong>-Newmann Foundation UCSF Fellow in <strong>Asthma</strong> <strong>Research</strong>, giving himthe opportunity to attain principal investigator status and to develop an independentresearch program in asthma immediately after obtaining his Ph.D. He was recently hiredinto a tenure-track Assistant Pr<strong>of</strong>essor position at UCSF.Dr. Allen’s research in the SABRE center focuses on the cellular immune response inasthma. He is using his expertise in cutting-edge two-photon microscopy to visualizeinteractions among cells in the lungs as well as in lymphoid organs that ‘prime’ cells forimmune responses in the respiratory tract. A particular emphasis <strong>of</strong> his research is on thedevelopment and function <strong>of</strong> IgE antibodies that contribute to allergic responses. IgE hasbeen shown to be important in human asthma, yet little is known about the events leadingto IgE production after inhaling allergen. The major goals <strong>of</strong> the research are to:1) Develop innovative new mouse models <strong>of</strong> asthma that will be useful for studies <strong>of</strong>IgE antibody responses to inhaled allergens.2) Define the early events leading to allergic sensitization and IgE antibodyproduction after inhalation <strong>of</strong> allergen.3) Characterize the interactions among inflammatory cells in the lung in asthma anddefine the features <strong>of</strong> the microenvironments in which these interactions occur.Publications1. Allen C.D.C., Ansel K.M., Low C., Lesley R., Tamamura H., Fujii N. Cyster J.G.(2004). Germinal center dark and light zone organization is mediated by CXCR4and CXCR5. Nature Immunology, 5(9), 943-952.2. Chen T.T., Li L., Chung D.H., Allen C.D.C., Torti S.V., Torti F.M., Cyster J.G.,Chen C.Y., Brodsky F.M., Niemi E.C., Nakamura M.C., Seaman W.E., Daws22
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>M.R. (2005). TIM-2 is expressed on B cells and in liver and kidney and is areceptor for H-ferritin endocytosis. The Journal <strong>of</strong> Experimental Medicine,202(7), 955-965.3. Allen C.D.C., Okada T., Tang H.L., Cyster J.G. (2007). Imaging <strong>of</strong> germinalcenter selection events during affinity maturation. Science, 315(5811), 528-531.4. *Allen C.D.C., *Okada T., *Cyster J.G. (2007). Germinal-center organizationand cellular dynamics. Immunity 27(2), 190-202. *co-corresponding author.PMCID: PMC2242846.5. Haynes N.M., Allen C.D.C., Lesley R., Ansel K.M., Killeen N., and Cyster J.G.(2007). Role <strong>of</strong> CXCR5 and CCR7 in follicular Th cell positioning andappearance <strong>of</strong> a programmed cell death gene-1 High germinal center-associatedsubpopulation. The Journal <strong>of</strong> Immunology, 179(8), 5099-5108.6. *Allen C.D.C., *Cyster J.G. (2008). Follicular dendritic cell networks <strong>of</strong> primaryfollicles and germinal centers: phenotype and function. Seminars in Immunology20(1), 14-25. *co-corresponding author PMCID: PMC2366796.7. Beltman J.B., Allen C.D.C., Cyster J.G., de Boer R.J. (2011). B cells withingerminal centers migrate preferentially from dark to light zone. Proceedings <strong>of</strong>the National Academy <strong>of</strong> Sciences, 108(21), 8755-8760. PMCID: PMC31023848. Green J.A., Suzuki K., Cho B., Willison L.D., Palmer D., Allen C.D.C., SchmidtT.H., Xu Y., Proia R.L., Coughlin S.R., Cyster J.G. (2011). The sphingosine 1-phosphate receptor S1P 2 maintains the homeostasis <strong>of</strong> germinal center B cells andpromotes niche confinement. Nature Immunology, 12(7), 672-680. PMCID:PMC3158008.9. Sullivan B.M., Liang H.E., Bando J.K., Wu D., Cheng L.E., McKerrow J.K.,*Allen C.D.C., *Locksley R.M. (2011). Genetic analysis <strong>of</strong> basophil function invivo. Nature Immunology, 12(6), 527-535. *co-corresponding author. PMCID:PMC3271435.10. Steiner D.F., Thomas M.F., Hu J.K., Yang Z., Babiarz J.E., Allen C.D.C.,Matloubian M., Blelloch R., Ansel K.M. (2011). MicroRNA-29 Regulates T-BoxTranscription Factors and Interferon-γ Production in Helper T Cells. Immunity,35(2), 169-181. PMCID: PMC3361370.11. Yang Z., Sullivan B.M., Allen C.D.C. (2012). Fluorescent in vivo detectionreveals that IgE + B cells are restrained by an intrinsic cell fate predisposition.Immunity, 36(5), 857-872.23
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Arron JR, Erle DJ, Woodruff PG. Airway Epithelial miRNA Expression isAltered in <strong>Asthma</strong>. Am J Respir Crit Care Med. 186:965-74 (2012)8. Thomas MF, Abdul-Wajid S, Panduro M, Babiarz JE, Rajaram M, Woodruff P,Lanier LL, Heissmeyer V, Ansel KM. Eri1 regulates microRNA homeostasis andmouse lymphocyte development and anti-viral function. Blood. 120:130-42(2012)9. Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, PanduroM, Huang X, Interlandi J, Djuretic IM, Brown DR, Sharpe AH, Rao A, AnselKM. Interleukin-4 Production by Follicular Helper T Cells Requires theConserved Il4 Enhancer Hypersensitivity Site V. Immunity 36:175-87 (2012).10. Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct Waves <strong>of</strong> BCL6Expression during T Follicular Helper Cell Development. J Immunol.187:2089-92(2011)11. Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CD, Matloubian M,Blelloch R, Ansel KM. MicroRNA-29 Regulates T-Box Transcription Factorsand Interferon-γ Production in Helper T Cells. Immunity 35:169-81 (2011)12. S<strong>of</strong>i MH, Qiao Y, Ansel KM, Kubo M, Chang CH. Induction and Maintenance <strong>of</strong>IL-4 Expression Are Regulated Differently by the 3' Enhancer in CD4 T Cells. J.Immunol. 186:2792-9 (2011)13. Thomas MF, Ansel KM. Construction <strong>of</strong> small RNA cDNA libraries for deepsequencing. Methods Mol Biol. 667:93-111 (2010)14. Ansel KM*, Pastor WA, Rath N, Lapan AD, Glasmacher E, Wolf C, Smith LC,Papadopoulou N, Lamperti E, Tahiliani M, Ellwart JW, Shi Y, Kremmer E, RaoA, Heissmeyer V*. Mouse ERI-1 interacts with the ribosome and catalyzes 5.8SrRNA processing. Nat Struct Mol Biol. 15:523-30 (2008) (*correspondingauthors)15. Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A,Frendewey D, Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N,Yancopoulos G, Rao A, Rajewsky K. Regulation <strong>of</strong> the germinal center responseby microRNA-155. Science 316:604-8 (2007)16. Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. T-bet andRunx3 cooperate to activate Ifng and silence Il4 in Th1 cells. Nat Immunol.8:145-53 (2007)17. Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation <strong>of</strong> Th2 differentiation andthe IL4 locus. Annu Rev Immunol. 24:607-56 (2006)18. *Monticelli S, *Ansel KM, *Xiao C, *Socci ND, Krichevsky AM, Thai, T-H,Rajewsky N, Marks DS, Sander C, Rajewsky K, Rao A, Kosik KS. MicroRNApr<strong>of</strong>iling <strong>of</strong> the murine hematopoietic system. Genome Biol. 6:R71 (2005)19. *Muljo SA, *Ansel KM, *Kanellopoulou C, Livingston DM, Rao A, RajewskyK. Aberrant T cell differentiation in the absence <strong>of</strong> Dicer. J Exp Med. 202:261-9(2005).20. Ansel KM, Greenwald RJ, Agarwal SA, Bassing CH, Monticelli S, Interlandi J,Djuretic IM, Lee DU, Sharpe AH, Alt FW, Rao A. Deletion <strong>of</strong> a conserved Il4silencer impairs Th1-mediated autoimmunity. Nat Immunol. 5:1251-9 (2004).26
Limin Liu, Ph.D.Associate Pr<strong>of</strong>essor, Department <strong>of</strong> Microbiology/Immunology<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong><strong>University</strong> <strong>of</strong> California San Francisco513 Parnassus Ave.HSE 201J, Box 0414San Francisco, CA 94143-0414Tel: 415-476-1466Fax: 415-476-8201email: Limin.Liu@ucsf.eduDr. Limin Liu is an Associate Pr<strong>of</strong>essor in the Department <strong>of</strong> Microbiology & Immunology. Heobtained his B.S. in Biology from <strong>University</strong> <strong>of</strong> Science and Technology <strong>of</strong> China and his Ph.D.in Molecular Biology from <strong>University</strong> <strong>of</strong> Missouri. He did postdoctoral research on the biology<strong>of</strong> nitric oxide (NO) at Duke <strong>University</strong> Medical <strong>Center</strong>.Dr. Liu’s laboratory focuses on enzymatic deactivation <strong>of</strong> NO bioactivity and its role in asthmaand other diseases. NO plays important roles in virtually every biological system. NO regulatesfunctions <strong>of</strong> numerous proteins through S-nitrosylation, the covalent addition <strong>of</strong> NO to cysteinethiol. Through the study <strong>of</strong> S-nitroso-glutathione reductase (GSNOR), the key protein for denitrosylationin cells, Dr. Liu and colleagues have demonstrated that S-nitrosylation and itsdeactivation exert critical functions in systematic inflammation, asthma, cancer, and many otherphysiological and pathological processes.GSNOR and asthma It has been demonstrated with GSNOR-deficient (GSNOR -/- ) mice thatincreased S-nitrosylation from GSNOR deficiency in a model <strong>of</strong> allergic asthma does not affectimmune responses but abolishes airway hyperresponsiveness. Dr. Liu’s laboratory isinvestigating the mechanism <strong>of</strong> S-nitrosylation-dependent protection to understand a keyquestion in asthma: How do allergic immune responses cause airway hyperresponsiveness?GSNOR and carcinogenesis NO is implicated in tumorigenesis by much circumstantialevidence, but little is known definitively about the mechanisms through which endogenous NOmight regulate the behavior <strong>of</strong> pre-cancerous or cancerous cells. Using GSNOR -/- mice Dr. Liu’slaboratory has discovered that dysregulated S-nitrosylation from GSNOR deficiency inactivates akey DNA repair system and promotes liver cancer (Science Translational Medicine 2:19ra13,2010). Investigation is underway to expend the findings and to further elucidate molecularmechanisms.New pathways in NO deactivation Dr. Liu and colleagues have demonstrated that GSNORand flavohemoglobin, the major NO-consuming enzyme, operate together to regulate NObioactivities and to protect against NO-related toxicity in the yeast Saccharomyces cerevisia.They are employing the yeast model to elucidate the roles <strong>of</strong> additional, novel genes that arerequired for protection against NO-related toxicity. Homologue proteins are also investigated inanimals.27
Selected Publications1. Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T,Marshall HE, Que LG, Stamler JS (2004). Essential roles <strong>of</strong> S-nitrosothiols in vascularhomeostasis and endotoxic shock. Cell 116:617-628.2. Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS (2005).Protection from experimental asthma by an endogenous bronchodilator. Science308:1618-1621.3. Choudhry S, Que LG, Yang Z, Liu L, Eng C, Kim SO, Kumar G, Thyne S, Chapela R,Meade K, Watson HG, LeNoir M, Rodriguez-Santana JR, Rodriguez-Cintron W, AvilaPC, Stamler JS, Burchard EG (2010). GSNO Reductase and β2 Adrenergic ReceptorGene-gene Interaction: Bronchodilator Responsiveness to Albuterol. Pharmacogeneticsand Genomics 20:351-8.4. Wei W, Li B, Hanes MA, Kakar S, Chen X, Liu L (2010). Deficiency <strong>of</strong> S-Nitrosoglutathione Reductase Impairs DNA Repair and Promotes Hepatocarcinogenesis.Science Translational Medicine 2: 19ra13.5. Yang Z, Wang Z, Doulias P, Wei W, Ischiropoulos H, Locksley RM, Liu L (2010).Lymphocyte development requires S-nitrosoglutathione reductase. J Immunol, 185:6664-6669.6. Wei W, Yang Z, Tang CH, Liu L (2011). Targeted deletion <strong>of</strong> GSNOR in hepatocytes <strong>of</strong>mice causes nitrosative inactivation <strong>of</strong> O6-alkylguanine-DNA alkyltransferase andincreased sensitivity to genotoxic diethylnitrosamine. Carcinogenesis 32:973–977.7. Na B, Huang ZM, Wang Q, Qi ZX, Tian YJ, Lu CC, Yu JW, Hanes MA, Sanjay KakaraS, Huanga EJ, Ou JHJ, Liu L‡,*, and Yen TSB‡,† (2011). Transgenic Expression <strong>of</strong>Entire Hepatitis B Virus in Mice Induces Hepatocarcinogenesis Independent <strong>of</strong> ChronicLiver Injury. PLoS One 6:e26240.8. ‡co-senior author; *Corresponding author; †Deceased August 31, 2009.9. Tang CH, Wei W, Liu L (2012). Regulation <strong>of</strong> DNA Repair by S-Nitrosylation. BiochimBiophys Acta 1820:730–735.10. Ozawa K*, Tsumoto H, Wei W, Tang CH, Komatsubara AT, Kawafune H, Shimizu K,Liu L*, Tsujimoto G (2012). Proteomic analysis <strong>of</strong> the role <strong>of</strong> S-nitrosoglutathionereductase in lipopolysaccharide-challenged mice. Proteomics 12, 2024-2035.*Corresponding author.11. Leung J, Wei W, Liu L (2013). S-nitrosoglutathione reductase deficiency increasesmutagenesis from alkylation in mouse liver. Carcinogenesis (in press).12. Tang CH, Wei W, Hanes MA, Liu L (2013). Hepatocarcinogenesis driven by GSNORdeficiency is prevented by iNOS inhibition. Cancer <strong>Research</strong> (in press).28
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Jeoung-Sook Shin, Ph.D.Assistant Pr<strong>of</strong>essor, Department <strong>of</strong> Microbiology & Immunology<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong><strong>University</strong> <strong>of</strong> California San Francisco513 Parnassus Ave, HSE-201San Francisco, CA 94143-0414Tel: 415-476-5451Fax: 415-476-3939email: jeoung-sook.shin @ ucsf.eduDr. Jeoung-Sook Shin is an Assistant Pr<strong>of</strong>essor in the Department <strong>of</strong> Microbiology &Immunology. She completed her B.S. and M.S. in Chemistry at Seoul National<strong>University</strong>, Korea. She received her Ph.D. from Duke <strong>University</strong> and her postdoctoraltraining at Yale <strong>University</strong> as a Jane C<strong>of</strong>fin Childs Memorial Fund Postdoctoral Fellow.The Shin laboratory is interested in understanding the molecular mechanism and function<strong>of</strong> dendritic cell-mediated antigen presentation. Dr. Shin has previously found that theantigen-presenting molecule MHCII is ubiquitinated by MARCH1 ubiquitin ligase indendritic cells, and this ubiquitination mediates MHCII endocytosis and lysosomaldegradation. Her laboratory has recently found that the costimulatory molecule CD86 isalso ubiquitinated by MARCH1 and that this ubiquitination also mediates endocytosisand degradation <strong>of</strong> CD86. More recently, Dr. Shin’s laboratory has studied the functionalrole <strong>of</strong> this ubiquitination. This study indicates that MHCII ubiquitination is required forproper production <strong>of</strong> regulatory T cells (Tregs) in the thymus. Currently, Dr. Shin isinvestigating how MHCII ubiquitination contributes to Treg development and whetherTregs generated in MHCII ubiquitination-dependent manner are distinct in theirrepertoire or function.The Shin laboratory is also interested in understanding the role <strong>of</strong> the high affinity IgEreceptor FceRI expressed in dendritic cells. Although the role <strong>of</strong> FceRI in thepathogenesis <strong>of</strong> allergy is well-known, its physiologic role remains unclear. Dr. Shin’slaboratory has recently found that FceRI is constitutively endocytosed and transported tothe lysosomes in human dendritic cells and monocytes, and that this FceRIendolysosomal trafficking mediates cellular entry <strong>of</strong> circulating IgE contributing to serumIgE clearance. These findings suggest that FceRI expressed by dendritic cells andmonocytes may play an important role in regulating serum IgE concentration in humans.Her laboratory is currently investigating whether unusually high blood IgE levels foundin some human diseases is attributed to the alteration in FceRI endolysosomal traffickingthat results circulating IgE not efficiently entering cells but accumulating in the blood.Dr. Shin’s research programs are greatly benefited by many <strong>of</strong> the excellent core facilitiessupported by SABRE. Flow cytometry core is being used in a daily basis for most <strong>of</strong> theprojects. Microscopy facility is helping in situ analysis <strong>of</strong> dendritic cells in human tissues29
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>and also the analysis <strong>of</strong> protein distribution inside dendritic cells. Mouse physiology coreis being used to test the therapeutic potential <strong>of</strong> human IgE derivative that Dr. Shin hasrecently found to be immune-regulatory.Selected Publications1. Shin, JS, Shelburne, CP, Jin, C, LeFurgey, EA, Abraham, SN. Harboring <strong>of</strong>particulat allergens within secretory compartments by mast cells followingIgE/FcεRI-lipid raft mediated phagocytosis, J Immunol. 177:5791-5800, 20062. Shin, JS, Ebersold, M, Pypaert, M, Delamarre, L, Hartley, A, and Mellman, I.Surface expression <strong>of</strong> MHC class II in dendritic cells is controlled by regulatedubiquitination, Nature. 444:115-8, 20063. Bloom, O, Unternaehrer, JJ, Jiang, A, Shin, JS, Delamarre, L, Allen, P, andMellman, I. Spinophilin participates in information transfer at immunologicalsynapses, J Cell Biol. 181:203-11, 20084. Baravalle, G, Park, H, McSweeney, M, Ohmura-Hoshino, M, Matsuki, Y, Shin,JS. Ubiquitination <strong>of</strong> CD86 is a key mechanism in regulating antigen presentationby dendritic cells, J Immunology. 187:2966, 20115. Ma, JK, Platt MY, Eastham-Anderson, J, Shin, JS*, and Mellman, I*. MHCclass II distribution in dendritic cells and B cells is determined by ubiquitin chainlength, PNAS. 109:8820, 20126. *Shin, JS and Mellman, I contributed equally to this work30
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core FacilitiesCORE FACILITIES31
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core FacilitiesMouse Physiology and Morphology CoreDirector: Xiaozhu Huang, M.D.ObjectiveThe objective <strong>of</strong> the <strong>Sandler</strong> animal physiology and morphology core facility is to providesupport to investigators at UCSF and other research institutes for their asthma related research.The core facility has extensive experience in generating and analyzing variety <strong>of</strong> allergic animalmodels. Investigators gain insight to the molecular mechanisms <strong>of</strong> asthma development througheither full or partial service provided by the core laboratory. The core also provides training tostudents, technicians and post-doctoral fellows in techniques relevant to the animal models andtheir analysis.AccomplishmentsDuring the year 2012, the Animal Physiology and Morphology Core provided assistance to manyUCSF investigators, including Drs Mark Ansel, Kamran Atabai, Anthony deFranco, David Erle,Xiaozhu Huang, Limin Liu, Steve Nishmura, Jason Rock, Dean Sheppard and Art Weiss for theirprojects studying airway physiology, immunology, cell and molecular biology and other relatedareas. The service provided by the core lab has significant impacts on many <strong>of</strong> these asthmarelated projects.Dr. Dean Sheppard has been interested in studying biologic role <strong>of</strong> integrin α9β1, an integrinexpressed on airway smooth muscle, in asthma development. The core conducted lung functionstudies in mice lacking integrin α9β1 specifically in smooth muscle. These mice had increasedairway responsiveness in vivo, and loss or inhibition <strong>of</strong> integrin α9β1 also increased in vitroairway narrowing and airway smooth muscle contraction in murine and human airways.Contraction was enhanced in control airways by the higher-order polyamine spermine or by cellpermeablePIP2, but these interventions had no effect on airways lacking integrin α9β1 or treatedwith integrin α9β1-blocking antibodies. Enhancement <strong>of</strong> SSAT activity or knockdown <strong>of</strong>PIP5K1γ inhibited airway contraction, but only in the presence <strong>of</strong> functional integrin α9β1.Therefore, integrin α9β1 appears to serve as a brake on airway smooth muscle contraction byrecruiting SSAT, which facilitates local catabolism <strong>of</strong> polyamines and thereby inhibits PIP5K1γ.Targeting key components <strong>of</strong> this pathway could thus lead to new treatment strategies forasthma. This work has been published in Journal <strong>of</strong> Clinical Investigation last year.The core assisted Dr. Rosemary Ahnkurst’s group in studying TGFβ activation and signaling inexperimental models <strong>of</strong> allergen-induced asthma as potential therapeutic targets. We show thatinnate variation within TGFβ1 genetic modifier loci, Tgfbm2 and Tgfbm3, alters diseasesusceptibility. Specifically, Tgfbm2(129) and Tgfbm3(C57) synergize to reverse accentuatedairway hyperresponsiveness caused by low TGFβ1 levels in Tgfb1(+/-) mice <strong>of</strong> the NIH/OlaHsdstrain and the epistatic interaction between Tgfbm2(129) and Tgfbm3(C57) uncouples theinflammatory response to ovalbumin from those <strong>of</strong> airway remodeling and airwayhyperresponsiveness, illustrating independent genetic control <strong>of</strong> these responses. The studysuggests that differential inheritance <strong>of</strong> genetic variants <strong>of</strong> Tgfbm genes alters biologicalresponses to reduced TGFβ1 signaling in an experimental asthma model and TGFβ antagonists32
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core Facilitiesfor treatment <strong>of</strong> lung diseases might therefore give diverse outcomes, dependent on geneticvariation. This work resulted in a publication last year in Proc Natl Acad Sci U S A.The animal physiology core also provides assistance to current as well as former AAF awardeesfor their asthma related studies. Continuing support <strong>of</strong> their asthma related projects has beenappreciated by many former awardees. Dr. Kenneth Rock is one <strong>of</strong> the former AAF awardeesand continues working on asthma related projects. To help solve one <strong>of</strong> variable allergen relatedoutcomes (Aspergillus induced pulmonary fibrosis) that his group has encountered, the coreperformed an Aspergillus sensitized and challenged experiment to prove that mice developsignificant lung fibrosis using this model. Dr. Rock’s group is currently working on the projectwith the helpful information provided by the core.In 2012, we continued our efforts in developing more regimens to suit the needs <strong>of</strong> investigators.One such effort was to use InExpose aerosol machine (Scireq, Canada) for OVA challenges.Our current intra-nasal OVA challenge has been working very well and produces consistentresults but it is not the natural route <strong>of</strong> sensitization and challenge. Some investigators haveexpressed interest <strong>of</strong> using aerosol route for challenge. We then tried 1% OVA aerosol challengeaccording to the literature but failed to achieve allergen-induced phenotype. We are in theprocess <strong>of</strong> trying different dosages and have just obtained preliminary data suggesting 6% OVAmay be sufficient to produce an allergen-induced airway hyperresponsiveness and lunginflammation.Training and Integration with <strong>Sandler</strong> ProgramThe core continues to provide training support for lab members whose PIs are interested inadapting the protocols and techniques that are useful for their studies. In 2012, investigators frommultiple laboratories (Labs from UCSF- Drs. Jeffery Cox and David Erle; other researchinstitutes - Dr. Michael Daines at <strong>University</strong> <strong>of</strong> Arizona, Dr. Daniel Conrad at VirginiaCommonwealth <strong>University</strong>) sent lab members to the core lab in observing the routineperformance and getting hands-on experience using the equipment and protocols. Jamie Sturgilland Jennifer Bradley from Virginia Commonwealth <strong>University</strong> made a special trip to visit us lastyear. They spent time observing core lab performing experiments and processing routinesamples, and obtained protocols used routinely by the core lab. Currently Dr. Conrad’s group hasbeen able to run lung function analysis in the studies <strong>of</strong> murine models <strong>of</strong> allergic asthma fortheir related studies. Core lab follows-up and communicates with former trainees the continuesthe information exchange that has benefitted studies for both sides.The animal core director attends the weekly UCSF pulmonary conference, monthly SABREmeeting, AAF and ATS annual meetings. This provides direct interaction between the animalcore and investigators who are interested in using the core service. The potential experiments canbe discussed and <strong>of</strong>tentimes can be arranged during the interaction.Future ActivitiesWe continually try to improve our techniques and develop allergic model regimens so that wemay accommodate the investigators needs. As mentioned above, we have been working on33
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core Facilitiesdeveloping protocol using the aerosol machine for allergen challenge. Our preliminary datasuggest that 6% OVA aerosol for 30 minutes once a day for 3 days may produce significantOVA-induced lung function change and lung inflammation. Further study is needed to validateour observation. We still need to improve the consistency and reduce the variability <strong>of</strong> our housedust mite model. We have been paying close attention to our key equipment. FlexiVent makesthe key lung function measurement possible. The machine model we are using has beendiscontinued and technical service will soon no longer be available. With the continuing supportfrom the SABRE foundation, the animal physiology and microscopy core facility will continueto provide UCSF investigators with high quality servicecritical to our research community.Grants and Publications:GrantThe animal physiology and morphology core continues to work with investigators at UCSF aswell as at other research institutes to provide consultations and support letters for their grantapplications. Core director generally meets in person (with local investigators) or has phoneconversations (with outside institutions) with grant applicants in helping them understand theprinciples <strong>of</strong> allergic animal models and carefully discuss the experimental protocols, animalnumbers and final outcomes. The AAF applicants very <strong>of</strong>ten have not worked with asthma, andsome not even with lung disease. For applicants who propose an in vivo animal experiment, abrief description <strong>of</strong> an allergic animal model will be provided so investigators can incorporate itinto their proposals. We not only help investigators funded by NIH, AAF and other grants butalso, if they are funded, make commitments to collaborate with investigators who submitproposals. The core staff receive direct salary support from <strong>Sandler</strong> foundation, NIAID and ourrecharge system.Publications1. Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ, HuangX, Locksley RM, Fahy JV. A protective role for periostin and TGF-β in IgE-mediatedallergy and airway hyperresponsiveness. Clin Exp Allergy. 2012 Jan; 42(1):144-55.PMID: 220931012. Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, RaymondWW, Erle DJ, Abrink M, Caughey GH, Huang X, Sheppard D. The αvβ6 integrinmodulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. JClin Invest. 2012 Feb 1; 122(2):748-583. Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M,Huang X, Interlandi J, Djuretic IM, Brown DR, Sharpe AH, Rao A, Ansel KM.Interleukin-4 production by follicular helper T cells requires the conserved Il4 enhancerhypersensitivity site V. Immunity. 2012 Feb 24;36(2):175-874. Makoto Kudo, Andrew C. Melton, Chun Chen, Mary B. Engler, Katherine E. Huang, XinRen, Yanli Wang, Xin Bernstein, John T. Li, Kamran Atabai, Xiaozhu Huang, Dean34
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core FacilitiesSheppard. IL-17A produced by ab T cells drives airway hyper-responsiveness in miceand enhances mouse and human airway smooth muscle contraction. Nature Medicine.2012 March 4;18(4)547-545. Thornton E, Looney M, Sheppard D, Locksley R, Huang X, Krummel M. Dynamics <strong>of</strong>antigen capture and presentation revealed by two-photon live imaging in the lung. J ExpMed. 2012 Jun 4;209(6):1183-996. Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ.AGR2 is Induced in <strong>Asthma</strong> and Promotes Allergen-Induced Mucin Overproduction. AmJ Respir Cell Mol Biol. 2012 Aug;47(2):178-857. Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A,Burlingame AL, Matthay MA, Sheppard D. IQGAP1 is necessary for pulmonary vascularbarrier protection in murine acute lung injury and pneumonia. Am J Physiol Lung CellMol Physiol. 2012 Jul 1;303(1):L12-98. Chun Chen, Makoto Kudo, Florentine Rutaganira, Hiromi Takano, Candace Lee, AmhaAtakilit, Toshimitsu Uede, Paul Wolters, Stephen Liggett, Kevan M. Shokat, XiaozhuHuang and Dean Sheppard . Integrin α9β1 in airway smooth muscle regulates a novelbrake on exaggerated murine and human airway narrowing. JCI 2012 2012 Aug1;122(8):2916-279. Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD,Donne ML, Huang X, Sheppard, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M,Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16A modulatesmucin secretion and airway smooth muscle contraction. Proc Natl Acad Sci U S A. 2012Oct 2;109(40):16354-9.10. Julia Freimuth, Frederic F. Clermont, Xiaozhu Huang, Angela De Sapio, TakuTokuyasu, Dean Sheppard, Rosemary J. Akhurst. Epistatic interactions between Tgfb1and genetic loci, Tgfbm2 and Tgfbm3, determine susceptibility to an asthmatic stimulus.Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18035
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core FacilitiesFunctional Genomics CoreDirector: David J. Erle, M.D.Associate Director: Andrea BarczakObjectiveTo make cutting-edge functional genomics technology readily available for investigatorsresearching questions relevant to basic biology <strong>of</strong> asthma.AccomplishmentsSupported projects: We continue to support many investigators studying immunology, airwaycell biology, lung development, and other relevant areas. This includes faculty members whoparticipate actively in a range <strong>of</strong> SABRE activities (including Ansel, Chapman, Erle, Krummel,Seaman, Sheppard, and Woodruff) and AAF-funded investigators (Keoki Williams and DanielConrad). Here is a partial listing <strong>of</strong> relevant projects that have been completed this year or arestill in progress:PIMark AnselMark AnselMark AnselHarold ChapmanHarold ChapmanDavid ErleDavid ErleDaniel Conrad 1 *Matthew KrummelMehrdad MatloubianJason RockWilliam SeamanDean SheppardProject (status)Effect <strong>of</strong> Eri1 on mRNA pr<strong>of</strong>ile and translation in natural killercells. Completed.The miR-17~92 cluster regulates TFH cell differentiation.Completed.Empirical determination <strong>of</strong> miRNA targets with comparativeRNA-Seq and PAR-CLIP. In Progress.Beta4 positive AECs vs. B4 negative AECs. Completed.Ephb2/B4 progenitor isolation. Completed.IL-13 and IL-17 responsive microRNAs in the airwayepithelium. Completed.RNA-Seq analysis <strong>of</strong> IL-13 treated airway epithelium.Completed.The Kainic Acid Receptor-ADAM10 Axis in <strong>Asthma</strong>. InProgress.Chemotactic circuits in a mouse tumor model. Completed.Plasmacytoid Dendritic Cell Response to a Viral InfectionCompleted.Gene expression differences between WT and MMP3 KO afterpneumonectomy. In Progress.Microglia expression pr<strong>of</strong>ile after TBI. Completed.RNA-Seq analysis <strong>of</strong> the role <strong>of</strong> integrin alpha-v in lung andliver fibrosis. In Progress.36
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core FacilitiesKeoki Williams 2 *Asa Wheelock 3Prescott WoodruffPharmacogenomics <strong>of</strong> asthma. Ongoing.COPD and Gender (COSMIC). Completed.miRNA pr<strong>of</strong>iling in asthma patients. Ongoing.1 Virginia Commonwealth <strong>University</strong>, 2 Henry Ford Health,3 Karolinska Institutet, *AAF awardeePersonnelAndrea Barczak (manager), Rebecca Barbeau (SRA), and Joshua Pollack (biostatistician)continue to provide outstanding service to core users. In aggregate, they have over 20 years <strong>of</strong>experience as core staff.New technologiesThe SABRE functional genomics core is a resource for the UCSF community (and beyond); wecollaborate on more than 65 projects per year. SABRE support is critical for development andimplementation <strong>of</strong> new methods. Priority is given to development <strong>of</strong> methods required forstudies requested by SABRE investigators. Although microarrays continue to be a very usefuland cost effective method for genome-wide pr<strong>of</strong>iling <strong>of</strong> mRNAs and miRNAs, recent advancesin sequencing technology <strong>of</strong>fer substantial advantages over arrays for most global mRNA andmiRNA pr<strong>of</strong>iling projects. RNA-Seq has superior sensitivity, specificity and reproducibility;<strong>of</strong>fers the potential for novel RNA discovery (especially important for non-coding RNAs); andcan be used for analyzing transcript variation (alternative splicing and polyadenylation, allelespecificexpression). Supply costs for RNA-Seq continue to drop and are now roughly similar tocosts for array experiments. Over the past two years, an increasing number <strong>of</strong> users haveswitched to RNA-Seq technologies. We have seen a rise from 2 projects in 2011 to 13 projects in2012 and anticipate that this trend will continue to grow at a rapid rate. This past year, we havefocused the majority <strong>of</strong> our efforts on implementing the following next generation sequencingservices: 1) methods for analysis <strong>of</strong> partially degraded or FFPE RNA, 2) improving samplethroughput for small RNA-Seq and 3) continued development <strong>of</strong> state-<strong>of</strong>-the-art methods foranalyzing next generation sequencing data.1) RNA-Seq analysis <strong>of</strong> degraded RNA: Standard mRNA-Seq library construction methodscontain an initial poly(A) RNA selection to remove highly abundant rRNA species beforeamplification; this method presents a challenge for clinical samples where degradation can be acommon problem. We have employed an alternative non-poly(A) based ribosomal depletionmethod (Ribo-Zero, Epicentre, Inc.) to accommodate samples that have compromised RNAintegrity. An additional benefit <strong>of</strong> this technology is that it recovers the 5’and 3’ ends <strong>of</strong> bothmRNAs and long non-coding RNAs. This is an attractive alternative to standard protocols forinvestigators who are interested novel gene discovery and expression <strong>of</strong> lncRNAs. We arecurrently analyzing data from our first set <strong>of</strong> ribosomal depleted samples.2) Improving sample throughput for small RNA-Seq: miRNA expression pr<strong>of</strong>iling accountsto approximately 20% <strong>of</strong> the projects that we process, including studies with Mark Ansel andPrescott Woodruff who are working on determining the miRNA expression patterns that areassociated with asthma pathology and response to therapy. Small RNA-Seq library preparation37
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core Facilitiesprotocols require a gel purification based size selection step for each sample library. This processis laborious, time consuming, and not amenable to higher-throughput methods. We haveimplemented a newer library preparation method that allows us to multiplex libraries before gelpurification, which cuts down the turn-around time substantially. Additionally, the ability tomultiplex small RNA-Seq libraries significantly decreases the associated costs for the samplepreparation and sequencing by up to as much as 50%. To date, we have processed over 24samples using this new method.3) Development <strong>of</strong> data analysis pipelines: RNA-Seq data analysis presents many newchallenges including alignment <strong>of</strong> millions <strong>of</strong> reads to a reference transcriptome (or genome),assembly <strong>of</strong> read alignments, and development <strong>of</strong> new algorithms for normalization andstatistical analysis <strong>of</strong> differential expression. Different tools are required to carry out each step <strong>of</strong>the analysis and the size and complexity <strong>of</strong> the datasets make the process computationallyexpensive and time consuming. This past year our biostatistician, Josh Pollack, has madesignificant progress towards developing pipelines that incorporate state-<strong>of</strong>-the-art algorithmsfrom publicly available s<strong>of</strong>tware, thereby streamlining the process and decreasing the turnaroundtime. We have implemented these new pipelines, and have analyzed data from bothmRNA and miRNA studies. NGS data analysis requires substantial processing power and it wasessential for us to acquire a new computer. SABRE funding contributed to the purchase <strong>of</strong> a 12-core workstation with 96 GB <strong>of</strong> RAM and 6TB <strong>of</strong> disk storage.Plans for the coming yearWe have optimized protocols for library generation and other steps in the process and now <strong>of</strong>fercomprehensive RNA-Seq services (including library preparation, coordination <strong>of</strong> sequencingruns, and data analysis), which relieves investigators <strong>of</strong> the need to develop and optimizeprotocols in their own laboratories. We have begun a new initiative to reduce turnaround time forRNA-Seq projects. SABRE support allows us to provide a substantial discount to SABREinvestigators and in recognition <strong>of</strong> this support SABRE projects receive higher priority than non-SABRE projects.We will continue to <strong>of</strong>fer assistance with whole-genome analysis <strong>of</strong> mRNA and miRNAexpression and ChIP analysis. In addition to the array-based analyses <strong>of</strong>fered in previous years,we are encouraging investigators to employ next generation sequencing-based approaches(RNA-Seq, small RNA-Seq, and ChIP-Seq) where appropriate. Further work is planned toimplement protocols that will allow the following: 1) analysis <strong>of</strong> very limited amounts <strong>of</strong> startingmaterial (starting with as little as 5 ng <strong>of</strong> total RNA), 2) strand-specific library preparation foranalysis <strong>of</strong> non-coding RNAs and antisense transcription and improved annotation <strong>of</strong> novelgenes, and 3) ChIP-Seq library preparation and analysis. We will also continue to implementstate-<strong>of</strong>-the-art methods for analyzing next generation sequencing data. Short-term goals includethe development <strong>of</strong> pipelines for the following: 1) analysis <strong>of</strong> differential is<strong>of</strong>orm expression, 2)analysis <strong>of</strong> non-coding RNAs and 3) implementation <strong>of</strong> visualization tools to display nextgenerationsequencing data more effectively.Training and Integration with SABRE and AAF programsWe provide extensive consultation and training for investigators using the core. We meet witheach group (PI and other group members, including students and postdoctoral fellows) to help38
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core Facilitieswith project planning. We help with grant preparation, sample size determination, appropriateselection <strong>of</strong> controls, and RNA extraction protocols. We meet with each group again after theinitial data analysis to discuss the results and provide guidance about further analysis. Whenwork is being readied for publication, we assist with preparation <strong>of</strong> figures and tables andsubmission <strong>of</strong> the results to a public database (NCBI’s Gene Expression Omnibus [GEO]). Thecore director attends the monthly UCSF SABRE <strong>Research</strong> Conference and the annual AAFmeeting. This allows many opportunities for raising awareness about the core services amongSABRE and AAF investigators. Although we continue to work with other investigators in orderto maintain the high volume needed to operate in an economical manner, SABRE projectsreceive the highest priority.In late 2012, David Erle (contact PI) and Esteban Burchard (co-PI) submitted a K12 proposal inresponse to an NIH RFA for “Career Development Programs in Omics <strong>of</strong> Lung Diseases.” 30UCSF faculty members with expertise in genomics (and other “omics”) and in asthma and otherlung diseases agreed to participate. If successful, this proposal will provide salary support for 2junior faculty K scholars per year to receive training in applying omics technologies to lungdiseases.Grants and PublicationsGrantsThe core is now supported by SABRE and by a recharge mechanism, which covers labor andreagent costs for projects performed by the core. Core staff members work closely withinvestigators as they apply for NIH funding via R01 and other mechanisms. The following listincludes asthma-related projects supported by the core that received new NIH funding during thelast year and projects now applying for additional funding from NIH:PI Project Number TitleAnsel, Karl Mark 1R01HL109102-01 Role <strong>of</strong> miRNAs In Th2-drivenInflammation in <strong>Asthma</strong>Chapman, Harold A 1U01HL111054-01Epithelial Progenitor Cells in LungRepair and RegenerationGerber, Anthony N 1R01 HL109557-01A1 Role <strong>of</strong> Klf15 in Airway Smooth Muscleand the Response to GlucocorticoidsPending:Sheppard, Dean 5U19AI077439 Mechanisms <strong>of</strong> Initiation and Persistence<strong>of</strong> Allergic <strong>Asthma</strong>(funding expected based on impact score <strong>of</strong> 20 and assurances from NIH program <strong>of</strong>ficial)Frank, James A R21 HL111707-01A1 A Claudin-18 Deficiency in thePathogenesis <strong>of</strong> <strong>Asthma</strong>Erle, David J and Burchard, E (multi-PI)UCSF Career Development Program in39
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core Facilities1K12HL119997-01 Omics <strong>of</strong> Lung DiseasesArjomandi, Mehrdad VA I21 Respiratory Tract Immune System in Gulf War IllnessPlanned for submission in early 2013:Rock, Jason AAF Tmem16a as a modulator <strong>of</strong> airway mucin dynamicsRock, Jason NIH (R21) Requirement for spontaneous contractions <strong>of</strong> the embryonicairway smooth muscle during lung developmentKeoki Williams NIH (R01) Combined transcriptomics and genomics to find asthmagenes in admixed populations(the core has a subcontract to perform RNA-Seq for roughly 800 samples on this grant proposal)The UCSF Clinical and Translational Sciences Institute provided some core funding until July2011, when CTSI elected not to continue to support any core laboratories. This reduced our nonrechargefunding. Hence continued support from SABRE is critical in allowing us to maintainservices and develop new technologies for support <strong>of</strong> SABRE investigators.PublicationsCore-supported projects led by SABRE-affiliated faculty:1. Bronevetsky Y, Villarino A, Eisley C, Barbeau R, Barczak AJ, Tarakhovsky A, Erle DJ,Rao A, Ansel KM. T cell activation induces proteasomal degradation <strong>of</strong> Argonaute andrapid remodeling <strong>of</strong> the microRNA repertoire. J Exp Med. In press.2. Diez D, Goto S, Fahy JV, Erle DJ, Woodruff PG, Wheelock ÅM, Wheelock CE.Network analysis identifies a putative role for the PPAR and type 1 interferon pathwaysin glucocorticoid actions in asthmatics. BMC Med Genomics. 2012 Jun 19;5:27.3. Levänen B, Bhakta NR, Paredes PT, Barbeau R, Hiltbrunner S, Pollack JL, Sköld CM,Svartengren, Grunewald J, Gabrielsson S, Eklund A, Larsson, Woodruff PG, David J ErleDJ, Wheelock AM. Altered MicroRNA pr<strong>of</strong>iles in Bronchoalveolar Lavage FluidExosomes in <strong>Asthma</strong> J Allergy Clin Immunol. In press.4. Seumois G, Vijayanand P, Eisley CJ, Omran N, Kalinke L, North M, Ganesan AP,Simpson LJ, Hunkapiller N, Moltzahn F, Woodruff PG, Fahy JV, Erle DJ, Djukanovic R,Blelloch R, Ansel KM. An Integrated nano-scale approach to pr<strong>of</strong>ile miRNAs in limitedclinical samples. Am J Clin Exp Immunol. 2012 Nov 30;1(2):70-89.5. Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M,Nguyen C, Barczak AJ, Zlock LT, Blagev DP, Finkbeiner WE, Ansel KM, Arron JR,Erle DJ, Woodruff PG. Airway epithelial miRNA expression is altered in asthma. Am JRespir Crit Care Med. 2012 Nov 15;186(10):965-74.6. Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, RaymondWW, Erle DJ, Abrink M, Caughey GH, Huang X, Sheppard D. The αvβ6 integrinmodulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. JClin Invest. 2012 Feb 1;122(2):748-58.40
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core Facilities7. Thomas MF, Abdul-Wajid S, Panduro M, Babiarz JE, Rajaram M, Woodruff P, LanierLL, Heissmeyer V, Ansel KM. Eri1 regulates microRNA homeostasis and mouselymphocyte development and antiviral function. Blood. 2012 Jul 5;120(1):130-42.Under review:Hsieh CL, Kim CC, Ryba BE, Niemi EC, Locksley RM, Liu J, Nakamura MC, Seaman WE.Traumatic brain injury induces unique macrophage subsets. Submitted.Sasse SK, Mailloux CM, Barczak AJ, Wang Q, Jain MK, Haldar SM, Gerber AN. GR and Klf15regulate gene expression dynamics and integrate signals through feed forward circuitry. Inrevision.Zhao W, Pollack JL, Blagev D, Erle DJ. Massively parallel mapping <strong>of</strong> 3’ UTR cis-regulatoryelements controlling mRNA stability. Submitted.Other core-supported projects:Broadhurst MJ, Ardeshir A, Kanwar B, Mirpuri J, Gundra UM, Leung JM, Wiens KE, Vujkovic-Cvijin I, Kim CC, Yarovinsky F, Lerche NW, McCune JM, Loke P. Therapeutic helminthinfection <strong>of</strong> macaques with idiopathic chronic diarrhea alters the inflammatory signature andmucosal microbiota <strong>of</strong> the colon. PLoS Pathog. 2012 Nov;8(11):e1003000.Petrillo LA, Wolf DM, Kapoun AM, Wang NJ, Barczak A, Xiao Y, Korkaya H, Baehner F,Lewicki J, Wicha M, Park JW, Spellman PT, Gray JW, van't Veer L, Esserman LJ. Xenograftsfaithfully recapitulate breast cancer-specific expression patters <strong>of</strong> parent primary breast tumors.Breast Cancer Res Treat. 2012 Oct;135(3):913-22.Under review:So AY, Garcia-Flores Y, Minisandram A, Martin A, Taganov K, Boldin M, Baltimore D.Regulation <strong>of</strong> APC development, immune response, and autoimmunity by Bach1/HO-1 pathwayin mice. Blood 2012 Sep 20;120(12):2428-37.Baraban SC, Dinday MT, Hortopan GA. Drug screening for Dravet syndrome using Scn1azebrafish mutants. Submitted.Hortopan GA and Baraban SC. Morpholino-based knockdown <strong>of</strong> LIS1 in zebrafish larvae:analysis <strong>of</strong> seizure activity and differential gene expression using microarrays. Submitted41
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core FacilitiesGenetics CoreDirector: Esteban González Burchard, M.D., M.P.H.,Pr<strong>of</strong>essor <strong>of</strong> Bioengineering & Therapeutic Sciences and MedicineVision: We built a multidisciplinary research center and we are taking a comprehensiveapproach to asthma research. Our approach integrates gene-environment studies with basicbiology, population genetics, social and environmental epidemiology. We built a network <strong>of</strong>“minority serving” providers to recruit well-phenotyped subjects from throughout the U.S. andPuerto Rico. As a result <strong>of</strong> our efforts we have assembled the largest gene-environment studypopulation <strong>of</strong> minority children with and without asthma in the U.S. (total sample n = 9,144).Finally, we have recruited two new faculty, Dara Torgerson, Ph.D. and Noah Zaitlen, Ph.D. intothe Lung Biology <strong>Center</strong>. Both scientists are up and coming leaders in their respective fields(described below).We have established the <strong>Asthma</strong> Genetics Core Facility to facilitate asthma genetic researchamong <strong>Sandler</strong>/AAF-sponsored investigators. In keeping with the Mission <strong>of</strong> the original<strong>Sandler</strong>-sponsored <strong>Asthma</strong> <strong>Research</strong> Program, we have made this resource “Open Source” byhaving an extensive range <strong>of</strong> national and international collaborations. We <strong>of</strong>fer investigators a“full service <strong>of</strong> genetic testing and analyses.” Specifically, we analyze promising candidate genesidentified by investigators using biologic material (DNA and plasma) from large wellphenotypedfamily-based and case-control asthma studies <strong>of</strong> racially/ethnically diverse subjects.This past year the Genetics Core Facility has focused on three main goals: 1) collaboration 2)well-phenotyped patient recruitment <strong>of</strong> Latino and African American subjects with and withoutasthma and 3) faculty recruitment.Accomplishments in 2012:1) Collaboration:a) UCSF <strong>Sandler</strong> Program and the AAF: In the era <strong>of</strong> large “Team Science” the value andimportance <strong>of</strong> collaboration cannot be overstated. We have made the existing cohortswidely available to <strong>Sandler</strong> sponsored investigators (UCSF <strong>Sandler</strong> Program and theAAF). The <strong>Asthma</strong> Genetics Core assists investigators with study design and providesgenotyping and expertise with statistical genetic analyses. We also allow our data to beused for replication <strong>of</strong> promising results from other investigative groups.b) Collaborating Faculty: In 2012 we have collaborated with the following faculty: RyanHernandez (UCSF), Carol Ober (Chicago), Kathleen Barnes (John’s Hopkins), EvanEicher (<strong>University</strong> <strong>of</strong> Washington), Jim Gauderman (USC), Charles Rotimi(NIH/NHGRI), Noah Zaitlen (UCSF), Mario Castro (Washington <strong>University</strong>), L. KeokiWilliams (Henry Ford), Fernando Martinez (<strong>University</strong> <strong>of</strong> Arizona), Max Seibold(National Jewish), Neil Trivedi (UCSF), Rajesh Kumar (Children’s Memorial Hospital,Chicago), Pedro Avila (Northwestern <strong>University</strong>), Stephanie London (NIEHS), ScottWeiss (Harvard), and Kathy Giacomini (UCSF). We have contributed our data to nationaland international consortiums to study asthma, obesity and eczema.42
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core Facilitiesc) We have generated genome wide association studies (GWAS) data from our studypopulations and provided our results as in silico replication to supplement initial findingsfor several <strong>Sandler</strong> investigators. In addition, we have worked closely with asthmainvestigators throughout the country to advance the field by collectively working towardstesting and replicating novel genetic associations identified from basic science models(animal and human) to GWAS.d) EVE <strong>Asthma</strong> Consortium: This past year we participated in the NHLBI-sponsored EVE<strong>Asthma</strong> Consortium, which was published in Nature Genetics. The lead author, DaraTorgerson, was recently recruited to UCSF as an Assistant Pr<strong>of</strong>essor in the Lung Biology<strong>Center</strong>.Admixture Mapping Meta-analysis in EVE: Through our work with the EVEConsortium we are currently working on a follow-up to the original GWAS to findadditional loci via ancestry association or admixture mapping. We have combined datafrom the African-American and Hispanic/Latino studies within EVE (9 studies in total),comprising over 7,000 individuals. For all individuals we have estimated local ancestryusing LAMP algorithms to have locus-specific ancestry calls for all individuals across thegenome. The studies comprise a diverse group <strong>of</strong> designs including case-control, triobased,and pedigrees. Within this project we have developed novel ancestry imputationmethods as well as methods for admixture mapping on large datasets. For replication wehave leveraged our study, GALA II, as it is the largest study <strong>of</strong> asthma with genome-widedata in U.S. minority pediatric populations. We identified SMAD2 as a novel risk factorfor asthma in Latino populations. Our results were supported by differential geneexpression between children with and without asthma. We did not find the sameassociation in African or European Americans. The manuscript has been approved by allco-authors and will be submitted to Nature Genetics by January 2013.e) Genentech (Joe Aaron): We have entered into a collaboration with Genentech to test theability to use biomarkers (periostin) and clinical phenotypes to stratify children withasthma into high and low drug responders to Genentech’s new anti-IL13 therapy(Lebrikizumab).f) Gene expression: We are collaborating with Mario Castro (Washington <strong>University</strong>) andMax Seibold (National Jewish Health <strong>Center</strong>) to compare gene expression in lungtissue/lavage vs. nasal epithelia vs. peripheral blood. We hypothesize that nasal epithelialtissue gene expression will be a good proxy for gene expression in the lung. If ourhypothesis is correct, it will facilitate large scale sampling <strong>of</strong> nasal epithelial tissue fromhundreds <strong>of</strong> children with and without asthma.2) Patient Recruitment: We recruited three study populations: 1) the Genetics <strong>of</strong> <strong>Asthma</strong> inLatino Americans (GALA I) Study (parent-child trios), a family-based study <strong>of</strong> Puerto Ricanand Mexican children with asthma 2) the Genes-environments & Admixture in Latino<strong>Asthma</strong>tics (GALA II) Study, a case-control study at 5 urban centers across the U.S. and 3)the Study <strong>of</strong> African Americans <strong>Asthma</strong>, Genes & Environments (SAGE), a case-controlstudy in the San Francisco Bay Area. Combined we now have the largest pediatric asthma43
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core Facilitiesgenetic study <strong>of</strong> Latino and African American pediatric populations in the U.S. (n > 9,144children with and without asthma). We are continuing to recruit and better characterizechildren with and without asthma.In collaboration with Genentech we are performing a longitudinal study <strong>of</strong> asthma treatmentand control in a subset <strong>of</strong> the study population (n~1000). All subjects are extensively wellphenotyped. We have detailed clinical measurements (spirometry, maximal bronchodilatorresponse testing, methacholine challenge, skin prick testing, and IgE measurements, exhalednitric oxide, skin color reflectance), biologic specimens (serum, RNA, DNA and nasalepithelia), geocoded air pollution measures and questionnaire-based information regardingenvironmental and social risk factors. We have genome-wide data available for all GALA Iand II participants. We will combine biomarkers and sub-phenotypes to identify high and lowdrug responders to anti-IL13 therapy.3) Faculty Recruitment: We have recruited two young scientists, who have significantresearch experience in minority populations and asthma genetics. Dara Torgerson, Ph.D. led thelargest genomewide meta-analysis <strong>of</strong> asthma in the U.S., which was published in NatureGenetics. She trained with Drs. Carole Ober and Dan Nicolae <strong>of</strong> the <strong>University</strong> <strong>of</strong> Chicago. Wealso recruited Dr. Noah Zaitlen from the Harvard <strong>University</strong> School <strong>of</strong> Public Health. Hisexpertise is gene-environment interactions and he will apply his theoretical methods to studyasthma in minority populations.Financial Support:We have been successful at leveraging <strong>Sandler</strong> funding with NIH and industry support. Theaforementioned efforts are supported by the equivalent <strong>of</strong> four NIH RO1 grants.Active Grants:1. 1R01 HL104608-01A1: subcontract ~ $400,000, September 28, 2011-June 30, 2015, PI:Barnes2. 1P60 MD006902: ~$1 million, August 27, 2012-February 28, 2017. PI, Bibbins-Domingo. Project PI: Burchard.Grants under review:1. K24 - Epigenomics <strong>of</strong> tobacco exposure on asthma severity among minority children2. RO1 – Sequencing Candidate Genes Under Admixture Mapping Peaks in Latino<strong>Asthma</strong>tics. Under review.3. RO1 - Pharmacogenomics <strong>of</strong> Bronchodilator Response in Minority Children with<strong>Asthma</strong>. Scored in the 9 th percentile. Pending council review.44
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core FacilitiesPublications supported by the Strategic Program for <strong>Asthma</strong> <strong>Research</strong> and/or the <strong>Sandler</strong>Family Foundation.2012 Publications1. Fejerman, L., Chen, G.K., Eng, C., Huntsman, S., Hu, D., Williams, A., Pasaniuc, B.,John, E.M., Via, M., Gignoux, C., Ingles, S., Monroe, K.R., Kolonel, L.N., Torres-Mejía,G., Pérez-Stable, E.J., González Burchard E., Henderson, B.E., Haiman, C.A., Ziv E.(2012) Admixture mapping identifies a locus on 6q25 associated with breast cancer riskin US Latinas. Hum Mol Genet. 2012 Jan 19.2. Kumar R, Tsai HJ, Hong X, Gignoux C, Pearson C, Ortiz K, Fu M, Pongracic JA,Burchard EG, Bauchner H, Wang X. African ancestry, early life exposures, andrespiratory morbidity in early childhood. Clin Exp Allergy. 2012 Feb;42(2):265-74. doi:10.1111/j.1365-2222.2011.03873.x. Epub 2011 Sep 25. PMID: 220930773. Xu H, Cheng C, Devidas M, Pei D, Fan Y, Yang W, Neale G, Scheet P, Burchard EG,Torgerson DG, Eng C, Dean M, Antillon F, Winick NJ, Martin PL, Willman CL, CamittaBM, Reaman GH, Carroll WL, Loh M, Evans WE, Pui CH, Hunger SP, Relling MV,Yang JJ. ARID5B genetic polymorphisms contribute to racial disparities in the incidenceand treatment outcome <strong>of</strong> childhood acute lymphoblastic leukemia. J Clin Oncol. 2012Mar 1;30(7):751-7. Epub 2012 Jan 30. PMID: 222910824. Galanter, J.M., Fernandez, J.C., Gignoux, C.R., Barnholtz-Sloan, J., Fernandez, C., Via,M., Hidalgo-Miranda, A., Contreras, A.V., Uribe Figueroa, L., Raska, P., Jimenez-Sanchez, G., Silva Zolezzi, I., Torres, M., Ruiz Ponte, C., Ruiz, Y., Salas, A., Nguyen, E.,Eng, C., Borjas, L., Zabala, W., Barreto, G., Rondón, F., Ibarra, A., Taboada, P., Porras,L., Moreno, F., Bigham,A., Gutierrez, G., Brutsaert, T., León-Velarde, F., Moore, L.G.,Vargas, E., Cruz, M., Escobedo, J., Rodriguez-Santana, J., Rodriguez-Cintrón, W.,Chapela, R., Ford, J.G., Bustamante, C.D., Seminara, D., Shriver, M.D., Ziv, E.,Burchard, E.G., Haile, R., Parra, E., Carracedo, A. for the Latin American CancerEpidemiology (LACE) Consortium. Development <strong>of</strong> a Panel <strong>of</strong> Genome-wide AncestryInformative Markers to Study Admixture Throughout the Americas. PLoS Genet. 2012Mar;8(3):e1002554. Epub 2012 Mar 8. PMID: 224123865. Avena S, Via M, Ziv E, Pérez-Stable EJ, Gignoux CR, Dejean C, Huntsman S, Torres-Mejía G, Dutil J, Matta JL, Beckman K, Burchard EG, Parolin ML, Goicoechea A,Acreche N, Boquet M, Ríos Part Mdel C, Fernández V, Rey J, Stern MC, Carnese RF,Fejerman L. Heterogeneity in genetic admixture across different regions <strong>of</strong> Argentina.PLoS One. 2012;7(4):e34695. 2012 Apr 10. PMID: 225060446. Fejerman L, Chen GK, Eng C, Huntsman S, Hu D, Williams A, Pasaniuc B, John EM,Via M, Gignoux C, Ingles S, Monroe KR, Kolonel LN, Torres-Mejía G, Pérez-Stable EJ,Burchard EG, Henderson BE, Haiman CA, Ziv E. Admixture mapping identifies a locuson 6q25 associated with breast cancer risk in US Latinas. Hum Mol Genet. 2012 Apr15;21(8):1907-17. Epub 2012 Jan 6. PMID: 222280987. Kenny EE, Timpson NJ, Sikora M, Yee MC, Moreno-Estrada A, Eng C, Huntsman S,45
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core FacilitiesBurchard EG, Stoneking M, Bustamante CD, Myles S. Melanesian blond hair is causedby an amino acid change in TYRP1. Science. 2012 May 4;336(6081):554. PMID:225562448. Baran Y, Pasaniuc B, Sankararaman S, Torgerson DG, Gignoux C, Eng C, Rodriguez-Cintron W, Chapela R, Ford JG, Avila PC, Rodriguez-Santana J, Burchard EG,Halperin E. Fast and accurate inference <strong>of</strong> local ancestry in Latino populations.Bioinformatics. 2012 May 15;28(10):1359-67. Epub 2012 Apr 11. PMID: 224957539. Torgerson DG, Capurso D, Ampleford EJ, Li X, Moore WC, Gignoux CR, Hu D, Eng C,Mathias RA, Busse WW, Castro M, Erzurum SC, Fitzpatrick AM, Gaston B, Israel E,Jarjour NN, Teague WG, Wenzel SE, Rodríguez-Santana JR, Rodríguez-Cintrón W,Avila PC, Ford JG, Barnes KC, Burchard EG, Howard TD, Bleecker ER, Meyers DA,Cox NJ, Ober C, Nicolae DL. Genome-wide ancestry association testing identifies acommon European variant on 6q14.1 as a risk factor for asthma in African Americansubjects. J Allergy Clin Immunol. 2012 May 17. PMID: 2260799210. Oh SS, Tcheurekdjian H, Roth LA, Nguyen EA, Sen S, Galanter JM, Davis A, Farber HJ,Gilliland FD, Kumar R, Avila PC, Brigino-Buenaventura E, Chapela R, Ford JG, LeNoirMA, Lurmann F, Meade K, Serebrisky D, Thyne S, Rodriguez-Cintron W, Rodriguez-Santana JR, Williams LK, Borrell LN, Burchard EG. Effect <strong>of</strong> secondhand smoke onasthma control among black and Latino children. J Allergy Clin Immunol (2012). 2012Jun;129(6):1478-83.e7. Epub 2012 Apr 30. PMID: 2255210911. Aldrich MC, Kumar R, Colangelo LA, Williams LK, Sen S, Kritchevsky SB, MeibohmB, Galanter J, Hu D, Gignoux CR, Liu Y, Harris TB, Ziv E, Zmuda J, Garcia M, LeakTS, Foreman MG, Smith LJ, Fornage M, Liu K, Burchard EG; for the Health ABC andCARDIA Studies. Genetic Ancestry-Smoking Interactions and Lung Function in AfricanAmericans: A Cohort Study. PLoS One. 2012 June;7(6):e39541. Epub 2012 Jun 21.PMID: 2273724412. Aminuddin F, Akhabir L, Stefanowicz D, Paré PD, Connett JE, Anthonisen NR, Fahy JV,Seibold MA, Burchard EG, Eng C, Gulsvik A, Bakke P, Cho MH, Litonjua A, LomasDA, Anderson WH, Beaty TH, Crapo JD, Silverman EK, Sandford AJ. Geneticassociation between human chitinases and lung function in COPD. Hum Genet. 2012Jul;131(7):1105-14. Epub 2011 Dec 28. PMID: 2220076713. Torgerson, DG, Gignoux, CR, Galanter, JM, Drake, KA, Roth, LA, Eng C, Huntsman, S,Torres, R, Avila, PC, Chapela, R, Ford, JG, Rodríguez-Santana, JR, Rodríguez-Cintrón,W, Hernandez, RD, and Burchard, EG. Case-control admixture mapping in Latinopopulations enriches for known asthma-associated genes. Journal <strong>of</strong> <strong>Asthma</strong> and ClinicalImmunology. J Allergy Clin Immunol. 2012 Jul;130(1):76-82.e12. Epub 2012 Apr 13.PMID: 2250279714. Nguyen EA, Burchard EG. <strong>Asthma</strong> <strong>Research</strong> for All <strong>of</strong> the United States. PediatrAllergy Immunol Pulmonol. 2012 Sep;25(3):128-131. PMID: 2297042246
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core Facilities15. Kumar R, Williams LK, Kato A, Peterson EL, Favoreto S Jr, Hulse K, Wang D,Beckman K, Thyne S, Lenoir M, Meade K, Lanfear DE, Levin AM, Favro D, Yang JJ,Weiss K, Boushey HA, Grammer L, Avila PC, Burchard EG, Schleimer R. Geneticvariation in B cell-activating factor <strong>of</strong> the TNF family (BAFF) and asthma exacerbationsamong African American subjects. J Allergy Clin Immunol. 2012 Oct. Epub 2012 June.PMID: 2272808016. Myers RA, Himes BE, Gignoux CR, Yang JJ, Gauderman WJ, Rebordosa C, XieJ, Torgerson DG, Levin AM, Baurley J, Graves PE, Mathias RA, Romieu I, RothLA, Conti D, Avila L, Eng C, Vora H, Lenoir MA, Soto-Quiros M, Liu J, CeledónJC, Farber HJ, Kumar R, Avila PC, Meade K, Serebrisky D, Thyne S, Rodriguez-CintronW, Rodriguez-Santana JR, Borrell LN, Lemanske RF Jr, Bleecker ER, MeyersDA, London SJ, Barnes KC, Raby BA, Martinez FD, Gilliland FD, WilliamsLK, Burchard EG, Weiss ST, Nicolae DL, Ober C. Further replication studies <strong>of</strong> theEVE Consortium meta-analysis identifies 2 asthma risk loci in European Americans. JAllergy Clin Immunol. 2012 Dec;130(6):1294-301. doi: 10.1016/j.jaci.2012.07.054. Epub2012 Oct 4.17. Levin AM, Mathias RA, Huang L, Roth LA, Daley D, Myers RA, Himes BE, RomieuI, Yang M, Eng C, Park JE, Zoratti K, Gignoux CR, Torgerson DG, GalanterJM,Huntsman S, Nguyen EA, Becker AB, Chan-Yeung M, Kozyrskyj AL, KwokPY, Gilliland FD, Gauderman WJ, Bleecker ER, Raby BA, Meyers DA, LondonSJ,Martinez FD, Weiss ST, Burchard EG, Nicolae DL, Ober C, Barnes KC, WilliamsLK. A meta-analysis <strong>of</strong> genome-wide association studies for serum total IgE in diversestudy populations. J Allergy Clin Immunol. 2012 Nov 10. doi:10.1016/j.jaci.2012.10.002. 2012 Nov [Epub ahead <strong>of</strong> print]Manuscripts currently under review:1. Rajesh Kumar, Elizabeth A Nguyen, Lindsey A Roth, Sam S Oh,Christopher R Gignoux,Scott Huntsman, Celeste Eng, Andres Moreno-Estrada, Karla Sandoval, RosendaPeñaloza-Espinosa, Marisol López-López, Pedro C Avila, Harold J Farber, HaigTcheurekdjian, William Rodriguez-Cintron, Jose R Rodriguez-Santana, DeniseSerebrisky, Shannon M Thyne, Keoki Williams, William Klitz, Cheryl Winkler, Carlos DBustamante, Eliseo J Pérez-Stable, Luisa N Borrell, Esteban G Burchard. Factorsassociated with degree <strong>of</strong> atopy in Latino children in a nationwide pediatric sample: TheGALA II Study. J Allergy Clin Immunol. (Under 2 nd review, December 2013).2. Joshua M Galanter, Christopher R Gignoux, Dara G Torgerson, Lindsey A Roth, CelesteEng, Sam S Oh, Elizabeth A Nguyen Scott Huntsman Donglei Hu, Saunak Sen. AdamDavis, Harold J. Farber, Pedro C. Avila, Emerita Brigino-Buenaventura, Michael A.LeNoir, Kelley Meade, Denise Serebrisky, Stephanie London, Frank Gilliland, FernandoMartinez, Luisa N Borrell, William Rodriguez-Cintron, Rajesh Kumar, Jose R.Rodriguez-Santana, Esteban G. Burchard, and the EVE Consortium. Genome-wideassociation and admixture mapping identify chromosomal regions 17q21 and 6p21 asasthma-associated loci in Latinos. AJRCCM. (Under review December 2013).47
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Core Facilities3. Luisa N. Borrell, Elizabeth A. Nguyen, Lindsey A. Roth,Sam S. Oh, HaigTcheurekdjian,Saunak Sen, Adam Davis, Harold J. Farber, Pedro C. Avila, EmeritaBrigino-Buenaventura, Michael A. LeNoir, Fred Lurmann, Kelley Meade, DeniseSerebrisky, William Rodriguez-Cintron, Rajesh Kumar, Jose R. Rodriguez-Santana,Shannon Thyne, Esteban G. Burchard. Childhood obesity and asthma control in adiverse sample: Examining age and racial/ethnic differences. AJRCCM. (Submitted for2nd review January 2013).4. Chris R Gignoux, Dara G Torgerson, Joshua Galanter, Lindsey A Roth, Celeste Eng,Elizabeth A Nguyen, Scott Huntsman, Sam S Oh,Raskia A Mathias, Adam Davis, HaroldJ Farber, Pedro C Avila, Emerita Brigino-Buenaventura, Michael A LeNoir, KelleyMeade, Denise Serebrisky, Luisa N Borrell, William Rodriguez-Cintron, Rajesh Kumar,Jose R Rodriguez-Santana, Andres Moreno, Karla Sandoval, Cheryl Winkler, CarlosBustamante, EVE Collaborators, Carole Ober, Dan Nicholae, Saunak Sen, Kathleen CBarnes, Esteban G Burchard. Meta-analysis <strong>of</strong> admixture mapping using existinggenome-wide association data implicates SMAD2 as a novel asthma-associated locus inLatinos. Nature Genetics. (Submitted January 2013).5. Katherine A. Drake, Dara G. Torgerson, Christopher R. Gignoux, Joshua M. Galanter,Lindsey A. Roth, Scott Huntsman, Celeste Eng, Sam S. Oh, Sook Wah Yee, LawrenceLin, Adam Davis, Luisa N. Borrell, Harold J. Farber, Rajesh Kumar, Pedro C. Avila,Emerita Brigino-Buenaventura, Rocio Chapela, Jean G. Ford, Michael A. LeNoir, FredLurmann, MS, Kelley Meade, Denise Serebrisky, Shannon Thyne, William Rodríguez-Cintrón, Saunak Sen, José R. Rodríguez-Santana, Kathleen M. Giacomini, and EstebanG. Burchard. A Genome-wide Association Study <strong>of</strong> Bronchodilator Response inLatinos Implicates Rare Variants. J Allergy Clin Immunol. (Submitted January 2013).6. Katherine K. Nishimura, Joshua M. Galanter, Lindsey A. Roth, Sam S. Oh, Harold J.Farber, Denise Serebrisky, Rajesh Kumar, Luisa N. Borrell, Emerita Brigino-Buenaventura, Adam Davis, Michael A. LeNoir, Kelley Meade, William Rodriguez-Cintron, Pedro C. Avila, Jose R. Rodriguez-Santana, Saunak Sen, Fred Lurmann, John R.Balmes, Esteban G. Burchard. Early Life Exposure to Air Pollution and the Risk <strong>of</strong><strong>Asthma</strong> in Latino and African American Children. ARJCCM. (Submitted January 2013).48
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core FacilitiesCell Sorting and Analysis CoreDirector: Zhien-WangObjectiveFlow cytometry is necessary to characterize, purify and separate cell types based on surfacecharacteristics. The objective <strong>of</strong> this core is to provide technical support and access to highlysophisticated instruments for persons at UCSF performing asthma-related research.Contributions from SABRE <strong>Center</strong> have been partnered with the Diabetes <strong>Center</strong>, individualinvestigator resources and institutional resources to support the space and maintenance <strong>of</strong> thecore largely through an integrated re-charge system. Students and postdocs participate intraining in the core at an area where training can be centralized in order to facilitate maintenanceand oversight.AccomplishmentsThe Core is located in S1067 in proximity to Dr. Locksley’s laboratory and centrally within theImmunology Program corridor above the SABRE <strong>Center</strong> space in HSE201. Scheduling is onlineand suitably trained individuals can access instruments in the core 24 hours a day, 7 days aweek.The space <strong>of</strong>fers an array <strong>of</strong> instrumentation that <strong>of</strong>fers unique capabilities in data acquisitionand enhances the depth <strong>of</strong> technical capacity:Two Beckman-Coulter MoFlo XDP High-Speed sorters with 4-laser, 17-color, 15-parametercapability centered around 488 nm, 532 mm, 561 mm and 647 nm multi-line air-cooled lasers.Two side-by-side instruments has enabled operation by a single individual, thus optimizing useand minimizing cost.Becton-Dickinson Biosciences LSR II Flow Cytometer with 4-laser, 10-color, 12-parametercapability centered around 488 nm OPSL, 637 nm (red), 403 nm (violet) and 535 nm (green)lasers. Total use > 1600 hrs.Becton-Dickinson Biosciences LSR II Flow Cytometer with 4-laser, 10-color, 12-parametercapability centered around 488 nm OPSL, 637 nm (red), 403 nm (violet) and 535 nm (green)lasers is maintained by the core but stationed in HSE201 in the SABRE core space. Total use >800 hrs.Training and Integration with <strong>Sandler</strong> ProgramUse <strong>of</strong> the core has stabilized over the past 2 years. Over the last 12 months, >45 laboratories atthe UCSF Parnassus and Mission Bay campuses have used the facility 2 or more times, with 32labs over 3 times. In turn, the numbers <strong>of</strong> users per laboratory has also stabilized at 2.3 users/lab,and reflects use by graduate students and postdocs. Nine <strong>of</strong> the labs are direct participants in theSABRE <strong>Center</strong>, and a number <strong>of</strong> the other users are affiliated investigators examining aspects <strong>of</strong>49
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core Facilitiesinflammation and tissue injury <strong>of</strong> relevance to asthma. All <strong>of</strong> the core SABRE <strong>Center</strong> scientistsparticipate in the Sorting Core. Budgetary requests from the SABRE <strong>Center</strong> are now restrictedto acute laser failure and maintenance, as personnel and renewables have all been coveredthrough recharge mechanisms.Grants and PublicationsData acquired in the Cell Sorting and Analysis core has contributed to almost a quarter <strong>of</strong> thepublications listed in the bibliography for the current annual report.50
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core FacilitiesMicroscopy CoreManaging Director: Kaitlin CorbinFaculty Director: Matthew Krummel, Ph.D.Objective/MandateThe objective <strong>of</strong> the SABRE Microscopy Core is to advance light-based imaging <strong>of</strong> the lung andassociated tissues. Our core operates under the premise that a critical understanding <strong>of</strong> diseasedtissues and organs such as the asthmatic lung will come with the study <strong>of</strong> the activities <strong>of</strong>component players (cell types, effector molecules) in their native environment. We alsorecognize that many existing imaging methods may require some additional development orelaboration before they can be successfully applied in studies <strong>of</strong> lung biology. We act both as arepository/resource <strong>of</strong> imaging technology and expertise and to develop novel technologies. Werepresent an emerging evolving example <strong>of</strong> a ‘Co-laboratory’ in which expertise in this activearea <strong>of</strong> scientific progress is shared rather than arbitrarily monetized.Strategic GoalsThe efforts <strong>of</strong> this center are being directed toward improved imaging technologies for cells <strong>of</strong>the normal and allergic lung. In 2013, the core will be trying to advance three specific goals forintravital imaging. Note that in the 2012 period, we have miniaturized and made less-invasiveour lung-stabilization rigs, have implemented a new microscope with photoablation andphotoactivation capability and have optimized mouse models for imaging increased numbers <strong>of</strong>cell types at the same time.1. To provide stabilized access to larger airways and trachea <strong>of</strong> living mice, using astabilized gradient- refractive index (GRIN) lens.2. To develop s<strong>of</strong>tware processing platforms for converting >100GB datasets into imagesand parametrized object-based data for rapid scoring <strong>of</strong> immune and cell-cell dynamicswithin large and long-time course experiments.3. To provide improved surgical stabilization to image lungs over many days withrepeated visits to the same regions.4. To extend usage <strong>of</strong> the human lung imaging method through expanded facility capacityand training/sample processing capabilities and through collaboration with asthmaresearchers with access to primary biopsy material.5. To provide ongoing technical and instrumentation support to the UCSF (and beyond)<strong>Asthma</strong> community in order to put existing and emerging imaging technologies topractical use in the study <strong>of</strong> asthma.51
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core FacilitiesPhotoablation and Photoactivation: In 2012, we extensively tested and subsequently purchased,microscopes with both a single-photon and two-photon ablation lasers that now allow us topurposefully modulate biology in the lung using spots <strong>of</strong> light. This is being developed as a wayto determine which cells are critical for the ongoing biology in allergic lungs. We were able toobtain grant money for this (NIH S10) as a result <strong>of</strong> preliminary data generated in this SABREfundingfacility.Introduction <strong>of</strong> demo equipment, (see below) and establishment <strong>of</strong> SOPs and training.This year, we served as a demo site for a Nikon ‘STORM’ microscope as well as the Zeiss&710MP. As with other demos in the past, we negotiated with vendors to ensure that equipmentwas onsite for at least one month (typically much longer).SpaceWe maintain instruments and development tools in three ‘core’ rooms in Medical Sciences S11and we also maintain additional microscopes in 6 additional sites throughout the campus,including behind the animal barrier.FundingThe following represent some <strong>of</strong> the lung-related grants which were funded in 2012, in partthrough our efforts and support:Sheppard/Woodruff/Erle/Krummel: NIH Headley:DoD Postdoctoral ($300K/yr 3 yrs)U01: AADCRC ($7.5M/5 years)Acerbi: Komen Postdoctoral ($60K/yr 3Maelig PBBR Postdoctoral ($10K/1yr)years)Maelig: DoD Postdoctoral ($300K/yr 3 yrs) Nystul: R01And these were submitted:Nishimura R01 resubmitted (likely funded)Looney/Krummel R01 submittedP.T. Chuang R01 submittedJ.S. Shin R01 submittedSchneider S10 submittedWerb R01 submittedWErb et al. PPG submittedRecent publicationsA number <strong>of</strong> recent and forthcoming publications, both methodological and research-orientated,have been produced with help <strong>of</strong> the facility during the past year. These include:1. Paszek MJ, DuFort CC, Rubashkin MG, Davidson MW, Thorn KS, Liphardt JT, WeaverVM. Scanning angle interference microscopy reveals cell dynamics at the nanoscale. NatMethods. 2012 Jul 1;9(8):825-7. doi: 10.1038/nmeth.2077. PubMed PMID: 22751201;PubMed Central PMCID: PMC3454456.2. Zhang Y, Chen YC, Krummel MF, Rosen SD. Autotaxin through lysophosphatidicacidstimulates polarization, motility, and transendothelial migration <strong>of</strong> naive Tcells. J53
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core Facilitiesquantify data has reached a limit with current tools. Our in-house s<strong>of</strong>tware programmer, HenryPinkard, has been developing methods to rapidly parameterize data to reduce complexity forfeature-tracking and analysis <strong>of</strong> cell-cell contacts. This work will expand in 2012.3. In 2012, we will be taking two steps toward applying subcellular imaging to human tissuesdirectly. First, we are continuing to evolve a ‘live biopsy’ method. Second, we are collaboratingwith the local and recently funded AADCRC (NIH <strong>Asthma</strong> consortium) to develop novel proteinaffinity reagents to specifically label and track key populations within the lung. This work willexpand into supporting imaging <strong>of</strong> bronchial pinch biopsies over the next five years.4. We plan to continue to provide ongoing technical and instrumentation support to the UCSF(and beyond) <strong>Asthma</strong> community in order to put existing and emerging imaging technologies topractical use in the study <strong>of</strong> asthma.Training and Integration with <strong>Sandler</strong> ProgramAs noted in previous updates, the center aims to provide ongoing technical and instrumentationsupport to the UCSF (and beyond) <strong>Asthma</strong> community. Specifically our goal is to put existingand emerging imaging technologies to practical use in the study <strong>of</strong> asthma. This year, we havespecifically undertaken training <strong>of</strong> Peck and Wong in performing lung-based assays from start t<strong>of</strong>inish so that they may be able to undertake experiments for/with new users that may not haveexperience with these assays. This is particularly important in some <strong>of</strong> the more complicatedprocedures in which technical expertise is critical to the success <strong>of</strong> the experiment.We have taught live lung imaging techniques to visitors from Michael Cahalan’s lab (UC Irvine)and from Paul Kubes’ lab (<strong>University</strong> <strong>of</strong> Calgary).To introduce imaging advances within the wider community, the core also sponsors a recurringpizza-talk at which groups from the Bay Area and beyond present novel imaging technology andadvances. Topics this year have included live lung imaging, 3D printing and design, Multidimensionalanalysis, and in vivo optical imaging. Details can be seen at the website:http://pathology.ucsf.edu/BIDC//seminars.html.Current EquipmentPermanent Equipment:1. Gen1 custom built 2-photon: 4 color2. Gen2 custom built 2-photon: 5 color3. *Gen3 custom built 2-photon: 8 color/2 laser (being elaborated with a DMD array forphoto-activation/ablation)4. *Spectral laser scanning confocal microscope (C1Si)5. Spinning-disk confocal microscope (Yokogawa 4-laser on a Zeiss 200M base)6. Upright Zeiss microscope set up for fluorescence interference contrast imaging.7. Zeiss Axiovert 100 timelapse microscope55
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Core Facilities8. IVIS live animal imager (animal colony)9. Nikon spinning-disk confocal with TIRF and photo-ablation (Wittman)10. Zeiss Cell Observer with Apotome (Nystul)11. *Alaris 3D printer12. Nikon A1R Multiphoton microscope.* Indicates SABRE is a partial owner <strong>of</strong> this instrument.Additional Equipment hosted on-site in 2012:1. Zeiss 710 Multi-photon confocal microscope2. Nikon ‘STORM’ ,microscope.Analysis Computers and S<strong>of</strong>tware Platforms:This year, as a result <strong>of</strong> increased demand, we purchased a third IMARIS license and associatedMatlab license and increased our workstations to four, installing a new desk to house this. Aspart <strong>of</strong> a cooperative agreement, MDS/Molecular Devices again supplied upgraded keys for PCbasedanalysis stations for image processing. We have partnered with and will support thefollowing commercial partners who supply working copies <strong>of</strong> their s<strong>of</strong>tware as part <strong>of</strong> thesponsorship program:• MDS/Molecular Devices 'Metamorph' (who are supplying the three <strong>of</strong>flinecomputers/keys as well as online keys)• Bitplane 'Imaris' (who have subsidized the purchase <strong>of</strong> s<strong>of</strong>tware used in the facility).• Solidworks who have supplied 2 discounted s<strong>of</strong>tware keys for our manufacturingpurposes.56
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong><strong>Asthma</strong> Related <strong>Research</strong> ProjectsASTHMA RELATED RESEARCH PROJECTS57
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong><strong>Asthma</strong> Related <strong>Research</strong> ProjectsEvolving Microenvironments in Airway InflammationProgram Director: George H. Caughey, M.D.Program Project Grant HL024136 is nearing completion <strong>of</strong> its 33nd year, the third <strong>of</strong> a plannedfive years <strong>of</strong> interdisciplinary study <strong>of</strong> airway inflammation competitively renewed on May 11,2010. Each <strong>of</strong> the component projects focuses on different populations <strong>of</strong> airway cells andmolecules mediating structural changes in the airway accompanying chronic airwayinflammation. The Projects are supported by two Cores, one administrative and one scientific(Tools for Analysis <strong>of</strong> Airway Inflammation).Summary <strong>of</strong> Advances this YearProject 1: Roles <strong>of</strong> Peptidases in Chronic Airway Inflammation (Project Leader: G.H. Caughey)One <strong>of</strong> Project 1’s major achievements was identification <strong>of</strong> a substrate andspectrophotometric technique allowing direct assay <strong>of</strong> prostasin activity in living humanairway epithelial apical and basolateral surfaces. Using this substrate in conjunction withselective inhibitors, we established that prostasin anchored to the cell surface by a lipid(glycosylphosphatidylinositol) is the major contributor to surface activity. For example, theapical surface <strong>of</strong> cultured human bronchial epithelial cells expresses 22% <strong>of</strong> total,extractable, aprotinin-inhibitable, tryptic activity and 16% <strong>of</strong> prostasin immunoreactivity.Similar results were obtained using cultured cystic fibrosis bronchial cells. Thus, weestablished that prostasin is present, mature and active on the apical surface <strong>of</strong> wild type andcystic fibrosis bronchial epithelial cells, where it can be targeted for inhibition via theairway lumen with the goal <strong>of</strong> inhibiting epithelial Na+ uptake and therapeuticallyincreasing hydration <strong>of</strong> airway secretions. We also established differences betweenprostasins (mouse and human) and other surface epithelial tryptic proteases (matriptase andmarapsin) in peptide cleavage preferences, inhibitor susceptibility, and modes <strong>of</strong> membraneanchoring. In contrast to mouse “knockouts” <strong>of</strong> prostasin and matriptase, a mouse wecreated that is genetically deficient <strong>of</strong> marapsin/Prss27 is fully viable, further supportingdifferent roles despite similar high degrees <strong>of</strong> evolutionary conservation in vertebrateanimals. Additional significant progress was made in testing our hypothesis that variationsin inheritance <strong>of</strong> active tryptase genes is common and that inheritance <strong>of</strong> dysfunctionalalleles is protective with respect to asthma phenotypes.Project 2: Imaging T cell Airway Responses during Inflammation (Project Leader: M.F. Krummel)In this year <strong>of</strong> funding, we established a labeling method for tracking and imaging incomingmonocytes using two genetically-encoded fluorescent proteins. We are now tracking in real-timethe differentiation <strong>of</strong> these cells as they move from alveoli towards airways. Our goal is toreveal key checkpoints that might be exploited for modulating asthma severity and this is a direct<strong>of</strong>fshoot <strong>of</strong> Aim 1 <strong>of</strong> this proposal. In a second project driven by our Aim 2, we are tracking thesite <strong>of</strong> Th2 differentiation in asthma models. Preliminary data on this front suggests that IL-4secretion is first found in lung, suggesting that dendritic cell-T cell interactions at that sitemodulate the response. Related to this, in the coming year, we will complete our study <strong>of</strong> therelative sites and dynamics <strong>of</strong> Th2 and regulatory (Treg) cells in asthma models. Finally, we58
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong><strong>Asthma</strong> Related <strong>Research</strong> Projectsbegan pilot work to examine interactions <strong>of</strong> allergic T cells with neurons and neuroendocrinecells in the allergic lung.Project 3: Lymphangiogenesis and Angiogenesis in Airway Inflammation (Project Leader: D.M.McDonald)During the past year, an important focus <strong>of</strong> Project 3 was to determine the extent anddistribution <strong>of</strong> lymphangiogenesis in the inflamed lung and the principal growth factors thatdrive it. Studies revealed that lung lymphatics increased 5-fold within 2 weeks afterMycoplasma pulmonis infection in mice. Strikingly, most <strong>of</strong> the new lymphatics were locatedwithin bronchus-associated lymphoid tissue (BALT) around large bronchi, pulmonaryarteries, and pulmonary veins. Few lymphatics were located near distal airways or alveoliunder normal conditions or in chronic inflammation. By using selective inhibitors <strong>of</strong> VEGFR-2 and VEGFR-3, experiments revealed that VEGFR-3 signaling was the dominant drivingmechanism for lymphangiogenesis after M. pulmonis infection. Because VEGF-C andVEGF-D are the principal ligands for VEGFR-3, these growth factors are likely to mediatelymphangiogenesis under these conditions. VEGFR-2 signaling could make a minorcontribution, but the underlying mechanism is still under investigation. VEGF-A appears notto have a significant contribution to lymphangiogenesis in the lung in this model. An essentialrole <strong>of</strong> VEGFR-3 signaling in lymphangiogenesis in the respiratory tract was also found in atransgenic mouse model, where IL-1b is overexpressed in the epithelium under doxycyclineregulation. Here, lymphatic growth was completely blocked by sequestration <strong>of</strong> the VEGFR-3 ligands VEGF-C and VEGF-D. Because <strong>of</strong> this key involvement <strong>of</strong> VEGF-C or VEGF-D,the role <strong>of</strong> IL-1b was explored further by determining the location <strong>of</strong> IL-1R1 receptor. Thesewere found to be preferentially located on epithelial basal cells and neuroendocrine cells, atleast under normal conditions. The identification <strong>of</strong> factors that link these epithelial targets <strong>of</strong>IL-1b to the cellular sources <strong>of</strong> VEGF-C and VEGF-D is underway. Chemokines that recruitmacrophages are being explored as candidates.Scientific Core ActivitiesIn this year <strong>of</strong> funding, we generated new and tittered stocks <strong>of</strong> mycoplasma for use in thethree projects and the Core continues to function in performing genotyping <strong>of</strong> key mousestrains. The Core also has performed and continues to perform confocal and 2-photonimaging experiments in addition to assisting in keeping these instruments maintained andoptimized. Personnel funded by this core component have also permitted pilot projects ontryptase and protease functions in airway remodeling, in neuroendocrine cells and in thedynamics <strong>of</strong> T cell-dendritic-mast cell interactions. In the next year, the core will alsocontribute to an effort to perform high-resolution intravital imaging <strong>of</strong> mouse trachea.Training and Integration with the <strong>Sandler</strong> ProgramThe SABRE <strong>Center</strong> continues to provide a focus to bring together all <strong>of</strong> the groups studyingfundamental questions relevant to asthma at UCSF. This focus includes a monthly researchmeeting <strong>of</strong> investigators with asthma-focused basic research. The <strong>Sandler</strong>-supported core alsoprovided advice and training in sensitization and challenge protocols for creating mouse models59
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong><strong>Asthma</strong> Related <strong>Research</strong> Projects<strong>of</strong> chronic allergic inflammation and for monitoring changes in airway resistance. It als<strong>of</strong>acilitated sharing <strong>of</strong> models and advanced imaging technologies.P01 HL024136-supported selected publications (2012-2013)1. Baluk P, Hogmalm A, Bry M, Alitalo K, Bry K, McDonald DM. Transgenicoverexpression <strong>of</strong> interleukin-1b induces persistent lymphangiogenesis but notangiogenesis in mouse airways. Am J Pathol (in press).2. Banfi A, von Degenfeld G, Gianni-Barrera R, Reginato S, Merchant MJ, McDonaldDM, Blau HM. Therapeutic angiogenesis due to balanced single-vector delivery <strong>of</strong>VEGF and PDGF-BB. FASEB J 26:2486-97, 2012, PMID 22391130; PMCIDPMC3360154.3. Caughey GH. “Chymases” in Handbook <strong>of</strong> Proteolytic Enzymes, 3 rd edition,Rawlings N and Salvesen G, eds., Elsevier, Oxford, in press.4. Caughey GH. “Mastins” in Handbook <strong>of</strong> Proteolytic Enzymes, 3 rd edition,Rawlings N and Salvesen G, eds., Elsevier, Oxford, in press.5. Cheng LE, Hartmann K, Roers A, Krummel MF, Locksley RM. Perivascular mastcells dynamically probe cutaneous blood vessels to capture Immunoglobulin E.Immunity (in press), PMID 23290520.6. Greenland JR, Jones KD, Hays SR, Golden JA, Urisman A, Jewell NP, CaugheyGH, Trivedi NN. Association <strong>of</strong> large-airway lymphocytic bronchitis withbronchiolitis obliterans syndrome. Am J Resp Crit Care Med, 187:417-23, 2013;PMID 23239157.7. Nimishakavi S, Besprozvannaya M, Raymond WW, Craik CS, Gruenert DC,Caughey GH. Activity and inhibition <strong>of</strong> prostasin and matriptase on apical andbasolateral surfaces <strong>of</strong> human airway epithelial cells. Am J Physiol Lung Cell MolPhysiol 303:L97-106, 2012; PMID 22582115.8. Orsenigo F, Giampietro C, Ferrari A, Corada M, Galaup A, Sigismund S, Ristagno G,Maddaluno L, Young Koh G, Franco D, Kurtcuoglu V, Poulikakos D, Baluk P,McDonald D, Grazia Lampugnani M, Dejana E. Phosphorylation <strong>of</strong> VE-cadherin ismodulated by haemodynamic forces and contributes to the regulation <strong>of</strong> vascularpermeability in vivo. Nat Commun 3:1208, 2012, PMID 23169049; PMCIDPMC3514492.9. Raman K, Caughey GH. “Marapsin” in Handbook <strong>of</strong> Proteolytic Enzymes, 3 rdedition, Rawlings N and Salvesen G, eds., Elsevier, Oxford, 2013.10. Raman K, Trivedi NN, Raymond WW, Ganesan R, Kirchh<strong>of</strong>er D, Verghese GM, CraikCS, Schneider EL, Nimishakavi S, Caughey GH. Mutational tail loss is an evolutionarymechanism for liberating marapsins and other type I serine proteases from transmembraneanchors. J Biol Chem, in press.11. Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V,Tabruyn SP, You WK, Chapman HA, Christensen JG, Aftab DT, McDonald DM.Suppression <strong>of</strong> tumor invasion and metastasis by concurrent inhibition <strong>of</strong> c-met andVEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov 2:270-87, 2012,PMID 22585997; PMCID PMC3354652.12. Sennino B, McDonald DM. Controlling escape from angiogenesis inhibitors. Nat Rev60
NIAID <strong>Asthma</strong> and Allergic Disease Cooperative <strong>Research</strong> <strong>Center</strong>Principal Investigator, Dean SheppardObjectiveThe major goal <strong>of</strong> this multi-project U19 <strong>Center</strong> Grant is to bring studies using human materialsand genetically modified mice together in innovative ways in order to define the overlapping anddistinctive roles for the cytokines IL-13 and IL-17 in airway pathology <strong>of</strong> relevance to humanasthma. This U19 was successfully renewed after an initial five year funding period, to extendour total recognition as an AADCRC to 10 years.Specific AimsOver the past decade, work from several centers, including our own, has identified critical rolesfor the effector cytokines, IL-13 and IL-17, in multiple models <strong>of</strong> allergic asthma. Recent datademonstrating the effectiveness <strong>of</strong> IL-13 inhibition in a subset <strong>of</strong> patients with asthma confirmsthe importance <strong>of</strong> this cytokine in the human disease. The demonstration that circulating levels<strong>of</strong> IL-17 and circulating Th17 cells correlate with asthma severity also support a role for IL-17 inpatients. It is now clear that IL-13 and IL-17 can be produced by multiple hematopoietic cells inthe airways, but that they exert their major disease-producing effects by acting directly onresident airway cells – principally airway epithelial cells and airway smooth muscle. During thecurrent funding period <strong>of</strong> this AADCRC, we have used mice engineered to mark cytokineproducingcells to identify key roles for innate helper cells (iH2 cells) and Th2 cells as early andsustained sources <strong>of</strong> IL-13 in allergic airways. We have also identified an important role forantigen-specific alpha-beta T cells as key effectors <strong>of</strong> airway hyperresponsiveness anddemonstrated that IL-17A released by these cells acts directly on murine (and human) airwaysmooth muscle to enhance contractility through induction <strong>of</strong> the small GTPase, RhoA and itseffector kinase, ROCK2. Finally, we have identified characteristic mRNA expression signaturesand a large number <strong>of</strong> miRNAs differentially expressed in cytokine-stimulated airway epithelialcells, work that has allowed identification <strong>of</strong> a subset <strong>of</strong> patients with asthma (Th2 high) whorespond favorably to treatment with IL-13 inhibitors. Work in the current funding period hasstrongly suggested that IL-13 and IL-17 act locally on airway epithelium and airway smoothmuscle, and has pointed to the importance <strong>of</strong> identifying where and when each cytokine is madeand released. It is also clear that we need to know which resident cells are the critical responders,and how these responses are regulated in individual patients. The current renewal is organizedaround 3 Projects driven by 3 Principle Investigators together with a Clinical Subjects andBiospecimen Core for standardizing the conjoined approaches.There are threee Specific Aims associated with the Grant:1. To identify critical miRNAs that are differentially expressed in the airway epithelium <strong>of</strong>patients with asthma at baseline and in response to allergen challenge or corticosteroidtreatment, to determine the roles <strong>of</strong> IL-13 and IL-17 in regulating these miRNAs and toidentify miRNAs that mediate cytokine-induced mucous metaplasia. (PI: David Erle)2. To determine the relative importance <strong>of</strong> responses <strong>of</strong> airway smooth muscle andepithelium to IL-17 in the induction <strong>of</strong> airway hyperresponsiveness and to determine the62
individual and combined effects <strong>of</strong> IL-13 and IL- 17 on airway smooth musclecontractility and on clinical responses <strong>of</strong> patients with severe and mild-to-moderateasthma and in response to allergen challenge. (PI: Dean Sheppard)3. To determine the temporal and spatial dynamics <strong>of</strong> the interactions <strong>of</strong> IL-13 and IL-17producing cells with antigen-presenting cells and with airway epithelium and airwaysmooth muscle in lung slices from allergen challenged mice and in human airwaybiopsies from patients with severe and mild-to-moderate asthma and in response toallergen challenge or treatment with corticosteroids. (PI: Matthew Krummel)The proposed studies will draw heavily from our large on-going clinical studies in patients withasthma and from the extensive experience <strong>of</strong> carefully collecting and utilizing biospecimensfrom these subjects by Prescott Woodruff, the PI and director <strong>of</strong> the Clinical Subjects andBiospecimen Core for the Grant. The work will benefit from the extensive experience withmicroarray and miRNA analysis and the study <strong>of</strong> mucous metaplasia in human airwayepithelium by David Erle, Project Leader <strong>of</strong> Project 1. We will also benefit from the experience<strong>of</strong> Dean Sheppard, PI and Project Leader <strong>of</strong> Project 2 in functional assessment <strong>of</strong> airway smoothmuscle from mice and humans. Finally, we will draw on pioneering approaches to intravitalmicroscopy in lung slices and human airway tissue, developed by Max Krummel, the ProjectLeader <strong>of</strong> Project 3. Through our joint efforts, we hope to better understand the dynamics <strong>of</strong>cytokine release and effects in the asthmatic airway, to develop new strategies to identifyclinically relevant sub-phenotypes in asthma, and to develop broadly useful methods to mark anddynamically follow antigen-specific T cell sub-types in readily accessible biopsies from patientswith asthma. In addition to addressing the specific important questions raised by this proposal,we expect these methods to be widely useful to investigators in each <strong>of</strong> the other nationwide<strong>Asthma</strong> and Allergic Diseases Cooperative <strong>Research</strong> <strong>Center</strong>s and to other investigators interestedin allergic airway diseases.Training and Integration with the <strong>Sandler</strong> <strong>Center</strong>This <strong>Center</strong> Grant provides training in basic biology and genetics <strong>of</strong> asthma for approximatelypost-doctoral fellows and graduate students working in the labs <strong>of</strong> the project directors. Theleaders <strong>of</strong> each <strong>of</strong> the Projects and the Core are already actively involved in SABRE. Much <strong>of</strong>the preliminary data that served as the basis for this successful application was generated at leastin part through support from SABRE. The studies using murine models <strong>of</strong> asthma that suppliedpreliminary data for project 2 were performed in the SABRE mouse physiology and morphologycore and the human genetic studies were performed together with the SABRE human geneticscore. Drs. Erle and Krummel, leaders <strong>of</strong> projects 1 and 3, have each received SABRE Innovativegrants in the past that contributed to generation <strong>of</strong> the preliminary data supporting their project.The infrastructure developed with SABRE participation for the Airway Clinical <strong>Research</strong> <strong>Center</strong>provided a critical aspect <strong>of</strong> support for this grant. This <strong>Center</strong> grant would not have been likelyto succeed without this extensive support from SABRE and thus represents a clear example <strong>of</strong>leveraging SABRE funds to further enhance research into the basic mechanisms underlyingasthma.63
Approved Funding for this AwardThis program project provides between $1.1 and $1.2 million in direct costs per year for 5 years,with a total indirect and direct over the 5 years <strong>of</strong> $8,642,948.64
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Contributions to Relevant Scientific ActivitiesCONTRIBUTIONS TO RELEVANTSCIENTIFIC ACTIVITIES65
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Immunology Seminar Series2012-2013 ScheduleMondays, 9amRoom: N-217Date Speaker HostSeptember 10 Amy Weinmann Mark AnselSeptember 17 Christopher Garcia Lewis LanierSeptember 24 Thaddeus Stappenbeck Averil MaOctober 1 Julie ZikhermanOctober 8 Taka Okada Mark AnselOctober 15 Harry Greenberg Mike McCuneOctober 22 Markus Müschen LowellOctober 29 Paul Crocker Jason CysterNovember 5 Andre Veillette Cliff LowellNovember 12 No SeminarNovember 19 Doreen Cantrell Art WeissNovember 26 No SeminarDecember 3 Erin Adams Frances BrodskyDecember10 Larry Samelson Art WeissDecember 17 Glenn Dran<strong>of</strong>f Lewis LanierJanuary 7 Boris Reizis Anthony DeFrancoJanuary 14 Mike Dustin Matthew KrummelJanuary 28 Hozefa Bandukwala Anthony DeFrancoFebruary 4 Emil Unanue Jeoung-Sook ShinFebruary 11 Hugh Rosen Matthew KrummelFebruary 25 Patrick Wilson Jason CysterMarch 4 David Underhill Cliff LowellMarch 11 Ann Marshak-Rothstein Matthias WablMarch 18 Pam Schwatzberg Mark Ansel and Averil MaMarch 25 Victor Engelhard Mark AndersonApril 1 Georg Lauer Mike McCuneApril 8 Judy Lieberman Averil MaApril 15 Kees Murre Averil MaApril 22 Judith Hellman - UCSFApril 29 John O’Shea Ansel /MaMay 6No SeminarMay 13 Hidde Ploegh Art Weiss66
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>UCSF PULMONARY RESEARCH CONFERENCE 2012-2013Mondays, 5:00 pm - Parnassus HSW-303Date Talk 1 (Clinical) Talk 2 (<strong>Basic</strong>) Moderator09/10/12 Anthony Shum Kamran Atabai Luke Davis09/17/12 Ted Omachi Nirav BhaktaDean Sheppard09/24/12 Erin Gordon Ed Ostrin Hal Collard10/01/12 Madori Kato-Maeda Suzaynn Schick Luke Davis10/08/12 Jon Singer Eric Seeley Prescott Woodruff10/15/12 Christina Yoon Mike LaFemina Luke Davis10/22/12 Yvonne Huang Aparna Sundaram Prescott Woodruff10/29/1211/05/12 Stephanie ChristensonCVRI Retreat (no conf)Dario Barbone James Frank11/12/1211/19/12 Neeta ThakurHolidayDara Torgerson Mallar Bhattacharya11/26/12 John Greenland Shaopeng (Leo) Yuan Mallar Bhattacharya12/03/12 Rama Mallampalli, MD (Visiting Pr<strong>of</strong>essor)Prescott Woodruff12/10/12 Nuala Meyer, MD (Assistant Pr<strong>of</strong>essor from Pennsylvania sponsored by M.Matthay) Michael Matthay12/17/12 Neil Trivedi Maria Del Mar Del Pino Yanes Hal Collard12/24/1212/31/1201/07/13Winter holidayWinter holidayLandon King, MD (Visiting Pr<strong>of</strong>essor)James Frank01/14/13 Charles Everett Peter Haggie Mallar Bhattacharya01/21/1301/28/13Martin Luther King holiday (no conf)Fellows feedback (no conf)Luke Davis (cancel)02/04/13 Julian Solway, MD (Visiting Pr<strong>of</strong>essor)Paul Wolters02/11/13 Robert Su Vikash Bhagwandin Prescott Woodruff02/18/1302/25/1303/04/13 Payam NahidPresident's Day holiday (no conf)Bruce Levy, MD (Visiting Pr<strong>of</strong>essor)Luke Bonser03/11/13 Kirsten Kangelaris Chun Chen Luke Davis03/18/13 Elizabeth Fair Anirban Datta Hal Collard03/25/13 Chris Gignoux Andrew Vaughan Hal Collard04/01/13 Serpil Erzurum (Visiting Pr<strong>of</strong>essor)Paul Wolters04/08/13 John Metcalfe Alexandra Greer Luke Davis04/15/1304/22/13 Noah ZaitlenDarrell Kotton, MD (Visiting Pr<strong>of</strong>essor)Binh Diep Prescott Woodruff04/29/13 Misha Agarwal Marrah Lachowicz-Scroggins Prescott Woodruff05/06/13 Xiang Xu Hassan Lemjabbar-Alaoui James Frank05/13/13 Sam Oh Mallar Bhattacharya Prescott Woodruff05/20/1305/27/1306/03/13 Josh GalanterATS Conf (no conf)Memorial Day holiday (no conf)John Li Mallar Bhattacharya06/10/13 Robert Blount Katherine Nishimura Mallar Bhattacharya06/17/1306/24/13Fellows feedback (no conf)Year end party67
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong> SABRE <strong>Asthma</strong> <strong>Research</strong> Conference ScheduleLocation: 513 PARNASSUS AVENUE, HSE-13032 nd Thursday <strong>of</strong> the Month for 2012 at 4 P.M.2 nd Monday <strong>of</strong> the Month for 2013 at 3 P.M.DatePresenter9/13/2012 Limin Liu, PhD10/11/2012 Chris Allen, PhD11/8/2012 Max Krummel, PhD12/13/2012 Esteban Burchard, MD, MPH2/11/2013 Kamran Atabai, MD3/11/2013 Mark Ansel, PhD4/8/2013 John Fahy, MD5/13/2013 Richard Locksley, MD6/10/2013 Jeoung-Sook Shin, PhD7/8/2013 David Erle, MD8/12/2013 Laurence Cheng, MD, PhD9/9/2013 Herbert DeBroski10/14/2013 Prescott Woodruff, MD, MPH11/18/2013 Dean Sheppard, MD12/9/2013 Chris Allen, PhD68
Immunology Seminar Series"Imaging <strong>of</strong> cross-presenting dendritic cellsin the lymph node”Takaharu Okada, Ph.D.Unit Leader, <strong>Research</strong> Unit for Immunodynamics<strong>Research</strong> <strong>Center</strong> for Allergy & ImmunologyRiken JapanMonday, October 8, 20129am, Parnassus, N-‐‐225Host: Mark Ansel (mark.ansel@ucsf.edu)BROADCAST | Mission Bay, Genentech Hall S-‐‐271 § /presentations/live § SPONSORS | Gladstone Institute <strong>of</strong> Virology &Immunology § Rosalind Russell Medical <strong>Research</strong> <strong>Center</strong> for Arthritis § <strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>, SABRE –INFORMATION (415) 502-‐‐1961 http://www.ucsf.edu/immuno Live stream and archive available (UCSF MyAccess login required)69
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>70
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Recent and New PublicationsRECENT AND NEW PUBLICATIONSSUPPORTED BY THE SANDLER ASTHMABASIC RESEARCH CENTER(2011-2013)71
Christopher C.D. Allen, Ph.D.Beltman JB, Allen CDC, Cyster JG, de Boer RJ (2011). B cells within germinal centers migratepreferentially from dark to light zone. Proceedings <strong>of</strong> the National Academy <strong>of</strong> Sciences,108(21), 8755-8760. PMCID: PMC3102384Green JA, Suzuki K, Cho B, Willison LD, Palmer D, Allen CDC, Schmidt TH, Xu Y, Proia RL,Coughlin SR, Cyster JG (2011). The sphingosine 1-phosphate receptor S1P 2 maintains thehomeostasis <strong>of</strong> germinal center B cells and promotes niche confinement. Nature Immunology,12(7), 672-680. PMCID: PMC3158008.Sullivan BM, Liang HE, Bando JK, Wu D, Cheng LE, McKerrow JK, *Allen CDC, *LocksleyRM (2011). Genetic analysis <strong>of</strong> basophil function in vivo. Nature Immunology, 12(6), 527-535.*co-corresponding author. NIHMSID352031Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CDC, Matloubian M, Blelloch R,Ansel KM (2011). MicroRNA-29 Regulates T-Box Transcription Factors and Interferon-γProduction in Helper T Cells. Immunity, 35(2), 169-181.Yang Z, Sullivan BM, Allen CDC (2012). Fluorescent in vivo detection reveals that IgE + B cellsare restrained by an intrinsic cell fate predisposition. Immunity, in press,doi:10.1016/j.immuni.2012.02.009K. Mark Ansel, Ph.D.Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct Waves <strong>of</strong> BCL6 Expression duringT Follicular Helper Cell Development. J Immunol.187:2089-92 (2011)Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CD, Matloubian M, Blelloch R,Ansel KM. MicroRNA-29 Regulates T-Box Transcription Factors and Interferon-γ Productionin Helper T Cells. Immunity 35:169-81 (2011)S<strong>of</strong>i MH, Qiao Y, Ansel KM, Kubo M, Chang CH. Induction and Maintenance <strong>of</strong> IL-4Expression Are Regulated Differently by the 3' Enhancer in CD4 T Cells. J. Immunol. 186:2792-9 (2011)Grégory Seumois, Pandurangan Vijayanand, Christopher J Eisley, Nada Omran, Lukas Kalinke,Mai North, Asha P. Ganesan, Laura J Simpson, Nathan Hunkapiller, Fleix Molzahan, Prescott GWoodruff, John V Fahy, David J Erle, Ratka Djukanovic, Robert Blelloch, K Mark Ansel: Anintegrated nano-scale approach to pr<strong>of</strong>ile miRNAs in limited clinical samples. Am J Clin ExpImmuol. 1:70-89 (2012)Hoefig KP, Rath N, Heinz GA, Wolf C, Dameris J, Schepers A, Kremmer E, Ansel KM,Heissmeyer V. Eri1 degrades the stem-loop <strong>of</strong> oligouridylated histone mRNAs to inducereplication-dependent decay. Nat Struct Mol Biol. 20:73-81 (2013)Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C,Barczak AJ, Zlock LT, Blaev DP, Finkbeiner WE, Ansel KM, Arron JR, Erle DJ, Woodruff PG.Airway Epithelial miRNA Expression is Altered in <strong>Asthma</strong>. Am. J. Respir. Crit. Care Med.186:965-74 (2012)Thomas MF, Abdul-Wajid S, Panduro M, Babiarz JE, Rajaram M, Woodruff P, Lanier LL,Heissmeyer , Ansel KM. Eri1 regulates microRNA homeostasis and mouse lymphocytedevelopment and anti-viral fuction. Blood. 120:130-42 (2012)Vijayanand P, Seumois G, Simpson LJ, Abdual-Wajid S, Baumjohann D, Panduro M, Huang X,Interlandi J, Djuretic IM, Brown DR, Sharpe AH, Rao A, Ansel KM. Interleukin-4 Production yFollicular Helper T Cells Requires the Conserved Il4 Enhancer Hypersensitivity Site V.Immunity 36:175-87 (2012)72
Homer Boushey, M.D.Huang YJ, Nelson CE, Brodie EL, DeSantis TZ, Baek MS, Liu J, Woyke T, Allaier M, BristowJ, Wiener-Kronish JP, Sutherland ER, King TS, Icitovic N, Martin RJ, Calhoun WJ, Castro M,Denlinger LC, DimangoE, Kraft M, Peters SP, Wasserman SI, Wechsler ME, Boushey HA, andLynch SV. Airway microbiota and bronchial hyperresponsiveness in patients with sub-optimallycontrolled asthma. JACI 2011; 127:372-381.Wootton SC, Kim DS, Kondoh Y, Chen E, Lee JS, Song JW, Huh JW, Taniguchi H, Chiu C,Boushey HA,Lancaster LH, Wolters PJ, Derisi J, Ganem D, Collard HR. “Viral Infection inAcute Exacerbation <strong>of</strong> Idiopathic Pulmonary Fibrosis.” Am J Respir Crit Care Med. 2011 Feb 25Havstad S, Wegienka G, Zoratti EM, Lynch SV, Boushey HA, Nicholas C, Ownby DR, JohnsonCC. "Effect <strong>of</strong> prenatal indoor pet exposure on the trajectory <strong>of</strong> total IgE levels in earlychildhood." Journal <strong>of</strong> Allergyand Clinical Immunology. 2011 Aug 4.McGrath KW, Icitovic N, Boushey HA, Lazarus SC, Sutherland ER, Chinchilli VM, Fahy JV;for the National Heart, Lung, and Blood Institute's <strong>Asthma</strong> Clinical <strong>Research</strong> Network <strong>of</strong> theNational Heart, Lung, and Blood Institute. "A Large Subgroup <strong>of</strong> Mild-to-Moderate <strong>Asthma</strong> isPersistently Non-Eosinophilic." Am J Respir Crit Care Med. 2012 Jan 20.Han MK, Huang YJ, Lipuma JJ, Boushey HA, Boucher RC, Cookson WO, Curtis JL, Erb-Downward J.Lynch SV, Sethi S, Toews GB, Young VB, Wolfgang MC, Huffnagle GB,Martinez FJ. "Significance <strong>of</strong> the microbiome in obstructive lung disease." Thorax. 2012 Feb 8.Calhoun WJ, Ameredes BT, King TS, Boushey HA. “Comparison <strong>of</strong> physician-based,biomarker-based, and symptom-based strategies for adjustment <strong>of</strong> inhaled corticosteroid therapyin adults with asthma: The Basalt Randomized Trial.” JAMA. 2012 Sept 12; 308:987-97.Esteban G. Burchard, M.D., M.P.H.Via, M, Gignoux C, Roth LA, Fejerman L, Galanter J, Choudhry S, Toro-Labradore G, Viera-Vera J, Oleksyke TK, Beckman K, Ziv E, Risch N, González Burchard E*, Martínez-CruzadoJC*. *These authors contributed equally to this manuscript. History shaped the geographicdistribution <strong>of</strong> genomic admixture on the island <strong>of</strong> Puerto Rico. PLoS One. 2011 Jan31;6(1):e16513.Galanter JM, Torgerson D, Gignoux CR, Sen S, Roth LA, Via M, Aldrich MC, Eng C,Huntsman S, Rodríguez-Santana J, Rodríguez-Cintrón W, Chapela R, Ford JG, Burchard, EG.Cosmopolitan and ethnic-specific replication <strong>of</strong> genetic risk factors for asthma in 2 Latinopopulations. J <strong>of</strong> Clinical Allergy & Immunology. 2011 Jul;128(1):37-43.Akuete K, Oh SS, Thyne S, Rodríguez-Santana J, Chapela R, Meade K, Rodríguez-Cintrón W,LeNoir M, Ford JG, Williams LK, Avila PC, Burchard EG†, Tcheurekdjian H†. EthnicVariability in Persistent <strong>Asthma</strong> after In Utero Tobacco Exposure. Pediatrics. 2011 In Press.†Shared senior authorship between Burchard and TcheurekdjianNHLBI EVE <strong>Asthma</strong> Genetics Consortium. Meta-analysis <strong>of</strong> genome-wide association studies<strong>of</strong> asthma in ethnically diverse North American populations. Nat Genet. 2011 Jul 31.Bustamante CD, Burchard EG, De la Vega FM. Genomics for the world. Nature. 2011 Jul13;475(7355):163-5.Schauberger EM, Ewart SL, Arshad SH, Huebner M, Karmaus W, Holloway JW, Friderici KH,Ziegler JT, Zhang H, Rose-Zerilli MJ, Barton SJ, Holgate ST, Kilpatrick JR, Harley JB, Lajoie-Kadoch S, Harley IT, Hamid Q, Kurukulaaratchy RJ, Seibold MA, Avila PC, Rodriguez-CintrónW, Rodriguez-Santana JR, Hu D, Gignoux C, Romieu I, London SJ, Burchard EG, Langefeld73
CD, Wills-Karp M. Identification <strong>of</strong> ATPAF1 as a novel candidate gene for asthma in children.J Allergy Clin Immunol. 2011 Jun 20.Caleshu C, Sakhuja R, Nussbaum RL, Schiller NB, Ursell PC, Eng C, De Marco T, McGlothlinD, Burchard EG, Rame JE. Furthering the link between the sarcomere and primarycardiomyopathies: restrictive cardiomyopathy associated with multiple mutations in genespreviously associated with hypertrophic or dilated cardiomyopathy. Am J Med Genet A. 2011Sep;155A(9):2229-35. doi: 10.1002/ajmg.a.34097. Epub 2011 Aug 5. PMID: 21823217Kovacic MB, Myers JM, Wang N, Martin LJ, Lindsey M, Ericksen MB, He H, Patterson TL,Baye TM, Torgerson D, Roth LA, Gupta J, Sivaprasad U, Gibson AM, Tsoras AM, Hu D, EngC, Chapela R, Rodríguez-Santana JR, Rodríguez-Cintrón W, Avila PC, Beckman K, SeiboldMA, Gignoux C, Musaad SM, Chen W, Burchard EG, Hershey GK. Identification <strong>of</strong> KIF3Aas a novel candidate gene for childhood asthma using RNA expression and population allelicfrequencies differences. PLoS One. 2011;6(8):e23714. Epub 2011 Aug 30. PMID: 21912604.Kumar R, Tsai HJ, Hong X, Gignoux C, Pearson C, Ortiz K, Fu M, Pongracic JA, BurchardEG, Bauchner H, Wang X. African ancestry, early life exposures, and respiratory morbidity inearly childhood. Clin Exp Allergy. 2011 Sep 25. doi: 10.1111/j.1365-2222.2011.03873.x. PMID:22093077.Williams LK, Peterson EL, Wells K, Ahmedani BK, Kumar R, Burchard EG, Chowdhry VK,Favro D, Lanfear DE, Pladevall M. Quantifying the proportion <strong>of</strong> severe asthma exacerbationsattributable to inhaled corticosteroid nonadherence. J Allergy Clin Immunol. 2011 Oct 20.PMID: 22019090.Kenny EE, Timpson NJ, Sikora M, Yee MC, Moreno Estrada A, Eng C, Huntsman S, GonzalezBurchard E, Stoneking M, Bustamante CD, Myles S, Blond hair is caused by an amino acidchange in TYRP1. Science. 2012 May 4;336(6081):554George Caughey, M.D.Caughey GH. “Mast cell proteases as protective and inflammatory mediators”, in Mast CellBiology: Contemporary and Emerging Topics, Gilfillan AM and Metcalfe DD, eds., LandesBioscience, 2011.Caughey GH. “Protease mediators <strong>of</strong> anaphylaxis”, in Anaphylaxis and HypersensitivityReactions: From Molecular Markers to Rapid Desensitizations, Castells A, ed., Springer Inc.,2011.Xu X, Zhang H, Song Y, Lynch SV, Lowell CA, Wiener-Kronish JP, Caughey GH. Straindependentinduction <strong>of</strong> neutrophil histamine production and cell death by Pseudomonasaeruginosa. J Leuk Biol, in press, epub 2011.Sutherland SE. Xu X. Kim SS, Seeley EJ, Caughey GH, Wolters PJ. Parasitic infectionimproves survival from septic peritonitis by enhancing mast cell responses to bacteria in mice.PLoS One 6(11):e27564, 2011.Xu X, Zhang H, Song Y, Lynch SV, Lowell CA, Wiener-Kronish JP, Caughey GH. Straindependentinduction <strong>of</strong> neutrophil histamine production and cell death by Pseudomonasaeruginosa. J Leuk Biol 91:275-84, 2012.Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, Raymond WW,Erle DJ, Åbrink M, Caughey GH, Huang X, Sheppard D. The α v β 6 integrin modulates airwayhyper-responsiveness in mice by regulating intra-epithelial mast cells. J Clin Invest 122:748-58, 2012.74
Nimishakavi S, Besprozvannaya M, Raymond WW, Craik CS, Gruenert DC, Caughey GH.Activity and inhibition <strong>of</strong> prostasin and matriptase on apical and basolateral surfaces <strong>of</strong>human airway epithelial cells. Am J Physiol: Lung Cell Mol Physiol 303:L97-106, 2012.Greenland JR, Jones KD, Hays SR, Golden JA, Urisman A, Jewell NP, Caughey GH, TrivediNN. Association <strong>of</strong> large-airway bronchitis with bronchiolitis obliterans syndrome. Am JRespir Crit Care Med, in pressHarold Chapman, M.D.Marmai C, Sutherland RE, Kim KK, Dolganov GM, Fang X, Kim SS, Jiang S, Golden JA,Hoopes CW, Matthay MA, Chapman HA, Wolters PJ. Alveolar Epithelial Cells ExpressMesenchymal Proteins in Patients With Idiopathic Pulmonary Fibrosis. Am J Physiol LungCell Mol Physiol 2011, 301:L71-8. PMID: 21498628Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A, Wei Y,Vu T. Integrin α6β4 identifies an adult distal lung epithelial population with regenerativepotential in mice. J Clin Invest. 2011, 121:2855-62. See Commentary same issue.Ulsamer A, Wei Y, Kim KK, Tan K, Wheeler S, Xi Y, Thies RS, Chapman HA. Axinpathway activity regulates in vivo pY654-b-catenin accumulation and pulmonary fibrosis. JBiol Chem 2012, 287: 5164-5172.Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, Bhagwandin V,Tabruyn SP, You WK, Chapman HA, Christensen JG, Aftab DR, McDonald DM.Suppression <strong>of</strong> tumor invasion and metastasis by concurrent inhibition <strong>of</strong> c-Met and VEGFsignaling in pancreatic neuroendocrine tumors. Cancer Discovery 2012, 2:270-287.Xi Y, Wei Y, Sennino, Ulsamer A, Kwan I, Brumwell AN, Tan K, Aghi MK, McDonald DM,Jablons DM, Chapman HA. Identification <strong>of</strong> pY654-b-catenin as a critical co-factor inhypoxia-inducible factor-1a signaling and tumor responses to hypoxia. Oncogene 2012, Dec17. doi: 10.1038/onc.2012.530.Smith KR, Dahl HH, Canafoglia L, Andermann E, Damiano J, Morbin M, Franceschetti S,Cossette P, Saftig P, Grötzinger J, Schwake M, Andermann F, Staropoli JF, Sims KB, MoleSE, Alexander NA, Cooper JD, Chapman HA, Carpenter S, Berkovic SF, Bahlo M.Cathepsin F mutations cause type B Kufs disease, an adult-onset neuronal ceroidlip<strong>of</strong>uscinosis. Human Molecular Genetics 2013, Jan 12. [Epub ahead <strong>of</strong> print].Anthony DeFranco, Ph.D.Hou B, A Benson, L Kuzmich, AL DeFranco and F Yarovinsky (2011). Critical coordination<strong>of</strong> innate immune defense against Toxoplasma gondii by dendritic cells responding via theirToll-like receptors. Proc. Natl. Acad. Sci. USA 108: 278-283. PMC3017180Hou B, P Saudan, G Ott, ML Wheeler, M Ji, L Kuzmich, LM Lee, RL C<strong>of</strong>fman, MF Bachmann,A L DeFranco (2011). Selective utilization <strong>of</strong> Toll-like receptor and MyD88 signaling in Bcells for enhancement <strong>of</strong> the anti-viral germinal center response. Immunity 34: 375-84.PMC3064721.Scapini P, Lamagna C, Hu Y, Lee K, Tang Q, DeFranco AL, Lowell CA. (2011) B cellderivedIL-10 suppresses inflammatory disease in Lyn-deficient mice. Proc. Natl. Acad Sci.108: E823-832. PMC3193193Gross AJ, I Proekt and AL DeFranco (2011). Elevated BCR signaling and decreased survival<strong>of</strong> Lyn-deficient transitional and follicular B cells. Eur. J. Immunol. 41: 3645-3655. PMC inprocess.Wheeler ML, and AL DeFranco (2012). Prolonged production <strong>of</strong> reactive oxygen species inresponse to BCR stimulation promotes B cell activation and proliferation. J. Immunol. 189:4405-16.75
Kirkland D, A Benson, J Mirpuri, R Pifer, B Hou, AL DeFranco, F Yarovinsky B cell-intrinsicMyD88 signaling prevents the lethal dissemination <strong>of</strong> commensal bacteria during colonicdamage (2012). Immunity 36: 228-238. PMC3288553.David Erle, M.D.Ritner C, Wong SS, King FW, Mihardja SS, Liszewski W, Erle DJ, Lee RJ, Bernstein HS. Anengineered cardiac reporter cell line identifies human embryonic stem cell-derived myocardialprecursors. PLoS One. 2011 Jan 4;6(1):e16004. PMID: 21245908Wang BT, Ducker GS, Barczak AJ, Barbeau R, Erle DJ, Shokat KM. The mammalian target <strong>of</strong>rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistanttranscriptional pr<strong>of</strong>ile. Proc Natl Acad Sci USA 108:15201-6, 2011. PMCID: PMC3174577.Zhao W, Blagev D, Pollack J, Erle DJ. Toward a systematic understanding <strong>of</strong> mRNA 3’untranslated regions. Proc Am Thorac Soc 8:163-6, 2011. PMCID: PMC3131834.Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, Raymond WW,Erle DJ, Abrink M, Caughey GH, Huang X, Sheppard D. The αvβ6 integrin modulates airwayhyperresponsiveness in mice by regulating intraepithelial mast cells. J Clin Invest 122:748-58,2012. PMCID: PMC3266785.Diez D, Goto S, Fahy JV, Erle DJ, Woodruff PG, Wheelock ÅM, Wheelock CE. Networkanalysis identifies a putative role for the PPAR and type 1 interferon pathways in glucocorticoidactions in asthmatics. BMC Med Genomics 5:27, 2012. PMCID: PMC3408345.Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ. AGR2 isinduced in asthma and promotes allergen-induced mucin overproduction. Am J Respir Cell MolBiol 47:178-85, 2012. PMCID: PMC3423459.Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C,Barczak AJ, Zlock LT, Blagev DP, Finkbeiner WE, Ansel KM, Arron JR, Erle DJ*, WoodruffPG*. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med 2012[Epub ahead <strong>of</strong> print] PubMed PMID: 22955319. * equal contributions.John Fahy, M.D.Innes AL, Wong McGrath K, Dougherty RH, McCulloch CE, Woodruff PG, Seibold MA,Okamoto KS, Kelsey J. Ingmundson KJ, Solon MC, Carrington SD, Fahy JV. The H Antigen atEpithelial Surfaces is Associated with Susceptibility to <strong>Asthma</strong> Exacerbation. Am J Respir CritCare Med. 2011;183:189-94. PMCID: PMC3040389.Choy DF, Modrek B, Abbas AR, Kummerfeld S, Clark HF, Wu LC, Fedorowicz G, Modrusan Z,Fahy JV, Woodruff PG, Arron JR. Gene expression patterns <strong>of</strong> Th2 inflammation andintercellular communication in asthmatic airways. J Immunol. 2011 Feb 1;186(3):1861-9.Fahy JV, Locksley RM. The airway epithelium as a regulator <strong>of</strong> Th2 responses in asthma.American Journal <strong>of</strong> Respiratory and Critical Care Medicine 2011;184: 390-392.Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ, Huang X,Locksley RM, Fahy JV. A protective role for periostin and TGFb in IgE-mediated allergy andairway hyperresponsiveness. Clinical and Experimental Allergy 2012;42:144-55.Wong McGrath K, Icitovic N, Boushey HA, Lazarus SC, Sutherland, ER, Chinchilli VM, FahyJV. A large subgroup <strong>of</strong> mild moderate asthma is persistently non-eosinophilic. AmericanJournal <strong>of</strong> Respiratory and Critical Care Medicine. 2012; 185(6):612-9.Xiaozhu Huang, M.D.Masuno K, Haldar SM, Jeyaraj D, Mailloux C, Huang X, Panettieri Jr RA, Jain MK, Gerber AN.76
Expression Pr<strong>of</strong>iling Identifies Klf15 as a Glucocorticoid Target that Regulates AirwayHyperresponsiveness. Am J Respir Cell Mol Biol. 2011 Jan 21Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X, Li JT,Atabai K, Huang X*, Sheppard D. IL-17A produced by αβ T cells drives airway hyperresponsivenessin mice and enhances mouse and human airway smooth muscle contraction. NatMed. 2012 Mar 4;18(4):547-54 (*co-authorship)Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M, Huang X,Interlandi J, Djuretic IM, Brown DR, Sharpe AH, Rao A, Ansel KM. Interleukin-4 production byfollicular helper T cells requires the conserved Il4 enhancer hypersensitivity site V. Immunity.2012 Feb 24;36(2):175-87Thornton E, Looney M, Sheppard D, Locksley R, Huang X, Krummel M. Dynamics <strong>of</strong> antigencapture and presentation revealed by two-photon live imaging in the lung. J Exp Med. 2012 Jun4;209(6):1183-99Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ. AGR2 isInduced in <strong>Asthma</strong> and Promotes Allergen-Induced Mucin Overproduction. Am J Respir CellMol Biol. 2012 Aug;47(2):178-85Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A,Burlingame AL, Matthay MA, Sheppard D. IQGAP1 is necessary for pulmonary vascular barrierprotection in murine acute lung injury and pneumonia. Am J Physiol Lung Cell Mol Physiol.2012 Jul 1;303(1):L12-9Chun Chen, Makoto Kudo, Florentine Rutaganira, Hiromi Takano, Candace Lee, Amha Atakilit,Toshimitsu Uede, Paul Wolters, Stephen Liggett, Kevan M. Shokat, Xiaozhu Huang and DeanSheppard . Integrin α9β1 in airway smooth muscle regulates a novel brake on exaggeratedmurine and human airway narrowing. JCI 2012 Aug 1;122(8):2916-27Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, Donne ML,Huang X, Sheppard, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, Jan LY,Rock JR. Calcium-activated chloride channel TMEM16A modulates mucin secretion and airwaysmooth muscle contraction. Proc Natl Acad Sci USA. 2012 Oct 2;109(40):16354-9.Julia Freimuth, Frederic F. Clermont, Xiaozhu Huang, Angela De Sapio, Taku Tokuyasu, DeanSheppard, Rosemary J. Akhurst. Epistatic interactions between Tgfb1 and genetic loci, Tgfbm2and Tgfbm3, determine susceptibility to an asthmatic stimulus. Proc Natl Acad Sci USA. 2012Oct 30;109(44):18042-7Kudo M, Khalifeh Soltani SM, Sakuma SA, McKleroy W, Lee TH, Woodruff PG, Lee JW,Huang K, Chen C, Arjomandi M, Huang X, Atabai K. Mfge8 suppresses airwayhyperresponsiveness in asthma by regulating smooth muscle contraction. Proc Natl Acad SciUSA. 2013 Jan 8;110(2):660Matthew Krummel, Ph.D.Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. 2011. StabilizedImaging <strong>of</strong> immune surveillance in the mouse lung. Nat Methods. 8(1):91-6. PMCID:PMC3076005Englehardt JE, Boldajipour B, Beemiller P, Werb Z, Pandurangi P, Sorenson C, Egelblad M,Krummel MF, Marginating dendritic cells <strong>of</strong> the tumor microenvironment cross-present tumorantigens and stably engage tumor-specific T cells. Cancer Cell. 2012 Mar 20;21(3):402-17. doi:10.1016/j.ccr.2012.01.008. PMCID: PMC3311997Thornton EE, Krummel MF, Looney, M.R. 2012. Live Imaging <strong>of</strong> the Lung. Curr ProtocCytom. Apr; Chapter 12:Unit12.28.77
Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X, Krummel MF.2012. Spatiotemporally Separated Antigen Uptake by Alveolar Dendritic Cells and AirwayPresentation to T Cells in the Lung. J Exp Med., 209(6):1183-99.Limin Liu, Ph.D.Wei W, Yang Z, Tang CH, Liu L (2011). Targeted deletion <strong>of</strong> GSNOR in hepatocytes <strong>of</strong> micecauses nitrosative inactivation <strong>of</strong> O 6 -alkylguanine-DNA alkyltransferase and increasedsensitivity to genotoxic diethylnitrosamine. Carcinogenesis 32:973–977.Lagoda G, Xie Y, Sezen SF, Hurt KJ, Liu L, Musicki B, Burnett AL (2011). FK506Neuroprotection after Cavernous Nerve Injury is Mediated by Thioredoxin and GlutathioneRedox Systems. J Sexual Medicine 8:3325-3334.Na B, Huang ZM, Wang Q, Qi ZX, Tian YJ, Lu CC, Yu JW, Hanes MA, Sanjay Kakara S,Huanga EJ, Ou JHJ, Liu L ‡, *, and Yen TSB ‡,† (2011). Transgenic Expression <strong>of</strong> Entire HepatitisB Virus in Mice Induces Hepatocarcinogenesis Independent <strong>of</strong> Chronic Liver Injury. PLoS One6:e26240.‡ co-senior author; *Corresponding author; † Deceased August 31, 2009.Khan O, Headley M, Gerard A, Wei W, Liu L, Krummel MF (2011). Regulation <strong>of</strong> T cellpriming by lymphoid-stromal inducible nitric oxidesynthase. PLoS ONE 6:e26138.Tang CH, Wei W, Liu L (2011). Regulation <strong>of</strong> DNA Repair by S-Nitrosylation. Biochimica etBiophysica Acta (In press; Published online: http://dx.doi.org/10.1016/j.bbagen.2011.04.014).Tang CH, Wei W, Liu L (2012). Regulation <strong>of</strong> DNA Repair by S-Nitrosylation. Biochim BiophysActa 1820:730–735 (PMC3170507; http://dx.doi.org/10.1016/j.bbagen.2011.04.014).Ozawa K*, Tsumoto H, Wei W, Tang CH, Komatsubara AT, Kawafune H, Shimizu K, Liu L*,Tsujimoto G (2012). Proteomic analysis <strong>of</strong> the role <strong>of</strong> S-nitrosoglutathione reductase inlipopolysaccharide-challenged mice. Proteomics 12, 2024-2035. *Corresponding author.Leung J, Wei W, Liu L (2013). S-nitrosoglutathione reductase deficiency increases mutagenesisfrom alkylation in mouse liver. Carcinogenesis (in press).Richard Locksley, M.D.Wu D, AB Mol<strong>of</strong>sky, HE Liang, RR Ricardo-Gonzalez, HA Jouihan, JK Bando, A Chawla, RMLocksley. 2011. Eosinophils sustain adipose alternatively activated macrophages associatedwith glucose homeostasis. Science 332:243-247.Sullivan BM, H-E Liang, JK Bando, D Wu, LE Cheng, JK McKerrow, CDC Allen, RMLocksley. 2011. Genetic analysis <strong>of</strong> basophil function in vivo. Nature Immunol 12:527-535.Nguyen KD, Y Qui, X Cui, YP Sharon Goh, J Mwangi, T David, L Mukundan, F Brombacher,RM Locksley, A Chawla. 2011. Alternatively activated macrophages produce catecholaminesto sustain adaptive thermogenesis. Nature 480:104-108.Liang H-E, RL Reinhardt, JK Bando, BM Sullivan, I-C Ho, RM Locksley. 2011. Divergentexpression patterns <strong>of</strong> IL-4 and IL-13 define unique functions in allergic immunity. NatureImmunol 13:58-66.Dickgreber N, KJ Farrand, N van Panhuys, DA Knight, ML Chong, S Miranda-Hernandez, AGBaxter, RM Locksley, G Le Gros, IF Hermans. 2012. Immature murine NKT cells passthrough a stage <strong>of</strong> developmentally programmed innate IL-4 secretion. J Leukoc Biol (in press)Gordon E, S Sidhu, Z-E Wang, P Woodruff, S Yuan, M Solon, S Conway, X Huang, RMLocksley, J Fahy. 2012. A protective role for periostin and TGF-b in IgE-mediated allergy andairway hyperresponsiveness. Clin Exp Allergy 42:144-155. PMC327179278
Thornton EE, MR Looney, O Bose, D Sen, D Sheppard, RM Locksley, X Huang, MF Krummel.2012. Spatiotemporally separated antigen uptake by alveolar dendritic cells and airwaypresentation to T cells in the lung. J Exp Med 209:1183-1199. PMC3371730Price AE, RL Reinhardt, H-E Liang, RM Locksley. 2012. Marking and quantifying IL-17Aproducingcells in vivo. PLoS ONE 7:e39750. PMC3387253Cheng LE, K Hartmann, A Roers, MF Krummel, RM Locksley. 2013. Perivascular mast cellsdynamically probe cutaneous blood vessels to capture IgE. Immunity 38:166-175. PMC inprocessOghumu S, R Dong, S Varikuti, T Shawler, T Kampfrath, CA Terrazas, C Lezama-Davila,BMM Ahmer, CC Whitacre, S Rajagopalan, R Locksley, AH Sharpe, AR Sataskar. 2013.Distinct populations <strong>of</strong> innate CD8 T cells revealed in a CXCR3 reporter mouse. J Immunol (inpress).Mol<strong>of</strong>sky AB, JC Nussbaum H-E Liang, SJ Van Dyken, LE Cheng, A Mohapatra, A Chawla,RM Locksley. 2013. Innate lymphoid type 2 cells ILC2) sustain visceral adipose tissueeosinophils and alternatively activated macrophages. J Exp Med (in press). PMC in processVan Dyken SJ, RM Locksley. 2013. Interleukin-4- and interleukin-13-mediated alternativelyactivated macrophages: roles in homeostasis and disease. Annu Rev Immunol 31:317-43. PMCin processSpits H, D Artis, M Colonna, A Diefenbach, JP Di Santo, G Eberl, S Koyasu, RM Locksley,ANJ McKenzie, RE Mebius, F Powrie, E Vivier. 2013. Innate lymphoid cells – a proposal for auniform nomenclature. Nature Rev Immunol 13:145-149. PMC in processHeredia JE, L mukundan, FM Chen, AA Mueller, R Deo, RM Locksley, TA Radno, A Chawla.2013. Type 2 innate signals regulate functionality <strong>of</strong> fibro/adipogenic progenitors to facilitatemuscle regeneration. Cell (in press).William Seaman, M.D.Rebres, RA, Roach TIA, Fraser IDC, Philip F, Moon C, Lin KM, Liu J, Santat L, Cheadle L,Ross EM, Simon MI, Seaman WE: Synergistic Ca2+ responses by Gai- and Gaq-coupled G-protein-coupled receptors require a single PLCb is<strong>of</strong>orm that is sensitive to both Gbg and Gaq. JBiol Chem 286:1-10, 2011Dean Sheppard, M.D.Spassov DS, Wong CH, Sergina N, Ahuja D, Fried M, Sheppard D, Moasser MM. A src-drivenphosphotyrosine signaling switch determines the anchorage state <strong>of</strong> epithelial cells. Mol CellBiol 2011 31:776-82. PMCID: PMC3028653Su G, Atakilit A, Li T. J, Wu N, Bhattacharya M, Zhu J, Li E, Sun S, Chen R, Su C, SheppardD. Integrin αvβ3 prevents vascular leak in mice by regulating cortical actin formation inendothelial cells. Am J Resp Crit Care Med 2012 (in press)Kudo M, Melton AC, Chen C, Engler M, Huang KE, Ren X, Wang Y, Bernstein X, Li J, AtabaiK, Huang X, Sheppard D. IL-17A produced by ab T cells drives airway smooth musclecontraction. Nature Medicine 2012 (in press).Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, Raymond WW,Erle D, Abrink M, Caughey GH, Huang X, Sheppard D. The avb6 integrin modulates airwayhyperresponsiveness by regulating intra-epithelial mast cells. J Clin Invest 2012 (in press).Su G, Atakilit A, Li T. J, Wu N, Bhattacharya M, Zhu J, Li E, Sun S, Chen R, Su C, SheppardD. Integrin αvβ3 prevents vascular leak in mice by regulating cortical actin formation inendothelial cells. Am J Resp Crit Care Med 2012 (in press)79
Giacomini M, Travis M, Sheppard D. Epithelial cells utilize cortical actin/myosin to activatelatent TGF-β through integrin αvβ6-dependent physical force. Exp Cell Res 2012 318:716-22.PMID: 22309779.Chen C, Kudo M, Rutaganira F, Takano H, Lee C, Atakilit A, Robinett, KS, Uede T, Wolters P,Shokat KM, Huang X, Sheppard D. Integrin alpha9beta1 in airway smooth muscle regulates anovel brake on exaggerated murine and human airway narrowing J Clin Invest 2012 122:2916-27 PMID 22772469.Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A,Burlingame AL, Matthay M, Sheppard D. IQGAP1 is necessary for pulmonary vascular barrierprotection in murine acute lung injury and pneumonia Am J Physiol: Lung Cell and Mol Biol2012 303:L12-19 PMID: 22561460.Jeoung-Sook ShinBaravalle, G, Park, H, McSweeney, M, Ohmura-Hoshino, M, Matsuki, Y, Shin, JS.Ubiquitination <strong>of</strong> CD86 is a key mechanism in regulating antigen presentation by dendritic cells,J Immunology. 187:2966, 2011Ma JK, Platt MY, Eastham-Anderson J, Shin JS*, and Mellman I*. MHC class II distribution indendritic cells and B cells is determined by ubiquitin chain length, PNAS. 2012 May 7 [Epubahead <strong>of</strong> print]Zhi-En Wang, M.D., M.S.Gordon E, S Sidhu, Z-E Wang, P Woodruff, S Yuan, M Solonm S Conway, X Huang, RMLocksley, J Fahy. 2012. A protective role for periostin and TGF-b in IgE-mediated allergy andairway hyperresponsiveness. Clin Exp Allergy 42: 144-155. PMC3271792Arthur Weiss, M.D., Ph.D.Limnander A, Depeille P, Freedman TS, Liou J, Leitges M, Kurosaki T, Roose JP, and Weiss A.Stim1, PKCd, and RasGRP proteins set a threshold for pro-apoptotic Erk signaling during B celldevelopment. Nat. Immunol., 12:425-433. 2011. PMID: 21441934Zhu JW, Doan K, Park J, Chau AH, Zhang H, Lowell CA and Weiss A. Distinct functions <strong>of</strong>receptor-like tyrosine phosphatases CD45 and CD148 in chemoattractant-mediated neutrophilmigration and response to S. aureus infection. Immunity. 35:757-769. 2011. PMID: 22078799Zikherman J, Doan K, Parameswaran R, Raschke W, and Weiss A. Quantitative differences inCD45 expression unmask functions for CD45 in B-cell development, tolerance and survival.Proc. Natl. Acad. Sci. USA. ePub ahead <strong>of</strong> print. 2011. PMID: 22135465Mukherjee S, Zhu J, Zikherman J, Parameswaran R, Kadlecek TA, Wang Q, Au-Yeung B,Ploegh H, Kuriyan J, Das J*, and Weiss A* Monovalent and multivalent ligation <strong>of</strong> the B cellreceptor exhibit differential dependence upon Syk and Src family kinases. Sci. Signal. 2013.Jan 1;6(256):ra1 PMID: 23281368 *co-corresponding authorsZikherman J, Parameswaran R, Hermiston M, and Weiss A. The wedge domain <strong>of</strong> the receptorliketyrosine phosphatase CD45 enforces B cell tolerance by regulating substrate specificity. J.Immunol., in press. 2013.Jonathan Weissman, Ph.D.Churchman LS, Weissman JS. (2011) Nascent transcript sequencing visualizes transcription atnucleotide resolution. Nature, 469: 368-73. PMID 2124884480
Ingolia NT, Lareau LF, Weissman JS. (2011) Ribosome pr<strong>of</strong>iling <strong>of</strong> mouse embryonic stemcells reveals the complexity and dynamics <strong>of</strong> Mammalian proteomes. Cell, 147: 789-802.PMC3225288Oh E, Becker AH, Sandikci A, Huber D, Chaba R, Gloge F, Nichols RJ, Typas A, Gross CA,Kramer G, Weissman JS, Bukau B. (2011) Selective ribosome pr<strong>of</strong>iling reveals the cotranslationalchaperone action <strong>of</strong> trigger factor in vivo. Cell, 147: 1295-1308. PMID: 22153074Brar GA, Yassour M, Friedman N, Regev A, Ingolia NT, Weissman JS. (2012) High-resolutionview <strong>of</strong> the yeast meiotic program revealed by ribosome pr<strong>of</strong>iling. Science, 335: 552-7. PMID22194413Li GW, Oh E, Weissman JS. (2012) The anti-Shine-Dalgamo sequence drives translationalpausing and codon choice in bacteria. Nature, 484: 538-41. PMC3338875Ingolia NT, Brar GA, Rouskin S, McGeachy AM, Weissman JS. (2012) The ribosome pr<strong>of</strong>ilingstrategy for monitoring translation in vivo by deep sequencing <strong>of</strong> ribosome-protected mRNAfragments. Nature Protocols, 7: 1534-50. PMID 22836135Carvunis AR, Rolland T, Wapinski I, Calderwood MA, Yildirim MA, Simonis N, CharloteauxB, Hidalgo CA, Barbette J, Santhanam B, Brar GA, Weissman JS, Regev A, Thierry-Mieg N,Cusick ME, Vidal M. (2012) Proto-genes and de novo gene birth. Nature, 487: 370-4.PMC3401362Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S, King D,Shen PS, Weibezahn J, Dunn JG, Rouskin S, Inada T, Frost A, Weissman JS. (2012) Aribosome-bound quality control complex triggers degradation <strong>of</strong> nascent peptides and signalstranslation stress. Cell, 151: 1042-54. PMID 23178123Stern-Ginossar N, Weisburd B, Michalski A, Le VT, Hein MY, Huang SX, Ma M, Shen B, QianSB, Hengel H, Mann M, Ingolia NT, Weissman JS. (2012) Decoding human cytomegalovirus.Science, 338: 1088-93. PMID 23180859Zena Werb, M.D.Hong JS, KJ Greenlee, R Pitchumani, S-H Lee, L-z Song, M Shan, SH Chang, PW Park, CDong, Z Werb, A Bidani, DB Corry & F Kheradmand (2011). Dual protective mechanisms <strong>of</strong>matrix metalloproteinases 2 and 9 in immune defense against Streptococcus pneumoniae. J.Immunol. 186:6427-6436. [2011 Apr 20, Epub ahead <strong>of</strong> print]. PMID: 21508260.Lemieux GA., J Liu, N Mayer, RJ Bainton, K Ashrafi & Z Werb (2011). A whole-organismscreen identifies new regulators <strong>of</strong> fat storage. Nature Chem. Biol. 7: 206-213. [Mar 13. Epubahead <strong>of</strong> print]. PMID: 21390037; PMCID: PMC3076723.Phillips JJ, A Ward, E Huillard, A Robinson, DH Lum, M-Y Polley, SD Rosen, DH Rowitch &Z Werb (2012). Heparan sulfate sulfatase SULF2 regulates PDGFRa signaling and growth inmalignant glioblastoma. J. Clin. Invest. In press.Engelhardt JE, B Boldajipour, P Beemiller, P Pandurangi, C Sorenson, Z Werb, M Egeblad &MF Krummel. (2011). Marginating dendritic cells <strong>of</strong> the tumor microenvironment cross-presentantigens and stably engage tumor-specific T cells. Cancer Cell. In press.Slorach EM, J Chou & Z Werb (2011). Zeppo1 is a novel metastasis promoter that represses E-cadherin expression and regulates p120-catenin is<strong>of</strong>orm expression and localization. Genes Dev.25: 471-484. [Epub ahead <strong>of</strong> print February 11, 2011]. PMCID: PMC3049288.Caudrillier A, K Kessenbrock, BM Gilliss, JX Nguyen, MB Marques, M Monestier, P Toy, ZWerb & MR Looney (2012). Platelets induce neutrophil extracellular traps in transfusion-relatedacute lung injury. J. Clin. Invest. 122(7):2661–2671. [Epub ahead <strong>of</strong> print, Jun 11]. PMID:81
22684106; PMCID: PMC3386815.Engelhardt J, B Boldajipour, P Beemiller, P Pandurangi, C Sorenson, Z Werb, M Egeblad & MFKrummel (2012). Marginating dendritic cells <strong>of</strong> the tumor microenvironment cross-presentantigens and stably engage tumor-specific T cells. Cancer Cell. 21:402-417. PMID: 22439936;PMCID: PMC3311997.Littlepage LE, AS Adler, H Kouros-Mehr, G Huang, J Chou, SR Krig, OL Griffith, JE Korkola,K Qu, DA Lawson, Q Xue, MD Sternlicht, GJP Dijkgraaf, P Yaswen, Hope S Rugo, CASweeney, CC Collins, JW Gray, HY Chang & Z Werb (2012). The transcription factor ZNF217is a prognostic biomarker and therapeutic target during breast cancer progression. CancerDiscov. 2:638-651 [Epub June 22]. PMID: 22728437.Nakasone E, HA Askautrud, T Kees, V Plaks, AJ Ewald, MG Rasch, YX Tan, J Qin, M Fein, JPark, P Sinha, MJ Bissell, E Frengen, Z Werb & M Egeblad (2012). Imaging tumor-stromainteractions during chemotherapy reveals microenvironmental contributions to chemoresistance.Cancer Cell. 21:488-503. PMCID: PMC3332002.Phillips JJ, A Ward, E Huillard, A Robinson, DH Lum, MY Polley, SD Rosen, DH Rowitch & ZWerb (2012). Heparan sulfate sulfatase SULF2 regulates PDGFRa signaling and growth inmalignant glioblastoma. J. Clin. Invest. 122:911–922 [Epub Feb. 1]. PMID: 22293178; PMCID:PMC3287216.Chou J, JH Lin, A Brenot, JW Kim, S Provot & Z Werb (2013). GATA3 suppresses metastasisand modulates the tumor microenvironment by regulating miR-29 expression. Nat. Cell Biol. Inpress.Prescott WoodruffChoy DF, Modrek B, Abbas AR, Kummerfeld S, Clark HF, Wu LC, Fedorowicz G, Modrusan Z,Fahy JV, Woodruff PG, Arron JR. Gene expression patterns <strong>of</strong> th2 inflammation andintercellular communication in asthmatic airways. J Immunol. 2011 Feb 1;186(3):1861-9.Woodruff PG, Albert RK, Bailey WC, Casaburi R, Connett JE, Cooper JAD, Criner GJ, CurtisJL, Dransfield MT, Han MK, Harnden SM, Kim V, Marchetti N, Martinez FJ, McEvoy CE,Niewoehner DE, Reilly JJ, Rice K, Scanlon PD, Scharf SM, Sciurba FC, Washko GR, LazarusSC for the COPD Clinical <strong>Research</strong> Network. Randomized Trail <strong>of</strong> Zileuton for Treatment <strong>of</strong>COPD Exacerbations Requiring hospitalization. COPD. 2011 Feb:8(1):21-9. PMC Journal – InProcessBhakta NR, Woodruff PG. Human <strong>Asthma</strong> Phenotypes: From the Clinic, to Cytokines, andBack Again Immunol Rev. 2011 Jul;242(1):220-32.Koth LL, Solberg OD, Peng JC, Bhakta NR, Nguyen DCP, Woodruff PG. Sarcoidosis BloodTranscriptome Reflects Lung Inflammation and Overlaps with Tuberculosis. AM J Respir CritCare Med. 2011 Aug 18. [Epub ahead <strong>of</strong> print]Albert RK, Connett J. Bailey WC, Casaburi R, Cooper JAD, Criner GJ, Curtis JL. DransfieldMT, Han MK, Lazarus SC, Make B, Marchetti N. Martinez FJ, Madinger NA, McEvoy C,Niewoehner DE, Porsasz J, Price CS, Reilly J, Scanlon PD, Sciurba FC, Scaharf SM, WashkoGR, Woodruff PG, and Anthonisen NR, for the COPD Clinical <strong>Research</strong> Network.Azithromycin for Prevention <strong>of</strong> Exacerbations <strong>of</strong> COPD. N Engl J Med 2011; 365:689-698Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ, Huang X,Locksley RM, Fahy JV. A protective role for periostin and TGF-β in IgE-mediated allergy andairway hyper-responsiveness. Clin Exp Allergy. 2012 Jan;42(1):144-55.82
Eri1 regulates microRNA homeostasis and mouse lymphocyte development and antiviralfunction. Thomas MF, Abdul-Wajid S, Panduro M, Babiarz JE, Rajaram M, Woodruff P, LanierLL, Heissmeyer V, Ansel KM. Blood. 2012 Jul 5;120(1):130-42. Epub 2012 May 21.Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, Carter R,Audusseau S, Hamid Q, Bradding P, Fahy JV, Woodruff PG, Harris JM, Arron JR;Bronchoscopic Exploratory <strong>Research</strong> Study <strong>of</strong> Biomarkers in Corticosteroid-refractory <strong>Asthma</strong>(BOBCAT) Study Group. Periostin is a systemic biomarker <strong>of</strong> eosinophilic airway inflammationin asthmatic patients. J Allergy Clin Immunol. 2012 Sep;130(3):647-654Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C,Barczak AJ, Zlock LT, Blagev DP, Finkbeiner WE, Ansel KM, Arron JR, Erle DJ, WoodruffPG. Airway Epithelial miRNA Expression is Altered in <strong>Asthma</strong>. Am J Respir Crit Care Med.2012 Sep 6. [Epub ahead <strong>of</strong> print]Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD, DonneML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, Li M, Jan YN, JanLY, Rock JR. Calcium-activated chloride channel TMEM16A modulates mucin secretion andairway smooth muscle contraction. Proc Natl Acad Sci USA. 2012 Sep 17. [Epub ahead <strong>of</strong> print]83
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>84
Looking to the FutureRichard M. Locksley, M.D.The SABRE <strong>Center</strong> is evolving into a more mature entity. All but one <strong>of</strong> the recruitedfaculty/fellow has obtained R01 NIH funding (the one exception has re-submitted with avery close score and received bridge funding from the School <strong>of</strong> Medicine based onpromise <strong>of</strong> future success), the first recruit has been promoted to Associate Pr<strong>of</strong>essor andthe second, Mark Ansel, was just put up for promotion this summer. The Fellow, Dr.Allen, was recruited to the faculty at the Cardiovascular <strong>Research</strong> Institute at MissionBay and will begin to anchor asthma-related research at that site. The first postdocs andgraduate students have left to assume positions after training in the SABRE <strong>Center</strong>. TheSABRE <strong>Center</strong> is recognized nationally by virtue <strong>of</strong> participation in each <strong>of</strong> the majorasthma multi-institutional efforts <strong>of</strong> the NIH, including as a member <strong>of</strong> <strong>Asthma</strong>Net(under the leadership <strong>of</strong> Homer Boushey), the Severe <strong>Asthma</strong> <strong>Research</strong> Program (SARP;under the leadership <strong>of</strong> John Fahy) and the <strong>Asthma</strong> and Allergic Diseases Cooperative<strong>Research</strong> <strong>Center</strong> Network (under the leadership <strong>of</strong> Dean Sheppard). Members <strong>of</strong> theSABRE <strong>Center</strong> were awarded a Program Project Grant to investigate the role <strong>of</strong> innatelymphoid cells in human asthma (Fahy, Locksley, Ansel, Woodruff). Extramural fundingbrought in solely by the 5 core basic investigators has more than doubled over the past 5years; the totals accrued to the entire program have been leveraged to an even greaterextent. The conferences and seminars are well attended and publicized. Thus, by anumber <strong>of</strong> metrics, asthma-centered research is now well established and recognized atUCSF.Given the current political climate, it is likely that continued government support forhealth sciences will be capped or decline, such that unlimited growth from this sector willnot be possible. Although we continue to compete aggressively in this arena, prioritizingresearch dollars from the Foundation will become increasingly important. Highlysuccessful focused research models from the past, such as Bell Laboratories or theCavendish laboratory at Cambridge <strong>University</strong>, reveal several commonalities, includingsmall laboratories with a shared research goal; technological innovation; and an ability tochange directions quickly to follow-up new insights. Some <strong>of</strong> these aspects we share, butre-evaluating our strategies will be an important component for moving forwardsuccessfully. Ultimately, the SABRE <strong>Center</strong> will need to move more comprehensively tostudies on human patients utilizing human tissues; will need to capture more individualslike Chris Allen early in science when they can be attracted into the field in a lasting andsubstantive way; will need to move seamlessly into ‘big science’ technologically-drivenfields at low cost, necessitating an interactive and integrated group <strong>of</strong> scientists; will needto partner with industry in ways to move scientific understanding towards interventionalefforts that remain cost-prohibitive for a small <strong>Center</strong>; and will need to grow an endowedfinancial base that will enable nimble re-direction and follow-up using competitivepriorities to fund shifting emergent needs for equipment, personnel (including visitingscientists) and short-term needs in response to windows <strong>of</strong> opportunity. Funding fixedCores may not be an optimal resource allocation, as technology advances, cores becomeincreasingly mainstreamed and publicly available, and costs continue to decrease. Inshort, with the help <strong>of</strong> both the Executive Board and the Scientific Advisory Board, the90
SABRE <strong>Center</strong> will work to develop a Strategic Plan in order to shape the research andfinancial priorities for the coming decade. Our hope is to continue the trajectoryestablished by the last 5 years on a pathway <strong>of</strong> increasing success in our mission tounderstand and ultimately conquer asthma. These challenges we take seriously for thefuture in order to honor the extraordinary vision <strong>of</strong> the <strong>Sandler</strong> family in committingresources to asthma basic research at UCSF. We are most grateful for the opportunity torespond to the challenge and look forward to discoveries that will impact thisincreasingly prevalent disease <strong>of</strong> humans.91
<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Biographical sketchesBIOGRAPHICAL SKETCHES92
BIOGRAPHICAL SKETCHESChristopher Allen, Ph.D.K. Mark Ansel, Ph.D.Homer Boushey, M.D.Esteban Burchard, M.D., M.P.H.George Caughey, M.D.Harold Chapman, M.D.Anthony DeFranco, Ph.D.David Erle, M.D.John Fahy, M.D., M.Sc.Xiaozhu Huang, M.D., M.S.Matthew Krummel, Ph.D.Limin Liu, Ph.D.Richard Locksley, M.D.William Seaman, M.D.Dean Sheppard, M.D.Jeoung-Sook Shin, Ph.DZhi-En Wang, M.D., M.S.Arthur Weiss, M.D., Ph.D.Jonathan Weissman, PhD.Zena Werb, PhD.Prescott Woodruff, M.D., M.P.H.93
BIOGRAPHICAL SKETCHNAMEChristopher David Caballero Allen, Ph.D.POSITION TITLE<strong>Sandler</strong>-Newmann Foundation UCSF Fellow in<strong>Asthma</strong> <strong>Research</strong>EDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYMassachusetts Institute <strong>of</strong> Technology B.S. 2001 Biology<strong>University</strong> <strong>of</strong> California, San Francisco Ph.D. 2007 Biomedical<strong>University</strong> <strong>of</strong> California, San Francisco Postdoctoral 2007 Sciences ImmunologyaralralPositions and Honors1998-2000 Summer <strong>Research</strong> Intern, Department <strong>of</strong> Molecular and CellularPharmacology, Isis Pharmaceuticals, Carlsbad, CA2000 Undergraduate Student <strong>Research</strong>er, Laboratory <strong>of</strong> Herman Eisen, <strong>Center</strong>for Cancer <strong>Research</strong>, Massachusetts Institute <strong>of</strong> Technology2001-2007 Graduate Student <strong>Research</strong>er, Laboratory <strong>of</strong> Jason Cyster, BiomedicalSciences Graduate Program and Immunology Graduate Program,<strong>University</strong> <strong>of</strong> California, San Francisco, CA2007 Postdoctoral Scholar, Laboratory <strong>of</strong> Jason Cyster, Department <strong>of</strong>Microbiology and Immunology, <strong>University</strong> <strong>of</strong> California, San Francisco2007–2012 <strong>Sandler</strong>-Newmann Foundation UCSF Fellow in <strong>Asthma</strong> <strong>Research</strong>, <strong>Sandler</strong><strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong> and the Department <strong>of</strong> Microbiology andImmunology, <strong>University</strong> <strong>of</strong> California, San Francisco, CA2012 – Present Assistant Pr<strong>of</strong>essor Cardiovascular <strong>Research</strong> Institute, Department <strong>of</strong>Anatomy and Department <strong>of</strong> Microbiology and Immunology, <strong>Sandler</strong><strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>, <strong>University</strong> <strong>of</strong> California, San Francisco1994-1995 National Science Foundation Young Scholars Program Fellowship1997 National Hispanic Scholar1998 San Diego Biotech Employee Development Coalition (BEDC)Scholarship1999, 2000 Academic Excellence Award, Office <strong>of</strong> Minority Education,Massachusetts Institute <strong>of</strong> Technology2001 Phi Beta Kappa2001 Whitehead Prize in Biomedical <strong>Research</strong>2001–2002 <strong>University</strong> <strong>of</strong> California Regents Fellowship94
2002–2007 Howard Hughes Medical Institute Predoctoral Fellowship2010 Seminars in Immunology Top Cited Article 2008-20102012 NIH Director’s New Innovator AwardSelected peer-reviewed publications (in chronological order)1. Allen C.D.C., Ansel K.M., Low C., Lesley R., Tamamura H., Fujii N. Cyster J.G. (2004).Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5.Nature Immunology, 5(9), 943-952.2. Chen T.T., Li L., Chung D.H., Allen C.D.C., Torti S.V., Torti F.M., Cyster J.G., Chen C.Y.,Brodsky F.M., Niemi E.C., Nakamura M.C., Seaman W.E., Daws M.R. (2005). TIM-2 isexpressed on B cells and in liver and kidney and is a receptor for H-ferritin endocytosis. TheJournal <strong>of</strong> Experimental Medicine, 202(7), 955-965. PMC2213179.3. Allen C.D.C., Okada T., Tang H.L., Cyster J.G. (2007). Imaging <strong>of</strong> germinal center selectionevents during affinity maturation. Science, 315(5811), 528-531.4. *Allen C.D.C., *Okada T., *Cyster J.G. (2007). Germinal-center organization and cellulardynamics. Immunity 27(2), 190-202. *co-corresponding author. PMCID: PMC2242846.5. Haynes N.M., Allen C.D.C., Lesley R., Ansel K.M., Killeen N., and Cyster J.G. (2007). Role<strong>of</strong> CXCR5 and CCR7 in follicular Th cell positioning and appearance <strong>of</strong> a programmed celldeath gene-1 High germinal center-associated subpopulation. The Journal <strong>of</strong> Immunology,179(8), 5099-5108.6. *Allen C.D.C., *Cyster J.G. (2008). Follicular dendritic cell networks <strong>of</strong> primary folliclesand germinal centers: phenotype and function. Seminars in Immunology 20(1), 14-25. *cocorrespondingauthor PMCID: PMC2366796.7. Sullivan B.M., Liang H.E., Bando J.K., Wu D., Cheng L.E., McKerrow J.K., *Allen C.D.C.,*Locksley R.M. (2011). Genetic analysis <strong>of</strong> basophil function in vivo. Nature Immunology,12(6), 527-535. *co-corresponding author. PMCID: 3271435.8. Beltman J.B., Allen C.D.C., Cyster J.G., de Boer R.J. (2011). B cells within germinal centersmigrate preferentially from dark to light zone. Proceedings <strong>of</strong> the National Academy <strong>of</strong>Sciences, 108(21), 8755-8760. PMCID: PMC3102384.9. Green J.A., Suzuki K., Cho B., Willison L.D., Palmer D., Allen C.D.C., Schmidt T.H., XuY., Proia R.L., Coughlin S.R., Cyster J.G. (2011). The sphingosine 1-phosphate receptorS1P 2 maintains the homeostasis <strong>of</strong> germinal center B cells and promotes niche confinement.Nature Immunology, 12(7), 672-680. PMCID: PMC3158008.10. Steiner D.F., Thomas M.F., Hu J.K., Yang Z., Babiarz J.E., Allen C.D.C., Matloubian M.,Blelloch R., Ansel K.M. (2011). MicroRNA-29 Regulates T-Box Transcription Factors andInterferon-γ Production in Helper T Cells. Immunity, 35(2), 169-181. PMCD: PMC3361370.11. Yang Z., Sullivan B.M., Allen C.D.C. (2012). Fluorescent in vivo detection reveals that IgE +B cells are restrained by an intrinsic cell fate predisposition. Immunity, 36(5), 857-872.95
<strong>Research</strong> SupportOngoing <strong>Research</strong> Support1 DP2 HL117752-01 Allen (PI) 09/30/2012 – 08/31/2017NIH/NHLBICellular interactions in asthmaThis project is focused on the dynamic communication among inflammatory cells inasthmatic lungs. The major goals <strong>of</strong> this project are to develop technical approaches tosimultaneously visualize multiple different types <strong>of</strong> inflammatory cells in the lung, followedby characterization <strong>of</strong> relevant cellular interactions in a combinatorial fashion, and thendefinition <strong>of</strong> the stromal microenvironments in which these interactions occur.Role: PI1 R01 AI103146-01 Allen (PI) 12/01/2012 – 11/30/2017NIH/NIAIDAnalysis <strong>of</strong> basophil function in secondary immune responsesThe major goal <strong>of</strong> this project is to determine the functional role <strong>of</strong> basophils that havecaptured antigen via IgE antibodies in secondary immune responses. Specifically, this projectwill determine whether basophils contribute to antigen transport, to the enhancement <strong>of</strong>adaptive immunity, and to tissue damage and repair.Role: PICompleted <strong>Research</strong> SupportNone96
BIOGRAPHICAL SKETCHNAMEK. Mark AnseleRA COMMONS USER NAMEanselmPOSITION TITLEAssistant Pr<strong>of</strong>essor <strong>of</strong> Microbiology andImmunologyEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYVirginia Tech (Blacksburg, VA) B.S. 1992-1996 Biochemistry<strong>University</strong> <strong>of</strong> California, San Francisco Ph.D. 1996-2001 Biomedical SciencesImmune Disease Institute, Harvard Medical School 2001-2007 ImmunologyPositions2001 – 2005 Postdoctoral Fellow, Immune Disease Institute (p.k.a. <strong>Center</strong> for Blood<strong>Research</strong>), Harvard Medical School, Boston, MA2005 – 2007 Instructor, Department <strong>of</strong> Pediatrics, Children’s Hospital and ImmuneDisease Institute (p.k.a. <strong>Center</strong> for Blood <strong>Research</strong>), Harvard MedicalSchool, Boston, MA2008 – Present Assistant Pr<strong>of</strong>essor, Department <strong>of</strong> Microbiology and Immunology and<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>, <strong>University</strong> <strong>of</strong> California SanFranciscoOther Experience and Pr<strong>of</strong>essional Memberships1998 - American Association for the Advancement <strong>of</strong> Science2006 - American Association <strong>of</strong> Immunologists2007 - International Cytokine Society2011 - Board <strong>of</strong> Reviewing Editors, Science Signaling2011 - International Predoctoral Fellows Review Cmte.,Howard Hughes Medical Institute2012 - NIH peer review committee: Cellular & MolecularImmunology B, ad hoc reviewerAwards and Honors1997 – 2001 Howard Hughes Medical Institute Predoctoral Fellowship2001 – 2004 Damon Runyon Cancer <strong>Research</strong> Fund Postdoctoral Fellowship2005 – 2007 Leukemia and Lymphoma Society Special Fellow2006 Burroughs Wellcome Career Award in Biomedical Sciences97
2007 International Cytokine Society Outstanding Postdoctoral Fellow2009 Dana Foundation Human Immunology Scholar2012 American Association <strong>of</strong> Immunologists Young Investigator TravelAward2012 Leukemia & Lymphoma Society ScholarSelected Peer-reviewed Publications1. Grégory Seumois, Pandurangan Vijayanand, Christopher J Eisley, Nada Omran, LukasKalinke, Mai North, Asha P. Ganesan, Laura J Simpson, Nathan Hunkapiller, FleixMolzahan, Prescott G Woodruff, John V Fahy, David J Erle, Ratka Djukanovic, RobertBlelloch, K Mark Ansel: An integrated nano-scale approach to pr<strong>of</strong>ile miRNAs in limitedclinical samples. Am J Clin Exp Immuol. 1:70-89 (2012)2. Hoefig KP, Rath N, Heinz GA, Wolf C, Dameris J, Schepers A, Kremmer E, Ansel KM,Heissmeyer V. Eri1 degrades the stem-loop <strong>of</strong> oligouridylated histone mRNAs to inducereplication-dependent decay. Nat Struct Mol Biol. 20:73-81 (2013)3. Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, NguyenC, Barczak AJ, Zlock LT, Blaev DP, Finkbeiner WE, Ansel KM, Arron JR, Erle DJ,Woodruff PG. Airway Epithelial miRNA Expression is Altered in <strong>Asthma</strong>. Am. J. Respir.Crit. Care Med. 186:965-74 (2012)4. Thomas MF, Abdul-Wajid S, Panduro M, Babiarz JE, Rajaram M, Woodruff P, Lanier LL,Heissmeyer , Ansel KM. Eri1 regulates microRNA homeostasis and mouse lymphocytedevelopment and anti-viral fuction. Blood. 120:130-42 (2012)5. Vijayanand P, Seumois G, Simpson LJ, Abdual-Wajid S, Baumjohann D, Panduro M, HuangX, Interlandi J, Djuretic IM, Brown DR, Sharpe AH, Rao A, Ansel KM. Interleukin-4Productio y Follicular Helper T Cells Requires the Conserved Il4 Enhancer HypersensitivitySite V. Immunity 36:175-87 (2012)6. Baumjohann D, Okada T, Ansel KM. Cutting Edge: Distinct Waves <strong>of</strong> BCL6 Expressionduring T Follicular Helper Cell Development. J Immunol.187: 2089-92 (2011)7. Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CD, Matloubian M, Blelloch R,Ansel KM. MicroRNA-29 Regulates T-Box Transcription Factors and Interferon-γProduction in Helper T Cells. Immunity 35:169-81 (2011)8. S<strong>of</strong>i MH, Qiao Y, Ansel KM, Kubo M, Chang CH. Induction and Maintenance <strong>of</strong> IL-4Expression Are Regulated Differently by the 3' Enhancer in CD4 T Cells. J. Immunol.186:2792-9 (2011)9. Thomas MF, Ansel KM. Construction <strong>of</strong> small RNA cDNA libraries for deep sequencing.Methods Mol Biol. 667:93-111 (2010)10. Parameswaran P, Sklan E, Wilkins C, Burgon T, Samuel MA, Lu R, Ansel KM, HeissmeyerV, Einav S, Jackson W, Doukas T, Paranjape S, Polacek C, Barreto dos Santos F, Jalili R,Babrzadeh F, Gharizadeh B, Grimm D, Kay M, Koike S, Sarnow P, Ronaghi M, Ding SW,Harris E, Chow M, Diamond MS, Kirkegaard K, Glenn JS, Fire AZ. Six RNA Viruses andForty-One Hosts: Viral Small RNAs and Modulation <strong>of</strong> Small RNA Repertoires inVertebrate and Invertebrate Systems. PLoS Pathog. 6:e1000764 (2010)98
11. Ansel KM # , Pastor WA, Rath N, Lapan AD, Glasmacher E, Wolf C, Smith LC,Papadopoulou N, Lamperti E, Tahiliani M, Ellwart JW, Shi Y, Kremmer E, Rao A,Heissmeyer V # . Mouse ERI-1 interacts with the ribosome and catalyzes 5.8S rRNAprocessing. Nat. Struct. Mol. Biol. 15:523-30 (2008) ( # corresponding authors, equalcontribution)12. Haynes NM, Allen CD, Lesley R, Ansel KM, Killeen N, Cyster JG. Role <strong>of</strong> CXCR5 andCCR7 in Follicular Th Cell Positioning and Appearance <strong>of</strong> a Programmed Cell Death Gene 1High Germinal <strong>Center</strong>-Associated Subpopulation. J. Immunol. 179:5099-108 (2007)13. Thai TH, Calado DP, Casola S, Ansel KM, Xiao C, Xue Y, Murphy A, Frendewey D,Valenzuela D, Kutok JL, Schmidt-Supprian M, Rajewsky N, Yancopoulos G, Rao A,Rajewsky K. Regulation <strong>of</strong> the germinal center response by microRNA-155. Science316:604-8 (2007)14. Djuretic IM, Levanon D, Negreanu V, Groner Y, Rao A, Ansel KM. T-bet and Runx3cooperate to activate Ifng and silence Il4 in Th1 cells. Nat. Immunol. 8:145-53 (2007)15. Ansel KM, Djuretic I, Tanasa B, Rao A. Regulation <strong>of</strong> Th2 differentiation and the IL4 locus.Annu. Rev. Immunol. 24:607-56 (2006)16. Bronevetsky Y, Villarino AV, Eisley C, Barbeau R, Barczak A, Heinz GA, Kremmer E,Heissmeyer V, McManus MT, Erle DJ, Rao A, Ansel KM. T cell activation inducesproteasomal degradation <strong>of</strong> Argonaute and rapid remodeling <strong>of</strong> the micro RNA repertoire. J.Exp. Med., in press.17. Baumjohann D, Clingan JM, de Kouchkovsky D, Bannard O, Bluestone JA, Matloubian M,Ansel KM#, Jeker LT#. The microRNA cluster miR-17-92 is essential for follicular helperT cell differentiation. Under revision at Nature Immunology (#co-corresponding authors).<strong>Research</strong> SupportOngoing <strong>Research</strong> Support1PO1HL107202 Fahy (PI) 8/15/12 – 5-31-17NIH/NHLBIInnate and adaptive immune responses in Th2-high asthma (PI Dr. John Fahy, co-directorUCSF ACRC) Project 2: Role <strong>of</strong> miRNAs in Th2-Driven inflammation in <strong>Asthma</strong> (ProjectLeader Ansel) Project 3: Mechanisms <strong>of</strong> airway Th2 inflammation in asthma (Project LeaderFahy, Co- project Leader, Ansel). The major goal <strong>of</strong> this PPG is to elucidate cellular andmolecular mechanisms underlying the initiation and maintenance <strong>of</strong> Th2-high asthma. Thegoals <strong>of</strong> Project 2 are to identify miRNAs that regulate helper T cell functions relevant toasthma, to discover asthma associated T cell miRNA expression patterns in clinical samples,and to determine the mRNA targets and in vivo role <strong>of</strong> miR-29 in mouse model <strong>of</strong> asthma. Myrole in aim 3 is immunophenotyping <strong>of</strong> innate helper type II cells in human asthma.Role: Project 2 Leader, Project 3 co-project leader1R01HL109102-01 Ansel (PI) 8/1/11 – 6/30/16NIH/NHLBIRole <strong>of</strong> miRNAs in Th2-driven inflammation in asthma99
The major goals <strong>of</strong> this project are to discover asthma-associated T cell miRNA expressionpatterns in clinical samples, and to identify and characterize the in vivo activity <strong>of</strong> miRNAs thatregulate helper T cell functions relevant to asthma. The project will be conducted incollaboration with co-investigator Dr. Prescott Woodruff, co-director <strong>of</strong> the UCSF AirwayClinical <strong>Research</strong> <strong>Center</strong>.LLS Scholar Award Ansel (PI) 7/1/12 – 6/30/17Leukemia & Lymphoma SocietyMicroRNA regulation <strong>of</strong> lymphocyte growth and effector functionsThis career development program award would support our research program focus onmiRNAs that regulate essential helper T cell functions that contribute to immunity,immunopathology, and immune malignancies. In particular, we aim to identify andcharacterize miRNAs that regulate helper T cell growth, survival, and cytokine production.Pending <strong>Research</strong> Support1R21AI105270 Ansel (PI) 7/1/13 – 6/30/15NIH/NIAIDMolecular mechanisms <strong>of</strong> Eri1 regulation <strong>of</strong> antiviral immunityThe major goals <strong>of</strong> this project is to elucidate the molecular mechanisms by which theexoribonuclease Eri1 controls antiviral immune responses mediated by T and NK lymphocytes.Genetic and biochemical approaches will be used to determine the contribution <strong>of</strong> direct effectson mRNA stability and translation and the indirect effects <strong>of</strong> Eri1 regulation <strong>of</strong> microRNAhomeostasis.Role: PI1U19 CA179512 Blelloch (PI) 7/1/13 – 6/30/18NIH/Director’s OfficeIn Vivo Regulated Release and Function <strong>of</strong> Extracellular Small RNAsThis U19 <strong>Center</strong>’s long-term goal is to uncover paradigms <strong>of</strong> extracellular small RNA functionin health and disease and apply those paradigms to clinically relevant settings includingbiomarker discovery and therapeutic intervention. As leader <strong>of</strong> Project 1, I will conduct studiesto test the central hypothesis that immune cells release ex-miRNAs in response to inflammatorystimuli, and that this process is critical for their immune function.Completed <strong>Research</strong> Support1R56AI089828-01 Ansel (PI) 9/1/10 – 7/31/11NIH/NHLBIRegulation <strong>of</strong> miRNA expression during helper T cell differentiationThe goal <strong>of</strong> these studies is to dissect the mechanisms that govern changes in miRNAexpression during the activation <strong>of</strong> T lymphocytes, and to define how the global regulation <strong>of</strong>miRNA homeostasis affects T cell activation and immune function.100
Human Immunology Scholars Program Ansel (PI) 2/1/09 – 1/31/12Dana FoundationMicroRNA regulation <strong>of</strong> helper T cell function in asthmaThe major goals <strong>of</strong> this proposal are to optimize technology for miRNA pr<strong>of</strong>iling by qPCR andperform a pilot study <strong>of</strong> miRNA expression in a small set <strong>of</strong> clinical samples, to develop alentiviral miRNA expression library for studies <strong>of</strong> miRNA function in T cells, and to developsystems for miRNA inhibitor testing in lung explants.101
BIOGRAPHICAL SKETCHNAMEHomer A. Boushey, Jr., M.D.eRA COMMONS USER NAMEBousheyPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> MedicineEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYStanford <strong>University</strong>, Palo Alto, CA A.B. 1964 Biology<strong>University</strong> <strong>of</strong> California, San Fancisco, CA M.D. 1968 Medicine<strong>University</strong> <strong>of</strong> California, San Francisco, CA Reside 1970 Internal MedicineBeth Israel Hospital, Boston, MAncy Reside 1971 Internal MedicineOxford <strong>University</strong>, Oxford, Englandncy Fellow 1972 Pulmonary MedicineshipPositions and Honors1974-1981 Assistant Pr<strong>of</strong>essor <strong>of</strong> Medicine in Residence, <strong>University</strong> <strong>of</strong>California, San Francisco.1981-1987 Associate Pr<strong>of</strong>essor <strong>of</strong> Medicine in Residency, <strong>University</strong> <strong>of</strong>California, San Francisco.1986- Present Member, Senior Staff, Cardiovascular <strong>Research</strong> Institute, <strong>University</strong><strong>of</strong> California, San Francisco1987-1989 Pr<strong>of</strong>essor <strong>of</strong> Medicine in Residence, <strong>University</strong> <strong>of</strong> California, SanFrancisco.1989-Present Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California, San Francisco.1989-1995 Vice Chairman for Clinical Affairs, Department <strong>of</strong> Medicine,<strong>University</strong> <strong>of</strong> California, San Francisco1996-2009 Chief, Allergy/Immunology Division, Department <strong>of</strong> Medicine,<strong>University</strong> <strong>of</strong> California, San FranciscoMedical Licenses and Board CertificationCalifornia License Number A 023453American Board <strong>of</strong> Internal Medicine, June 1972Subspecialty Board <strong>of</strong> Pulmonary Medicine, October 1974102
Medical Society Memberships2003-May 2004Honors and AwardsPast-President - American Thoracic SocietyCalifornia Thoracic SocietyAmerican Federation for Clinical <strong>Research</strong>American Physiological SocietyCalifornia Academy <strong>of</strong> MedicineWestern Society for Clinical InvestigationWestern Association <strong>of</strong> Physicians1964 Phi Beta Kappa1967 AOA1964-1968 Regents' Scholar1968 Gold-Headed Cane Recipient1977 H. J. Kaiser Award for Excellence in Teaching1988, ’90, ’95, Faculty-Student Teaching Award for "An Outstanding Lecture"99, 20001993 Clean Air Award (Education/<strong>Research</strong>), American Lung Association,San Francisco1993 California Medal, American Lung Association-California1996 UCSF Alumnus <strong>of</strong> the Year Award1997-2000 Bay Area’s Best Physicians, San Francisco Focus Magazine2000 Medical Student Teaching Award: “An Outstanding ClinicalCorrelation Lecturer”Selected peer-reviewed publications (in chronological order)1. Fahy JV, Wong H, Liu J, Boushey HA. Comparison <strong>of</strong> samples collected by sputuminduction and bronchoscopy from asthmatic and healthy subjects. Am J Respir Crit CareMed 1995;152:53-58.2. Fahy JV, Kim KW, Liu J, Boushey HA. Prominent neutrophilic inflammation in sputumfrom subjects with asthma exacerbation. J Allergy Clin Immunol 1995;95(4): 843-852.3. Drazen JM, Israel E, Boushey HA, Chinchilli VM, Fahy JV, Fish JE, Lazarus SC,Lemanske RF, Martin RJ, Peters SP, Sorkness C, Szefler SJ, for the National Heart,Lung, and Blood Institute’s <strong>Asthma</strong> Clinical <strong>Research</strong> Network. Comparison <strong>of</strong>regularly scheduled with as-needed use <strong>of</strong> albuterol in mild asthma. N Engl J Med 1996;335:841- 47.4. Fahy JV, Fleming HE, Wong HH, Liu JT, Su JQ, Reimann J, Fick RB, Boushey HA.The effect <strong>of</strong> an anti-IgE monoclonal antibody on the early and late phase responses toallergen inhalation in asthmatic subjects. Am J Respir Crit Care Med 1997; 155:1828-34.5. Fleming HE, Little FF, Schnurr D, Avila PC, Wong H, Liu J, Yagi S, Boushey HA.Rhinovirus-16 colds in healthy and asthmatic subjects: similar changes in upper andlower airways. Am J Respir Crit Care Med 1999; 160:100-8.103
6. Lazarus S, Boushey H, Fahy J, Chinchilli V, Lemanske R, Sorkness C, Kraft M, Fish J,Peters S, Craig T, Drazen J, Ford J, Israel E, Martin R, Mauger E, Nachman S, Spahn J,Szefler S, and the <strong>Asthma</strong> Clinical <strong>Research</strong> Network. A randomized study <strong>of</strong> longactingbeta-agonist in patients with persistent asthma: I. monotherapy? JAMA 2001 May;23; 285: 2583-93.7. Wang D, Coscoy L, Zylberberg M, Avila PC, Boushey HA, Ganem D, DeRisi JL.Microarray-based detection and genotyping <strong>of</strong> viral pathogens. PNAS-2002; 99:15687-92.8. Lopez-Souza N, Dolganov G, Dubin R, Sporer H, Yagi S, Schurr D, Boushey H, et al.Resistance <strong>of</strong> Differentiated human airway epithelium to infection by rhinovirus. Am JPhysiol Lung Cell Mol Physiol. 2004 Feb; 286: L373-81.9. Boushey HA, Sorkness CA, King TS, Sullivan SD, Fahy JV, Lazarus SC, et al. “Dailyversus As-Needed Corticosteroids for Mild Persistent <strong>Asthma</strong>” New Eng J Med, 2005;352:1519-28.10. Stol<strong>of</strong>f SW, Boushey HA. Severity, Control, and Responsiveness in <strong>Asthma</strong>. J AllergyClin Immunol 2006: 117:544-8 (“Rostrum”)11. Kistler A, Avila PC, Rouskin S, Wang D, Ward T, Yagi S, Schnurr D, Ganem D, DeRisiJ, Boushey HA. “Pan-viral screening <strong>of</strong> respiratory tract infections in asthmatic and nonasthmaticadults reveals unexpected coronavirus and human rhinovirus diversity” J InfectDis. 2007 Sep 15:196(6):817-2512. Kistler A, Webster D, Rouskin S, Magrini V, Credle J, Schnurr D, Boushey HA, MardisE, Li H, DeRisi JL. “Genome-wide diversity and selective pressure in the humanrhinovirus.” Virol J. 2007 May 3; 4:40.13. Huang YJ, Nelson CE, Brodie EL, DeSantis TZ, Baek MS, Liu J, Woyke T, Allaier M,Bristow J, Wiener-Kronish JP, Sutherland ER, King TS, Icitovic N, Martin RJ, CalhounWJ, Castro M, Denlinger LC, DimangoE, Kraft M, Peters SP, Wasserman SI, WechslerME, Boushey HA, and Lynch SV. Airway microbiota andbronchial hyperresponsivenessin patients with sub-optimally controlled asthma. JACI 2011; 127:372-381.14. Wootton SC, Kim DS, Kondoh Y, Chen E, Lee JS, Song JW, Huh JW, Taniguchi H,Chiu C, Boushey HA, Lancaster LH, Wolters PJ, Derisi J, Ganem D, Collard HR. “ViralInfection in Acute Exacerbation <strong>of</strong> Idiopathic Pulmonary Fibrosis.” Am J Respir CritCare Med. 2011 Feb 2515. Calhoun WJ, Ameredes BT, King TS, Boushey HA. “Comparison <strong>of</strong> physician-based,biomarker-based, and symptom-based strategies for adjustment <strong>of</strong> inhaled corticosteroidtherapy in adults with asthma: The Basalt Randomized Trial.” JAMA. 2012 Sept 12;308:987-97.104
<strong>Research</strong> SupportOngoing <strong>Research</strong> SupportU10 HL098107(Boushey, HA) 09/30/09-06/30/16NIH/NHLBIUCSF <strong>Asthma</strong>Net Clinical <strong>Center</strong>The major goals are to serve as a clinical center participating in the conduct <strong>of</strong> NHLBIsupportedmulti-center clinical trials <strong>of</strong> asthma therapies in children and adults with asthma, and toconductsmaller, focused studies <strong>of</strong> mechanisms <strong>of</strong> action <strong>of</strong> asthma therapies, <strong>of</strong> novel treatments forsevere asthma, and <strong>of</strong> concepts <strong>of</strong> asthma pathophysiology that could lead to the development<strong>of</strong> new asthma treatments. Role: Co-InvestigatorU10 HL0981(Boushey, HA) 09/30/09-06/30/16NIH/NHLBIUCSF <strong>Asthma</strong>Net Clinical <strong>Center</strong>The major goals are to serve as a clinical center participating in the conduct <strong>of</strong> NHLBIsupportedmulti-center clinical trials <strong>of</strong> asthma therapies in children and adults with asthma, andto conduct smaller, focused studies <strong>of</strong> mechanisms <strong>of</strong> action <strong>of</strong> asthma therapies, <strong>of</strong> noveltreatments for severe asthma, and <strong>of</strong> concepts <strong>of</strong> asthma pathophysiology that could lead to thedevelopment <strong>of</strong> new asthma treatments. Role: Co-InvestigatorHHS N272200900052C(Boushey, HA) 09/30/09-09/29/14NIH/NIAIDInner-City <strong>Asthma</strong> Consortium II / UCSF ICAC-II <strong>Basic</strong> Science Site -The major goal is to serve as a <strong>Basic</strong> Science Site for the ICAC, enabling examination <strong>of</strong>relationships <strong>of</strong> the mirocrobial environment <strong>of</strong> inner city households, the development <strong>of</strong>immune function in infancy, and the development <strong>of</strong> allergic disease, especially asthma, inchildhood. Role: Principal InvestigatorP01 HL070831-06A1 (Lemanske, R) 05/01/08-04/30/13NIHRhinovirus Infection and Childhood <strong>Asthma</strong>The major goals <strong>of</strong> this study are to apply the Virochip microarray to search for novel viruses inrespiratory secretions obtained from children with severe clinical illnesses with the features <strong>of</strong> arespiratory infection but in whom standard PCR tests have not detected a virus, and further toexpand the ViroChip to detect regions <strong>of</strong> the rhinovirus genome associated with virulence.Role: Co-Investigator5 U10 HL074204-05NIH/NHLBI(Boushey, HA) 09/15/03-07/31/11105
<strong>Asthma</strong> Clinical <strong>Research</strong> Network <strong>Center</strong> at UCSFTo link the established clinical research group at the <strong>University</strong> <strong>of</strong> California, San Franciscowith other clinical research groups in an interactive network conducting collaborative studies <strong>of</strong>novel therapeutic approaches for asthma and disseminating the findings on optimalmanagement <strong>of</strong> asthmatic patients to practitioners and other health care pr<strong>of</strong>essionals. Role:Principal Investigator5 U10 HL074431-05(Lazarus, SC) 08/15/03-07/31/11NIH/NHLBICOPD Clinical <strong>Research</strong> Network at UCSFHL-03-002 COPD Clinical <strong>Research</strong> NetworkThe purpose <strong>of</strong> the NIH-sponsored COPD Clinical <strong>Research</strong> Network is to evaluate new andexisting approaches for the management <strong>of</strong> COPD and to disseminate the findings <strong>of</strong> thisnetwork to the medical community. Role: Co-InvestigatorCompleted <strong>Research</strong> SupportR01 HL080414-05 (Fahy, JV) 07/01/05-05/31/10NIHHistoblood group antigens, viruses & asthmaThe major goals are to understand how expression <strong>of</strong> histo-blood groups antigens by airwayepithelial cells and airway mucins influences susceptibility to asthma exacerbations. Role: Co-InvestigatorR01 HL080074-01 (Cabana, M) 07/01/04-06/30/10NIHTrial <strong>of</strong> Infant Probiotic Exposure on Developing <strong>Asthma</strong>This trial will measure the effect <strong>of</strong> a 6-month daily exposure <strong>of</strong> Lactobacillus, as an infantformula supplement, on immune system and asthma development during the first 3 years <strong>of</strong> life.Role: Co-InvestigatorDoris Duke Foundation (Ganem, DE) 10/01/03-6/30/09Genomics-based Approaches to New Pathogen Discovery in Chronic Human DiseasesTo use a DNA microarray designed to detect any known virus to explore whether viruses areassociated with liver and lung diseases that are currently <strong>of</strong> unknown etiology. Role: PIAI050496-01(Boushey, HA) 09/01/01-04/31/06NIHAI-00-012 <strong>Asthma</strong> and Allergic Diseases <strong>Research</strong> <strong>Center</strong>s -- Rhinoviruses, Epithelial Cells,and Airway Function The purpose <strong>of</strong> this Program Project Grant is to examine the hypothesisthat the nature and intensity <strong>of</strong> the nasal and bronchial responses to Rhinovirus infection isdetermined by properties inherent to the infecting strain, and/or properties inherent to theepithelial cells infected. Role: PI106
NAMEEsteban González Burchard, M.D., M.P.H.eRA COMMONS USER NAMEEburchardBIOGRAPHICAL SKETCHPOSITION TITLEPr<strong>of</strong>essor, Bioengineering & Therapeutic Sciences andMedicineDirector, <strong>Center</strong> on Genes, Environments & HealthEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYSan Francisco State <strong>University</strong>, San Francisco, CA B.S. 1984-1990Cellular & MolecularBiologyStanford <strong>University</strong> School <strong>of</strong> Medicine,Stanford, CAM.D.1990-1995 MedicineProgram in ClinicalHarvard School <strong>of</strong> Public Health, Boston, MA Certificate 1997EffectivenessBrigham and Women's Hospital, Boston, MA Resident 1995-1998 Internal Medicine<strong>University</strong> <strong>of</strong> California, San Francisco, SF, CA Fellow 1998-2001Pulmonary & Critical CareMedicineStanford <strong>University</strong>, Stanford, CA2001-2002 Genetic Epidemiology<strong>University</strong> <strong>of</strong> California, Berkeley M.P.H. 2005-2006 EpidemiologyPositions and Honors2001 - 2010 Director, UCSF DNA Bank and <strong>Asthma</strong> Genetics Core Facility2008 - Director, UCSF <strong>Center</strong> on Genes, Environments & Health2009 - Director, UCSF Clinical Pharmacology Training Program2010 - Vice Chair, Department <strong>of</strong> Bioengineering & Therapeutic Sciences, UCSF2011 - Pr<strong>of</strong>essor, Bioengineering & Therapeutic Sciences and Medicine, UCSF1988, 1989 National Collegiate Athletic Association (NCAA)Div. II Academic All-American, Wrestling2005 – 2010 RWJ Amos Medical Faculty Development Award2008 NIH Study Section Member, Genetics <strong>of</strong> Health and Disease (GHD)2009 American Society <strong>of</strong> Clinical Investigation (ASCI), inducted member2009 Guest Speaker, Tavis Smiley Show2010 Guest Speaker, NPR’s Science Friday, hosted by Ira Flatow2011 Athletic Hall <strong>of</strong> Fame, San Francisco State <strong>University</strong>107
Selected Peer-reviewed Publications (selected from 86 publications)1. Burchard EG, Ziv E, Coyle N, Lin-Gomez S, Tang H, Karter AJ, Mountain J, Perez-Stable EJ, Sheppard D, Risch N. (2003) The Importance <strong>of</strong> Race and Ethnic Backgroundin Biomedical <strong>Research</strong> and Clinical Practice. N. Engl. J. Med. 348:1077-1192.2. Burchard EG, Avila PC, Nazario S, Casal J, Torres A, Rodriguez-Santana JR, ToscanoM, Senter-Sylvia J, Alioto ME, Salazar M, Gomez I, Fagan JK, Salas J, Lilly C,Matallana H, Ziv E, Castro R, Selman M, Chapela R, Sheppard D, Weiss ST, Ford JG,Boushey HA, Rodriguez-Cintron W, Drazen JM, Silverman EK (2004) LowerBronchodilator Responsiveness in Puerto Rican than in Mexican <strong>Asthma</strong>tic Subjects. Am.J. Respir. Crit. Care Med. 169:386-92.3. Salari K, Choudhry S, Tang H, Naqvi M, Lind D, Avila PC, Coyle NE, Ung N, NazarioS, Casal J, Torres-Palacios A, Clark S, Phong A, Gomez I, Matallana H, Perez-Stable EJ,Shriver MD, Kwok PY, Sheppard D, Rodriguez-Cintron W, Risch NJ, Ziv E, BurchardEG. (2005) Genetic admixture and asthma-related phenotypes in Mexican American andPuerto Rican asthmatics. Genet. Epidemiol. 29:76-86.4. Burchard EG, Borrell LN, Choudhry S, Naqvi M, Tsai HJ, Rodriguez-Santana JR,Chapela R, Rogers SD, Mei R, Rodriguez-Cintron W, Arena JF, Kittles R, Perez-StableEJ, Ziv E, Risch N. (2005) Latino Populations: A unique opportunity for the study <strong>of</strong>race, genetics and social environment in epidemiologic research. Am. J. Public Health95:2161-8.5. Tsai HJ, Kho JY, Shaikh N, Choudhry S, Naqvi M, Navarro D, Matallana H, Castro R,Lilly CM, Watson HG, Meade K, Le Noir M, Thyne S, Ziv E, Burchard EG. (2006)Admixture-matched case-control study: A practical approach for genetic associationstudies in admixed populations. Hum. Genet. 118:626-39.6. Tang H, Choudhry S, Mei R, Morgan M, Rodriguez-Cintron W, Burchard EG, Risch NJ(2007) Recent genetic selection in the ancestral admixture <strong>of</strong> Puerto Ricans. Am. J. Hum.Genet. 81:626-33 (PMC1950843).7. Choudhry S, Taub M, Mei R, Rodriguez-Santana J, Rodriguez-Cintron W, Shriver MD,Ziv E, Risch NJ, Burchard EG (2008) Genome-wide screen for asthma in PuertoRicans: Evidence for association with 5q23 region. Hum. Genet. 123:455-68(PMC2664533).8. Galanter J, Choudhry S, Eng C, Nazario S, Rodriguez-Santana J, Casal J, Torres A, SalasJ, Chapela R, Watson HG, Meade K, LeNoir M, Rodriguez-Cintron W, Avila PC,Burchard EG (2008) ORMDL3 gene is associated with asthma in three ethnicallydiverse populations. Am. J. Resp. Crit. Care Med. 177:1194-200 (PMC2408437).9. Seibold MA, Reese TA, Choudhry S, Salam MT, Beckman K, Eng C, Atakilit A, MeadeK, Lenoir M, Watson HG, Thyne S, Kumar R, Weiss KB, Grammer LC, Avila P,Schleimer RP, Fahy JV, Rodriguez-Santana J, Rodriguez-Cintron W, Boot RG, SheppardD, Gilliland FD, Locksley RM, Burchard EG (2009) Differential enzymatic activity <strong>of</strong>common haplotypic versions <strong>of</strong> the human acidic mammalian chitinase protein. J. Biol.Chem. 284:19650-8 (PMC2740590).108
10. Hancock DB, Romieu I, Shi M, Sienra-Monge JJ, Wu H, Chiu GY, Li H, del Rio-Navarro BE, Willis-Owens SA, Weiss ST, Raby BA, Gao H, Eng C, Chapela R,Burchard EG, Tang H, Sullivan PF, London SJ (2009) Genome-wide association studyimplicates chromosome 9q21.31 as a susceptibility locus for asthma in Mexican children.PLoS Genet. 5:e1000623 (PMC2722731).11. Risch N, Choudhry S, Via M, Basu A, Sebro R, Eng C, Beckman K, Thyne S, Chapela R,Rodriguez-Santana J, Rodriguez-Cintron W, Avila PC, Ziv E, Burchard EG (2009)Ancestry-related assortative mating in Latino populations. Genome Biol. 10:R132.12. Mathias RA, Grant AV, Rafaels N, Hand T, Gao L, Vergara C, Tsai YJ, Yang M,Campbell M, Foster C, Gao P, Togias A, Hansel NN, Diette G, Adkinson NF, Liu MC,Faruque M, Dunston GM, Watson HR, Bracken MB, Hoh J, Maul P, Maul T, JedlickaAE, Murray T, Hetmanski JB, Ashworth R, Ongaco CM, Hetrick KN, Doheny KF, PughEW, Rotimi CN, Ford J, Eng C, Burchard EG, Sleiman PM, Hakonarson H, Forno E,Raby BA, Weiss ST, Scott AF, Kabesch M, Liang L, Abecasis G, M<strong>of</strong>fatt MF, CooksonWO, Ruczinski I, Beaty TH, Barnes KC (2010) A genome-wide association study onAfrican-ancestry populations for asthma. J. Allergy Clin. Immunol. 125:336-46. *13. Kumar R*, Seibold MA*, Aldrich MC*, Williams KL*, Reiner AP, Colangelo L,Galanter J, Gignoux C, Hu D, Sen S, Choudhry S, Peterson EL, Rodriguez-Santana J,Rodriguez-Cintron W, Nalls MA, Leak TS, O'Meara E, Meibohm B, Kritchevsky SB, LiR, Harris TB, Nickerson DA, Fornage M, Enright P, Ziv E, Smith LJ, Liu K, BurchardEG. (2010) Genetic ancestry and lung function predictions. New England Journal <strong>of</strong>Medicine. 2010 Jul 22;363(4):321-30. Epub 2010 Jul 7.14. Tcheurekdjian, H.*, Via, M.*, De Giacomo, A., Corvol, H., Eng, C., Thyne, S., Chapela,R., Rodriguez-Cintron, W., Rodriguez-Santana, J., Avila, P.C., González Burchard, E.,on behalf <strong>of</strong> the Genetics <strong>of</strong> <strong>Asthma</strong> in Latino Americans (GALA) Study. *These authorscontributed equally to this manuscript. ALOX5AP and LTA4H polymorphisms modifyaugmentation <strong>of</strong> bronchodilator responsiveness by leukotriene modifiers in Latinos. JAllergy Clin Immunol. 2010 Oct; 126(4):853-8.*15. Kenny, E.E., Timpson, N.J., Sikora, M., Yee, M.C., Moreno Estrada, A., Eng, C.,Huntsman, S., Gonzalez Burchard, E., Stoneking, M., Bustamante, C.D., Myles, S.,Blond hair is caused by an amino acid change in TYRP1. Science. 2012 May4;336(6081):554Manuscripts in under review1. Luisa N. Borrell, Elizabeth A. Nguyen, Lindsey A. Roth,Sam S. Oh, HaigTcheurekdjian,Saunak Sen, Adam Davis, Harold J. Farber, Pedro C. Avila, EmeritaBrigino-Buenaventura, Michael A. LeNoir, Fred Lurmann, Kelley Meade, DeniseSerebrisky, William Rodriguez-Cintron, Rajesh Kumar, Jose R. Rodriguez-Santana,Shannon Thyne, Esteban G. Burchard. Childhood obesity and asthma control in adiverse sample: Examining age and racial/ethnic differences. AJRCCM. (Under 2ndreview January 2013).109
2. Rajesh Kumar, Elizabeth A Nguyen, Lindsey A Roth, Sam S Oh,Christopher R Gignoux,Scott Huntsman, Celeste Eng, Andres Moreno-Estrada, Karla Sandoval, RosendaPeñaloza-Espinosa, Marisol López-López, Pedro C Avila, Harold J Farber, HaigTcheurekdjian, William Rodriguez-Cintron, Jose R Rodriguez-Santana, DeniseSerebrisky, Shannon M Thyne, Keoki Williams, William Klitz, Cheryl Winkler, Carlos DBustamante, Eliseo J Pérez-Stable, Luisa N Borrell, Esteban G Burchard. Factorsassociated with degree <strong>of</strong> atopy in Latino children in a nationwide pediatric sample: TheGALA II Study. J Allergy Clin Immunol. (Under 2 nd review, December 2013).3. Joshua M Galanter, Christopher R Gignoux, Dara G Torgerson, Lindsey A Roth, CelesteEng, Sam S Oh, Elizabeth A Nguyen Scott Huntsman Donglei Hu, Saunak Sen. AdamDavis, Harold J. Farber, Pedro C. Avila, Emerita Brigino-Buenaventura, Michael A.LeNoir, Kelley Meade, Denise Serebrisky, Stephanie London, Frank Gilliland, FernandoMartinez, Luisa N Borrell, William Rodriguez-Cintron, Rajesh Kumar, Jose R.Rodriguez-Santana, Esteban G. Burchard, and the EVE Consortium. Genome-wideassociation and admixture mapping identify chromosomal regions 17q21 and 6p21 asasthma-associated loci in Latinos. AJRCCM. (Under review December 2013).4. Chris R Gignoux, Dara G Torgerson, Joshua Galanter, Lindsey A Roth, Celeste Eng,Elizabeth A Nguyen, Scott Huntsman, Sam S Oh,Raskia A Mathias, Adam Davis, HaroldJ Farber, Pedro C Avila, Emerita Brigino-Buenaventura, Michael A LeNoir, KelleyMeade, Denise Serebrisky, Luisa N Borrell, William Rodriguez-Cintron, Rajesh Kumar,Jose R Rodriguez-Santana, Andres Moreno, Karla Sandoval, Cheryl Winkler, CarlosBustamante, EVE Collaborators, Carole Ober, Dan Nicholae, Saunak Sen, Kathleen CBarnes, Esteban G Burchard. Meta-analysis <strong>of</strong> admixture mapping using existinggenome-wide association data implicates SMAD2 as a novel asthma-associated locus inLatinos. Nature Genetics. (Submitted Jan 2013).5. Katherine A. Drake, Dara G. Torgerson, Christopher R. Gignoux, Joshua M. Galanter,Lindsey A. Roth, Scott Huntsman, Celeste Eng, Sam S. Oh, Sook Wah Yee, LawrenceLin, Adam Davis, Luisa N. Borrell, Harold J. Farber, Rajesh Kumar, Pedro C. Avila,Emerita Brigino-Buenaventura, Rocio Chapela, Jean G. Ford, Michael A. LeNoir, FredLurmann, MS, Kelley Meade, Denise Serebrisky, Shannon Thyne, William Rodríguez-Cintrón, Saunak Sen, José R. Rodríguez-Santana, Kathleen M. Giacomini, and EstebanG. Burchard. A Genome-wide Association Study <strong>of</strong> Bronchodilator Response inLatinos Implicates Rare Variants. J Allergy Clin Immunol. (Submitted January 2013).6. Katherine K. Nishimura, Joshua M. Galanter, Lindsey A. Roth, Sam S. Oh, Harold J.Farber, Denise Serebrisky, Rajesh Kumar, Luisa N. Borrell, Emerita Brigino-Buenaventura, Adam Davis, Michael A. LeNoir, Kelley Meade, William Rodriguez-Cintron, Pedro C. Avila, Jose R. Rodriguez-Santana, Saunak Sen, Fred Lurmann, John R.Balmes, Esteban G. Burchard. Early Life Exposure to Air Pollution and the Risk <strong>of</strong><strong>Asthma</strong> in Latino and African American Children. ARJCCM. (Submitted January 2013).110
<strong>Research</strong> SupportOngoing <strong>Research</strong> Support1R01 HL104608-01A1 (PI: Barnes) 09/28/2011-6/30/2015Source: Johns Hopkins <strong>University</strong>-SubcontractRole: SubcontractorProject title: New Approaches for Empowering Studies <strong>of</strong> <strong>Asthma</strong> in Populations <strong>of</strong> African DescentThe major goal <strong>of</strong> this project is to identify genetic variants specific to African populations. We willsequence Latino and African American samples to design a custom GeneChip for populations withAfrican ancestry.P60 MD006902 (PI: Bibbins-Domingo) 03/01/13-02/28/17Source: NIH/NIMHDRole: Project PIProgram title: Addressing Disparities in Chronic Disease with a Teen and Young AdultFocusProject title: The Genetics <strong>of</strong> <strong>Asthma</strong> and Obesity Using Admixture Mapping in LatinosThe major goal <strong>of</strong> this proposal is to identify novel genetic variants associated with bothasthma and obesity by deep re-sequencing <strong>of</strong> candidate regions identified throughadmixture mapping.R01 ES015794 (PI: Burchard) 9/01/08-5/31/13Source: NIH/NIEHSProject title: Genes-environments & Admixture in Latino <strong>Asthma</strong>tics (GALA 2)The major goal <strong>of</strong> this project is to identify genetic, social and environmental riskfactors for asthma among various Latino groups recruited throughout the U.S.R01 HL088133 (PI: Burchard) 3/01/08-2/28/13Source: NIH/NHLBIProject title: Whole Genome Analyses for <strong>Asthma</strong> in Latino PopulationsThe major goal <strong>of</strong> this project is to perform genome-wide association analyses toidentify genetic factors associated with asthma and related phenotypes in PuertoRicans and Mexicans.U19 AI077439 (PI: Sheppard) 04/01/08-03/31/13Source: NIH/NIAIDRole: Project 3 PIProgram title: Mechanisms <strong>of</strong> Initiation and Persistence <strong>of</strong> Allergic <strong>Asthma</strong>Project 3 title: Chitinases and TGFb in Human <strong>Asthma</strong>The goal is to analyze the effects <strong>of</strong> genetic variation on genes in the TGF beta andChitinase pathways and their role in the initiation and persistence <strong>of</strong> asthma acrossracial and ethnically diverse populations.111
Completed <strong>Research</strong>(Neal Benowitz, Program PI) 07/01/07-06/30/12Role on project: Project 7 PISource: Flight Attendants Medical <strong>Research</strong> Institute (FAMRI)Program title: UCSF <strong>Center</strong> <strong>of</strong> Excellence on Secondhand SmokeProject title: Tobacco Gene–Environment Interactions in African American and Latino<strong>Asthma</strong>tics. The goal is to identify gene-environment interactions between asthma andsecondhand smoke.RC2 HL101651 (Co-PIs: Ober, Nicolae) 09/30/09-09/29/11NIH/R01Role on project: SubcontractorThe EVE <strong>Asthma</strong> Genetics Consortium: Building Upon GWASTo replicate the most significant GWAS (meta-analysis) results in >15,000 asthma casesand controls <strong>of</strong> European American, African American, and U.S. Hispanic ethnicities, resequence5-10 genes associated with asthma in European Americans but not in AfricanAmericans or Hispanics, to study additional asthma-associated phenotypes and examineinteractions, and develop methods to facilitate gene discovery.U01 GM61390 (PI:Giacomini) 07/20/05-06/30/10Role on project: Co-InvestigatorSource: NIH/NIGMSProgram title: Pharmacogenetics <strong>of</strong> Membrane TransportersProject title: Study <strong>of</strong> Pharmacogenetics in Ethically Diverse GroupsThe goal was to develop an ethnically diverse cohort <strong>of</strong> subjects to participate inpharmacogenetic studies.R01 HL078885 (PI: Burchard) 08/15/05-07/31/10Source: NIH/NHLBIProject title: Case-control Association Studies and Genetic ConfoundingThe goal was to test and compare methods <strong>of</strong> detecting and correcting for populationstratification.112
BIOGRAPHICAL SKETCHNAMEGeorge H. CaugheyeRA COMMONS USER NAMEgcaugheyPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> MedicineINSTITUTION AND LOCATIONEDUCATION/TRAININGDEGREEYEAR(s)FIELD OF STUDYArizona State <strong>University</strong> BS 1975 ChemistryStanford <strong>University</strong> School <strong>of</strong> Medicine MD 1979 MedicinePennsylvania Hospital/UPenn 1982 Internal Medicine<strong>University</strong> <strong>of</strong> California, San Francisco 1986 Pulmonary MedicinePositions and Honors1988-92 Assistant Pr<strong>of</strong>essor, Dept. <strong>of</strong> Medicine, UCSF1988-98 Associate Staff, Cardiovascular <strong>Research</strong> Institute, UCSF1992-98 Associate Pr<strong>of</strong>essor, Dept. <strong>of</strong> Medicine, UCSF1992- Molecular Medicine Program Faculty, UCSF1995- Editorial Board, American Journal <strong>of</strong> Respiratory Cell and Molecular Biology1996- Member <strong>of</strong> UCSF Graduate Program in Biomedical Sciences1998- Pr<strong>of</strong>essor, Dept. <strong>of</strong> Medicine, UCSF1999- Investigator, Cardiovascular <strong>Research</strong> Institute, UCSF2002- Member, UCSF Cancer <strong>Center</strong> and <strong>Center</strong> for Neurobiology <strong>of</strong> Digestive Disease2004- Editorial Board, Current Respiratory Medicine Reviews2004- Chief <strong>of</strong> Pulmonary and Critical Care and Sleep Medicine,San Francisco VA Medical <strong>Center</strong>2012- Associate Chief <strong>of</strong> <strong>Research</strong> and Academic Affairs, Medical ServiceSan Francisco VA Medical <strong>Center</strong>Honors and Awards1974 American Chemical Society Outstanding Undergraduate Award, ASU1975 Phi Beta Kappa and Merck Award in Chemistry, ASU1986 NIH Clinical Investigator Award1992 American Lung Association Career Investigator Award1992 Elected to American Society for Clinical Investigation113
2000 Elected to American Association <strong>of</strong> Physicians2004- Julius and Lillian Nadel Endowed Chair <strong>of</strong> Medicine2010 Elected to Collegium Internationale AllergologicumPublications in chronological order (selected from 135)1. Vanderslice P, Ballinger SM, Tam EK, Goldstein SM, Craik CS, Caughey GH. Humanmast cell tryptase: multiple cDNAs and genes reveal a multigene protease family. ProcNatl Acad Sci USA 87:3811-5, 1990.2. Rubinstein I, Nadel JA, Graf PD, Caughey GH. Mast cell chymase potentiateshistamine-induced wheal formation in the skin <strong>of</strong> ragweed-allergic dogs. J Clin Invest86:555-9, 1990.3. Ruoss SJ, Hartmann T, Caughey GH. Mast cell tryptase is a mitogen for culturedfibroblasts. J Clin Invest 88:493-9, 1991.4. Caughey GH, Raymond WW, Bacci E, Lombardy RJ, Tidwell RR. BABIM and relatedamidines are potent, reversible inhibitors <strong>of</strong> mast cell tryptases. J Pharmacol Exp Therap264:676-82, 1993.5. Caughey GH, Schaumberg TH, Zerweck EH, Butterfield JH, Hanson RD, SilvermanGA, Ley TJ. The human mast cell chymase gene (CMA1): mapping to the cathepsinG/granzyme gene cluster and lineage-restricted expression. Genomics 15:614-20, 1993.6. Fang KC, Raymond WW, Lazarus SC, Caughey GH. Dog mastocytoma cells secrete a92 kDa gelatinase activated extracellularly by mast cell chymase. J Clin Invest 97:1589-96, 1996.7. Fang KC, Raymond WW, Blount JL, Caughey GH. Dog mast cell a-chymase activatesprogelatinase B by cleaving Phe 88 -Gln 89 and Phe 91 -Glu 92 bonds <strong>of</strong> the propeptide domain.J Biol Chem 272:25628-35, 1997.8. Wolters PJ, Raymond WW, Blount JL, Caughey GH. Regulated expression, processingand secretion <strong>of</strong> dog mast cell dipeptidyl peptidase I. J Biol Chem 273:15514-20, 1998.9. Pallaoro M, Fejzo MS, Shayesteh L, Blount JL, Caughey GH. Characterization <strong>of</strong> genesencoding known and novel human tryptases on chromosome 16p13.3. J Biol Chem274:3355-62, 1999.10. Ma Y, Longley BJ, Blount JL, Langley K, Caughey GH. Clustering <strong>of</strong> activatingmutations in c-kit’s juxta-membrane coding region in canine mastocytomas. J InvestDermatol 112:165-70, 1999.11. Coussens LM, Raymond WW, Bergers G, Behrendtsen O, Werb Z, Caughey GH,Hanahan D Inflammatory mast cells potentiate the angiogenic switch during squamousepithelial carcinogenesis. Genes & Development 13:1382-97, 1999.12. Caughey GH, Raymond WW, Blount JL. Hau LW-T, Pallaoro M, Wolters PJ, VergheseGM. Characterization <strong>of</strong> human g-tryptases, novel members <strong>of</strong> the chromosome 16p mastcell tryptase and prostasin gene families. J Immunol 164:6566-75, 2000.13. Steinh<strong>of</strong>f M, Vergnolle N, Young S, Ennes H, Hollenberg MD, Wallace JL, CaugheyGH, Williams LM, Geppetti P, Mayer EA, Bunnett NW. Agonists <strong>of</strong> proteinaseactivatedreceptor 2 induce inflammation by a neurogenic mechanism. Nature Med6:151-8, 2000.114
14. Frank BT, Rossall JC, Caughey GH, Fang KC. Mast cell tissue inhibitor <strong>of</strong>metalloproteinase-1 is cleaved and inactivated extracellularly by a-chymase. J Immunol166:2783-92, 2001.15. Wolters PJ, Muilenburg D, Pham CTN, Ley TJ, Caughey GH. Dipeptidylpeptidase I isessential for in vivo activation <strong>of</strong> mast cell chymases, but not tryptases. J Biol Chem276:18551-6, 2001.16. Muilenburg DJ, Raymond WW, Wolters PJ, Caughey GH. Lys 40 but not Arg 143influences selectivity <strong>of</strong> angiotensin conversion by human a-chymase. Biochim BiophysActa 1596:346-56, 2002.17. Soto D, Malmsten C, Blount JL, Muilenberg DJ and Caughey GH. Genetic deficiency <strong>of</strong>human mast cell a-tryptase. Clin Exp Allergy 32:1000-6, 2002.18. Bhagwandin VJ, Hau L W-T, Mallen-St. Clair J, Wolters PJ, Caughey GH. Structureand activity <strong>of</strong> pancreasin, a novel tryptic serine peptidase expressed by pancreas. J BiolChem 278:3363-71, 2003.19. Reiling KK, Krucinski J, Miercke LJW, Raymond WW, Caughey GH, Stroud RM.Structure <strong>of</strong> human pro-chymase. Biochemistry 42:2616-24, 2003.20. Raymond WW, Ruggles Waugh S, Craik CS, Caughey GH. Albumin is a substrate <strong>of</strong>human chymase: prediction by combinatorial peptide screening and development <strong>of</strong> aselective inhibitor based on the cleavage site. J Biol Chem 278:34517-24, 2003.21. Mallen-St. Clair J, Pham CTN, Villalta SA, Caughey GH, Wolters PJ. Mast celldipeptidyl peptidase I mediates survival from sepsis. J Clin Invest 113:628-34, 2004.22. Verghese GM, Tong ZY, Bhagwandin V, Caughey GH. Mouse prostasin gene structure,promoter function, and restricted expression in lung and kidney. Am J Respir Cell MolBiol 30:519-29, 2004.23. Baluk P, Raymond WW, Ator E, Coussens LM, McDonald DM, Caughey GH. MMP-2and -9 expression increases in mycoplasma-infected airways but is not required formicrovascular remodeling. Am J Physiol 287:L307-17, 2004.24. Raymond WW, Sommerh<strong>of</strong>f CP, Caughey GH. Mastin is a gelatinolytic mast cellpeptidase resembling a mini-proteasome. Arch Biochem Biophys 435:311-22, 2005.25. Tong ZY, Illek B, Bhagwandin VK, Verghese GM, Caughey GH. Prostasin, amembrane-anchored serine peptidase, regulates sodium currents in JME/CF15 cells, acystic fibrosis epithelial cell line. Am J Physiol 287:L928-35, 2004.26. Wolters PJ, Mallen-St. Clair J, Lewis CC, Villalta SA, Baluk P, Erle DJ, Caughey GH.Tissue-selective mast cell reconstitution and differential lung gene expression in mastcell-deficient Kit W-sh /Kit W-sh sash mice. Clin Exp Allergy 35:82-8, 2005.27. Xu X, Golden JA, Dolganov G, Jones KD, Donnelly S, Weaver T, Caughey GH.Transcript signatures <strong>of</strong> lymphocytic bronchitis in lung allograft biopsies. J Heart LungTransplant 24:1055-66, 2005.28. Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, Baluk P, McDonald DM,Hawgood S, Caughey GH. Mast cells protect mice from mycoplasma pneumonia. Am JResp Crit Care Med 173:219-25, 2006.29. Raymond WW, Cruz AC, Caughey GH. Mast cell and neutrophil peptidases attack aninactivation segment in hepatocyte growth factory to generate NK4-like antagonists. JBiol Chem 281:1489-94, 2006.115
30. Caughey GH. Tryptase genetics and anaphylaxis. J Allergy Clin Immunol 117:1411-4,2006.31. Mallen-St Clair J, Shi G-P, Sutherland RE, Chapman HA, Caughey GH, Wolters PJ.Cathepsins L and S are not required for activation <strong>of</strong> dipeptidyl peptidase I in mice. BiolChem 387:1143-6, 2006.32. Verghese GM, Gutknecht MF, Caughey GH. Prostasin regulates epithelial monolayerfunction: cell-specific Gpld1-mediated secretion and role <strong>of</strong> GPI anchor. Am J PhysiolCell Physiol 291:C1258-70, 2006.33. Caughey GH. A pulmonary perspective on GASPIDs: Granule-Associated SerinePeptidases <strong>of</strong> Immune Defense. Curr Resp Med Rev 2:263-77, 2006.34. Xu X, Zhang D, Zhang H, Wolters PJ, Killeen NP, Sullivan BM, Locksley RM, LowellCA, Caughey GH. Neutrophil histamine contributes to inflammation in mycoplasmapneumonia. J Exp Med 203:2907-17, 2006.35. Planès C and Caughey GH. Regulation <strong>of</strong> the epithelial Na+ channel by peptidases. CurrTop Dev Biol 78:23-46, 2007.36. Caughey GH. Mast cell tryptases and chymases in inflammation and host defense.Immunol Rev 217:114-54, 2007.37. Akin C, Soto D, Brittain E, Chhabra A, Schwartz LB, Caughey GH, Metcalfe DD.Tryptase haplotype in mastocytosis: Relationship to disease variant and diagnostic utility<strong>of</strong> total tryptase levels. Clin Immunol 123:268-71, 2007.38. Trivedi NN, Tong Q, Raymond WW, Bhagwandin VJ, Caughey GH. Mast cell tryptaseschanged rapidly during primate speciation and evolved from g-like transmembranepeptidases in ancestral vertebrates. J Immunol 179:6072-9, 2007.39. Caughey GH. “Mast Cells and Basophils” in <strong>Asthma</strong> and COPD—<strong>Basic</strong> Mechanismsand Clinical Management, Barnes PJ, Drazen JM, Rennard S, Thompson NC, eds.,Academic Press, London, 2008.40. Trivedi NN, Raymond WW, Caughey GH. Chimerism, point mutation and truncationdramatically transformed mast cell d tryptases during primate evolution. J Allergy ClinImmunol 121:1262-8, 2008.41. Caughey GH, Beauchamp J, Schlatter D, Raymond WW, Trivedi NN, Banner D, MauserH, Fingerle J. Guinea pig chymase is leucine-specific: a novel example <strong>of</strong> functionalplasticity in the chymase/granzyme family <strong>of</strong> serine peptidases. J Biol Chem 283:13943-51, 2008.42. Raymond WW, Su S, Makarova A, Wilson TM, Carter MC, Metcalfe DD, Caughey GH.a 2 -Macroglobulin capture allows detection <strong>of</strong> mast cell chymase in serum and creates areservoir <strong>of</strong> angiotensin II-generating activity. J Immunol 182:5770-7, 2009.43. Innes AL, Carrington SD, Thornton DJ, Kirkham S, Dougherty RH, Raymond WW,Caughey GH, Muller SJ, Fahy JV. Ex vivo sputum analysis reveals impairment <strong>of</strong>protease-dependent mucus degradation by plasma proteins in acute asthma. Am J RespCrit Care Med 180:203-10, 2009.44. Trivedi NN, Tamraz B, Chu C, Kwok PY, Caughey GH. Humans are protected frommast cell tryptase deficiency despite frequent inheritance <strong>of</strong> loss-<strong>of</strong>-function mutations. JAllergy Clin Immunol 124:1099-105, 2009.116
45. Trivedi NN and Caughey GH. Mast cell peptidases: chameleons <strong>of</strong> innate immunity andhost defense. Am J Respir Cell Mol Biol 42:257-67, 2010.46. Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, WoodruffPG, Fahy JV. Accumulation <strong>of</strong> intraepithelial mast cells with a unique proteasephenotype in Th2-high asthma. J Allergy Clin Immunol, 125:1046-53, 2010.47. Raymond WW, Trivedi NN, Makarova A, Ray M, Craik CS, Caughey GH. Howimmune peptidases change specificity: Cathepsin G gained tryptic function but lostefficiency during primate evolution. J Immunol 185:5360-8, 2010.48. Caughey GH. “Mast cell proteases as protective and inflammatory mediators”, in MastCell Biology: Contemporary and Emerging Topics, Gilfillan AM and Metcalfe DD, eds.,Landes Bioscience, 2011.49. Caughey GH. “Protease mediators <strong>of</strong> anaphylaxis”, in Anaphylaxis and HypersensitivityReactions: From Molecular Markers to Rapid Desensitizations, Castells A, ed., SpringerInc., 2011.50. Xu X, Zhang H, Song Y, Lynch SV, Lowell CA, Wiener-Kronish JP, Caughey GH.Strain-dependent induction <strong>of</strong> neutrophil histamine production and cell death byPseudomonas aeruginosa. J Leuk Biol, in press, epub 2011.51. Sutherland SE. Xu X. Kim SS, Seeley EJ, Caughey GH, Wolters PJ. Parasitic infectionimproves survival from septic peritonitis by enhancing mast cell responses to bacteria inmice. PLoS One, 6(11):e27564, 2011.52. Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, RaymondWW, Erle DJ, Åbrink M, Caughey GH, Huang X, Sheppard D. The α v β 6 integrinmodulates airway hyperresponsiveness in mice by regulating intra-epithelial mast cells. JClin Invest, in press.53. Nimishakavi S, Besprozvannaya M, Raymond WW, Craik CS, Gruenert DC, CaugheyGH. Activity and inhibition <strong>of</strong> prostasin and matriptase on apical and basolateral surfaces<strong>of</strong> human airway epithelial cells. Am J Physiol: Lung Cell Mol Physiol 303:L97-106,2012.54. Greenland JR, Jones KD, Hays SR, Golden JA, Urisman A, Jewell NP, Caughey GH,Trivedi NN. Association <strong>of</strong> large-airway bronchitis with bronchiolitis obliteranssyndrome. Am J Respir Crit Care Med, in press117
<strong>Research</strong> SupportOngoing <strong>Research</strong> Support"Evolving Microenvironments in Airway Inflammation"Project 1: Roles <strong>of</strong> Peptidases in Chronic Airway Inflammation; and Administrative Core AProgram Director/Project 1 Leader/Core Leader: George H. CaugheyAgency: NIH-NHLBI; Type: Program Project P01 HL024136; 05/11/2010-3/30/2015Project 1 goals are to determine roles <strong>of</strong> secreted proteases in airway inflammation.“Roles <strong>of</strong> Mast Cells in Primary Dysfunction <strong>of</strong> Lung Allografts”Principal Investigator: George H. CaugheyAgency: UCSF Nina Ireland Lung <strong>Research</strong> Program; 01/1/2012-12/31/2013The goals <strong>of</strong> this project are to explore roles <strong>of</strong> mast cells in primary graft dysfunction."Alloimmune Monitoring <strong>of</strong> Lung Transplant Recipients"Principal Investigator: Qizhi Tang; Co-I: George H. CaugheyAgency: UCSF Nina Ireland Lung <strong>Research</strong> Program; 01/1/2013-12/31/2015The goals <strong>of</strong> this project are to identify means <strong>of</strong> predicting and preventing graft dysfunction in lungallograft recipients.118
BIOGRAPHICAL SKETCHNAMEHarold A. Chapman, M.D.eRA COMMONS USER NAMEHalchapmanPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> MedicineEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYTulane <strong>University</strong> 1968 Premedical<strong>University</strong> <strong>of</strong> Alabama School <strong>of</strong> Medicine M.D. 1972 MedicinePositions and HonorsPositions1972-1975 Residency Training in Internal Medicine, <strong>University</strong> <strong>of</strong> Utah Affiliated Hospitals,Salt Lake City, UT1975-1977 Associate Investigator, V.A. Medical <strong>Center</strong>, Salt Lake City, UT1978-1979 Pulmonary Fellow, <strong>University</strong> <strong>of</strong> Utah Affiliated Hospitals, Salt Lake City, UT1979-1985 Assistant Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> Utah, Department <strong>of</strong> Medicine,Salt Lake City, UT1985 Associate Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> Utah, Department <strong>of</strong> Medicine,Salt Lake City UT1985-1999 Associate Pr<strong>of</strong>essor <strong>of</strong> Medicine, Harvard Medical School, Department <strong>of</strong>Medicine, Boston, MA1992-1999 Physician, Brigham and Women's Hospital, Boston, MA1992-1999 Associate Pr<strong>of</strong>essor <strong>of</strong> Environmental Health, Harvard School <strong>of</strong> Public Health,Boston, MA2000-2008 Chief, Division <strong>of</strong> Pulmonary and Critical Care Medicine, <strong>University</strong> <strong>of</strong>California, San Francisco2000 Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California, San Francisco2000 Senior Member, Cardiovascular <strong>Research</strong> Institute, <strong>University</strong> <strong>of</strong> California SanFranciscoHonors1985-1990 Career Investigator Award, American Lung Association1987 American Society for Clinical Investigation1998 American Association <strong>of</strong> Physicians119
2001-2011 MERIT Award, NIH/NHLBIAd Hoc member <strong>of</strong> various NIH study sectionsEditorial Board <strong>of</strong> Journal <strong>of</strong> Clinical Investigation and Associate Editor,American Journal <strong>of</strong> Respiratory, Cell, and Molecular Biology.Selected Peer-reviewed Publications (Selected from ~140)1. Shi GP, Munger JS, Meara JP, Rich DH, Chapman HA. Molecular cloning andexpression <strong>of</strong> human alveolar macrophage cathepsin S, an elastinolytic cysteineprotease. J Biol Chem 1992 15; 267:7258-62.2. Wei Y, Waltz D, Rao N, Drummond R, Rosenberg S, Chapman HA. Identification<strong>of</strong> the urokinase receptor as an adhesion receptor for vitronectin. J Biol Chem, 1994;209:32380-32388.3. Riese R, Wolf P, Bromme D, Natkin L, Villadangos JA, Ploegh H, and HAChapman. Essential role for cathepsin S in MHC Class II-associated invariant chainprocessing and antigen presentation. Immunity 1996;4:357-366.4. Gelb BD, Shi GP, Chapman HA Jr, Desnick RJ. Pycnodysostosis, a lysosomaldisease caused by cathepsin K deficiency. Science 1996; 273:1236-1238.5. Wei Y, Lukasev M, Simon DI, Bodary SC, Rosenberg S, Doyle MV, Chapman HA.Regulation <strong>of</strong> integrin function by the urokinase receptor. Science 1996; 273:1551-1555.6. Shi GP, Villadangos J, Dran<strong>of</strong>f G, Ploegh H, Chapman HA. Cathepsin S required fornormal MHC class II peptide loading and germinal center development. Immunity,1999;10:196-206.7. Tang CH, Lee JW, Galvez MG, Robillard L, Mole SE, Chapman HA. Murinecathepsin F deficiency causes neuronal lip<strong>of</strong>uscinosis and late-onset neurologicaldisease. Mol Cell Biol. 2006; 26 :2309-16.8. Kim KK, Kugler MC, Wolters PJ, Robillard L, Galvez MG, Brumwell AN, SheppardD, Chapman HA. Alveolar epithelial cell mesenchymal transition develops in vivoduring pulmonary fibrosis and is regulated by the extracellular matrix. Proc NatlAcad Sci 2006; 103(35):13180-5. Epub 2006 Aug 219. Ewald SE, Lee BL, Lau L, Wickliffe KE, Shi GP, Chapman HA, Barton GM. Theectodomain <strong>of</strong> Toll-like receptor 9 is cleaved to generate a functional receptor.Nature. 2008 Dec 4;456(7222):658-62.10. Kim KK, Wei Y, Szekeres C, Kugler MC, Wolters PJ, Hill ML, Frank JA, BrumwellAN, Wheeler SE, Kreidberg JA, Chapman HA. Epithelial cell alpha3beta1 integrinlinks beta-catenin and Smad signaling to promote my<strong>of</strong>ibroblast formation andpulmonary fibrosis. J Clin Invest. 2009 Jan;119(1):213-24. See Commentary sameissue.11. Kim Y, Kugler MC, Wei Y, Kim KK, Li X, Brumwell AN, Chapman HA. Integrinalpha3beta1-dependent beta-catenin phosphorylation links epithelial Smad signalingto cell contacts. J Cell Biol. 2009 Jan 26;184(2):309-22.12. Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, Sonnenberg A,Wei Y, Vu T. Integrin α6β4 identifies an adult distal lung epithelial population with120
egenerative potential in mice. J Clin Invest. 2011, 121:2855-62. See Commentarysame issue.13. Ulsamer A, Wei Y, Kim KK, Tan K, Wheeler S, Xi Y, Thies RS, Chapman HA.Axin pathway activity regulates in vivo pY654-b-catenin accumulation andpulmonary fibrosis. J Biol Chem 2012, 287: 5164-5172.14. Sennino B, Ishiguro-Oonuma T, Wei Y, Naylor RM, Williamson CW, BhagwandinV, Tabruyn SP, You WK, Chapman HA, Christensen JG, Aftab DR, McDonaldDM. Suppression <strong>of</strong> tumor invasion and metastasis by concurrent inhibition <strong>of</strong> c-Metand VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discovery 2012,2:270-287.15. Xi Y, Wei Y, Sennino, Ulsamer A, Kwan I, Brumwell AN, Tan K, Aghi MK,McDonald DM, Jablons DM, Chapman HA. Identification <strong>of</strong> pY654-b-catenin as acritical co-factor in hypoxia-inducible factor-1a signaling and tumor responses tohypoxia. Oncogene 2012, Dec 17. doi: 10.1038/onc.2012.530.16. Smith KR, Dahl HH, Canafoglia L, Andermann E, Damiano J, Morbin M,Franceschetti S, Cossette P, Saftig P, Grötzinger J, Schwake M, Andermann F,Staropoli JF, Sims KB, Mole SE, Alexander NA, Cooper JD, Chapman HA,Carpenter S, Berkovic SF, Bahlo M. Cathepsin F mutations cause type B Kufsdisease, an adult-onset neuronal ceroid lip<strong>of</strong>uscinosis. Human Molecular Genetics2013, Jan 12. [Epub ahead <strong>of</strong> print].<strong>Research</strong> SupportOngoing <strong>Research</strong> Support5 R37 HL67204 (Chapman, HA) No cost extension 6/1/2001 – 5/31/2013NIH/NHLBI MERIT AWARDRole <strong>of</strong> Elastolytic Cathepsins in EmphysemaThe major goal <strong>of</strong> this project is define the role <strong>of</strong> cysteine proteases in smoking-relatedemphysema. The project focuses on pathways <strong>of</strong> elastase expression in lung mesenchymalcells and on the genetics <strong>of</strong> emphysema in human subjects with early-onset emphysema andnormal alpha-1-antitrypsin.5 R01 HL44712 (Chapman, HA) $250,000 per yr directRegulation <strong>of</strong> Integrin Function 1/1/1991 – 12/31/2014The major goals <strong>of</strong> this project are to understand the molecular basis and importance <strong>of</strong>integrin function in promoting TGFb1 signaling and pulmonary fibrosis. The hypothesisthat epithelial to mesenchymal transition is an important component <strong>of</strong> pulmonary fibrosis,and regulated by integrins, is the main idea tested in this grant.5 R01 CA125564 (Chapman, HA) No cost extension 7/1/2007 – 5/31/2013121
NIH/NHLBIUrokinase Receptor Integrin Interactions in Lung CancerThe major goals <strong>of</strong> this project are to define the physical basis <strong>of</strong> urokiinase receptor beta1interactions and the influence <strong>of</strong> these interactions on tumor cell signaling and lung tumorprogression. Primary tumor cells from human tumors will be examined for their expressionand dependence on urokinase receptors for adhesion and migration.U01 HL111054-01 (Chapman HA, PI) $250,000 per year directNIH/NHLBIEpithelial Progenitor Cells in Lung Repair and Regeneration 1/1/2012-12/31/2016The major goals <strong>of</strong> this project are (1) To define the transcriptional program <strong>of</strong> heret<strong>of</strong>oreuncharacterized distal airway and alveolar progenitors and test the hypothesis that differentialexpression <strong>of</strong> adhesion receptors underlies the capacity <strong>of</strong> epithelial subtypes to self-organizeand promote repair. (2) Define the requirement for neuroendocrine cells (PNECS )andalveolar progenitor cells in maintenance and reconstitution <strong>of</strong> distal airway and alveolar cellsfollowing lung injury. (3) Analyze and further develop a novel, single cell in vivo lungorganoid assay in kidney capsules in order to optimize the capacity <strong>of</strong> adult epithelialprogenitor cells to generate functional respiratory units de novo.PO1 HL108794 (Sheppard PI, Chapman HA, project leader) 250,000 per year directTargeting epithelial cells to treat pulmonary fibrosis. 8/1/2012-7/31/2017The major goal <strong>of</strong> this project is to deliver one or more novel therapeutics based on recentlyidentified regulators <strong>of</strong> EMT in lung epithelial cells for further drug development.122
BIOGRAPHICAL SKETCHNAMEAnthony L. DeFranco, Ph.D.eRA COMMONS USER NAMEDeFrancoPOSITION TITLEPr<strong>of</strong>essor,Department <strong>of</strong> Microbiology & ImmunologyEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYHarvard <strong>University</strong>, Cambridge, MA A.B. 6/75 Biochem. & Molec.<strong>University</strong> <strong>of</strong> California, Berkeley, CA Ph.D. 10/79 Biol. BiochemistryNational Institutes <strong>of</strong> Health, Bethesda, MD Postdoctoral 11/79-8/83 ImmunologyPositions and Honors1972-1975 Undergraduate research, laboratory <strong>of</strong> Dr. Jack Strominger. HLA antigens.1976-1979 Graduate research, laboratory <strong>of</strong> Dr. Daniel E. Koshland, Jr. Bacterialchemotaxis.1979-1983 Postdoctoral research, laboratory <strong>of</strong> Dr. William E. Paul. B cell activation.1983-1988 Assistant Pr<strong>of</strong>essor, UCSF, Department <strong>of</strong> Microbiology & Immunology,1988-1994 Associate Pr<strong>of</strong>essor, UCSF, Department <strong>of</strong> Microbiology & Immunology1989-1990 Sabbatical with David Baltimore, Whitehead Insititute, MIT, Cambridge, MA1994-present Pr<strong>of</strong>essor, UCSF, Department <strong>of</strong> Microbiology & Immunology1997-1998 Sabbatical with Suzanne Cory, Walter and Eliza Hall Institute, Melbourne,Australia1998-2004 Scientific Advisory Board, Abgenix, Inc. Fremont, CA1999-2009 Chairman, Department <strong>of</strong> Microbiology & Immunology, UCSF2012- Scientific Advisory Board, UCB Celtech, Slough, UK1974 Dreyfuss Foundation Fellow1975 Phi Beta Kappa, Harvard <strong>University</strong>1975-1978 NSF Predoctoral Fellow1979-1982 Helen Hay Whitney Postdoctoral Fellow; 2nd Rose Lieberman Lecturer, NIH1993 1994 NIAID Merit Award1997-1998 NIH Fogarty Senior International Award.123
Pr<strong>of</strong>essional Service (selected list)Member Exptl. Immunol. Study Section (1993-97), Chair (1995-1997); NIH grant reviewer, adhoc (average 3 committees/yr since 2005, including TTT 2008, 2009); J. Immunol. AssociateEditor (1986-90), Section Editor (1990-1994), Deputy Editor (1999-2001); Annual Review <strong>of</strong>Immunology, Editorial Committee for Volumes 9,13, 15, 17 (1991, ‘95, ‘97, ‘99); CurrentBiology, Editorial Board (1993-99). Lecturer, American Assoc. <strong>of</strong> Immunologists, AdvancedCourse 1995-97; Leukemia Society <strong>of</strong> America, Career Development Program Grant ReviewSubcommittee (1999-2005); Co-organizer: “B cell Immunobiology and Disease”, a Keystonesymposium, 2001. Faculty <strong>of</strong> 1000 contributor 2001-present (www.faculty<strong>of</strong>1000.com/start.asp).Selected peer-reviewed publications (in chronological order)Original <strong>Research</strong>/Peer-reviewed journals (from total <strong>of</strong> 75 original research publications)1. Gold, M.R., D.A. Law and A.L. DeFranco. (1990) Stimulation <strong>of</strong> protein tyrosinephosphorylation by the B lymphocyte antigen receptor. Nature 345: 810-813.2. Yu, C.C.K., T.S.B. Yen, C.A. Lowell, and A.L. DeFranco (2001). Lupus-like kidneydisease in mice deficient in Src-family protein tyrosine kinases Lyn and Fyn. Curr. Biol.11:34-38.3. Gupta, N., B. Wollscheid, J.D. Watts, B. Scheer, R. Aebersold, and A.L. DeFranco(2006). Quantitative proteomic analysis <strong>of</strong> B cell lipid rafts reveals that ezrin regulatesantigen receptor-mediated lipid raft dynamics. Nature Immunol. 7: 625-633.4. Mamchak, A.A., B.M. Sullivan, B. Hou, L.M. Lee, J.K. Gilden, M.F. Krummel, R.M.Locksley, and A.L. DeFranco (2008). Normal development and activation but alteredcytokine production <strong>of</strong> Fyn-deficient CD4+ T cells. J. Immunol. 181: 5374-5385.PMC26575555. Gross, A.J., J.R. Lyandres, A.K. Panigrahi, E.T.L. Prak, and A.L. DeFranco (2009).Developmental acquisition <strong>of</strong> the Lyn-CD22-SHP-1 inhibitory pathway promotes B Celltolerance. J. Immunol. 182: 5382-92. PMC28400416. Gross, A.J., I. Proekt, and A.L. DeFranco (2011). Elevated BCR signaling and decreasedsurvival <strong>of</strong> Lyn-deficient transitional and follicular B cells. Eur. J. Immunol. 41: 3645-3655. PMC in process.7. Hou, B., P. Saudan, G. Ott, M.L. Wheeler, M. Ji, L. Kuzmich, L.M. Lee, R.L. C<strong>of</strong>fman,M.F. Bachmann, A. L. DeFranco (2011). Selective utilization <strong>of</strong> Toll-like receptor andMyD88 signaling in B cells for enhancement <strong>of</strong> the anti-viral germinal center response.Immunity 34: 375-84. PMC3064721.8. Scapini, P., Lamagna, C., Hu, Y., Lee, K., Tang, Q., DeFranco, A. L., Lowell, C.A.(2011) B cell-derived IL-10 suppresses inflammatory disease in Lyn-deficient mice.Proc. Natl. Acad Sci. 108: E823-832. PMC31931939. Wheeler, M.L., and A.L. DeFranco (2012). Prolonged production <strong>of</strong> reactive oxygenspecies in response to BCR stimulation promotes B cell activation and proliferation. J.Immunol. 189: 4405-16.10. Kirkland, D., A. Benson, J. Mirpuri, R. Pifer, B. Hou, A.L. DeFranco, F. Yarovinsky Bcell-intrinsic MyD88 signaling prevents the lethal dissemination <strong>of</strong> commensal bacteriaduring colonic damage (2012). Immunity 36: 228-238. PMC3288553.124
Additional recent publications <strong>of</strong> importance to the field (in chronological order)11. Nishiya, T. and A.L. DeFranco (2004). Ligand-regulated chimeric receptor approachreveals distinctive subcellular localization and signaling properties <strong>of</strong> the Toll-likereceptors. J. Biol. Chem. 279: 19008-19017.12. Nishiya, T. Kajita, E., Miwa, S. and A.L. DeFranco (2005). TLR3 and TLR7 aretargeted to the same intracellular compartments by distinct regulatory elements. J. Biol.Chem. 280: 37107-17.13. Hou, B., B. Reizis, and A.L. DeFranco (2008). Toll-like receptors activate innate andadaptive immunity using dendritic cell-dependent and -independent mechanisms.Immunity 29: 272-82. PMC284779614. Scapini, P., Y. Hu, C.L. Chu, T.S. Migone, A.L. DeFranco, M.A. Cassatella, and C.A.Lowell (2010). Myeloid cells, BAFF, and IFN-g establish an inflammatory loop thatexacerbates autoimmunity in Lyn-deficient mice. J. Exp. Med. 207:1757-73.PMC291612415. Hou, B., A. Benson, L. Kuzmich, A.L. DeFranco and F. Yarovinsky (2011). Criticalcoordination <strong>of</strong> innate immune defense against Toxoplasma gondii by dendritic cellsresponding via their Toll-like receptors. Proc. Natl. Acad. Sci. USA 108: 278-283.PMC3017180<strong>Research</strong> projects ongoing or completed during the last the three yearsActive1. “Regulation <strong>of</strong> B lymphocyte proliferation by antigen”Principal Investigator: Anthony DeFrancoAgency: National Institute for Allergy and Infectious DiseaseType: R01 (AI20038-26). Period: 12/1/05-11/30/10 (12/10-11/30/11 no cost extension)The major goals <strong>of</strong> this project are: 1. Define the roles <strong>of</strong> ezrin and non-muscle myosins inlipid raft coalescence following BCR stimulation. 2. Define the role <strong>of</strong> B144, Rho-familyGTPases, and non-muscle myosin motors in directing outgrowth <strong>of</strong> long membrane processesin BCR-stimulated B cells. 3. Determine the mechanism <strong>of</strong> assembly <strong>of</strong> the NF-kBsignalosome at lipid rafts in mature B cells stimulated through the BCR. 4. Characterize thedifferences between immature B cells and mature B cells with regard to BCR-induced lipidraft coalescence and assembly <strong>of</strong> the Carma1/Bcl10 signaling complex.2. “Cell Type-Specific Roles <strong>of</strong> TLR Signaling in Immune Responses”Principal Investigator: Anthony DeFrancoAgency: NIAIDType: R01 (R01AI0720585-3). Period: 12/1/07-11/30/2012The major goals <strong>of</strong> this project are: Aim 1: To define the role <strong>of</strong> TLR signaling in dendriticcells for initiating adaptive T cell immune responses. Aim 2: To define the role <strong>of</strong> TLRsignaling in phagocytic cells for inducing inflammation and promoting microbial killingduring bacterial infection. Aim 3: To define the role <strong>of</strong> TLR signaling in B lymphocytes foramplifying antibody responses.3. “Innate Immune Regulation <strong>of</strong> Inflammation and Adaptive Immunity”125
Program Director: Anthony L. DeFranco. Project #1 “Cellular Basis <strong>of</strong> TLR Signaling forMucosal Immune Responses” (A.L. DeFranco, PI)Agency: NIAIDType: P01 (AI078869-02). Period: 7/1/08-6/30/13The major goals <strong>of</strong> Project 1 are: Aim 1: To define the role <strong>of</strong> TLR signaling in dendriticcells for initiating adaptive T cell immune responses to antigen challenge via the airways.Aim 2: To define the role <strong>of</strong> TLR signaling in immature dendritic cells and in neutrophils andmacrophages for innate and adaptive immune responses to fungal cell walls. Aim 3: Todefine the role <strong>of</strong> TLR signaling in immature dendritic cells and in neutrophils andmacrophages for immune defense against systemic infection with the fungal pathogenCandida albicans.Completed (last 3 years)1. “Cytoskeleton and Signal Transduction in Host Defense”Principal Investigator: Anthony DeFranco Agency: NIAIDType: R01 (R01 AI35811-11). Period: 12/1/06-1/31/10The goals <strong>of</strong> this project are to characterize the functional alterations <strong>of</strong> L-plastin-deficiencyon neutrophil and T cell function (This grant was awarded to Dr. Eric Brown and I becamethe PI to finish the project when Dr. Brown took a position at Genentech, Inc.).126
BIOGRAPHICAL SKETCHNAMEDavid J. Erle, M.D.eRA COMMONS USER NAMEDJERLEPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> MedicineEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYHarvard College, Cambridge, MA A.B. 1980 Biochemistry<strong>University</strong> <strong>of</strong> California, San Francisco, CA M.D. 1984 Medicine<strong>University</strong> <strong>of</strong> California, San Francisco, CA Resident 1984-87 Internal Medicine<strong>University</strong> <strong>of</strong> California, San Francisco, CA Fellow 1987-90 Pulmonary DiseasePositions1984-1987 Resident in Internal Medicine, <strong>University</strong> <strong>of</strong> California Hospitals,San Francisco1987-1988 Clinical Pulmonary Fellow, <strong>University</strong> <strong>of</strong> California Hospitals, San Francisco1988-1990 <strong>Research</strong> Fellow, Lung Biology <strong>Center</strong> and Cardiovascular <strong>Research</strong>Institute, UCSF1990-1992 Adjunct Assistant Pr<strong>of</strong>essor <strong>of</strong> Medicine, UCSF1990-present Attending Physician, San Francisco General Hospital1992-1998 Assistant Pr<strong>of</strong>essor <strong>of</strong> Medicine in Residence, UCSF1996-present Faculty, UCSF Immunology and Biomedical Sciences Graduate Programs1997-2001 UCSF/SFGH General Clinical <strong>Research</strong> <strong>Center</strong> (GCRC) AdvisoryCommittee1998-2004 Associate Pr<strong>of</strong>essor <strong>of</strong> Medicine, UCSF1999-present Investigator, Cardiovascular <strong>Research</strong> Institute, UCSF2000-present Director, Functional Genomics Core Facility, UCSF SABRE <strong>Center</strong>2004-present Pr<strong>of</strong>essor <strong>of</strong> Medicine, UCSF2006-2011 Associate Director, UCSF Clinical and Translational Sciences InstituteBioinformatics ProgramHonors1977 Detur Prize1977, 1978 John Harvard Scholarship1984 Alpha Omega Alpha, elected1990-1993 Edward Livingston Trudeau Award <strong>of</strong> the American Lung Association2008-2012 Member, LCMI Study Section (chair 2010-2012)127
Selected peer-reviewed publicationsFifteen most relevant to my role as director <strong>of</strong> the SABRE <strong>Center</strong> Functional Genomics Core(all include microarray studies):1. Kuperman DA, Huang X, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA,Sheppard D, Erle DJ. Direct effects <strong>of</strong> interleukin-13 on epithelial cells cause airwayhyperreactivity and mucus overproduction in asthma. Nat Med 8:885-9, 2002. PMID:12091879.2. Barczak A, Rodriguez MW, Hanspers K, Koth LL, Tai YC, Bolstad BM, Speed TP, ErleDJ. Spotted long oligonucleotide arrays for human gene expression analysis. GenomeRes 13:1775-85, 2003. PMCID: PMC403751.3. Woodruff PG, Koth LL, Yang YH, Rodriguez MW, Favoreto S, Dolganov GM, PaquetAC, Erle DJ. A distinctive alveolar macrophage activation state induced by cigarettesmoking. Am J Respir Crit Care Med 172:1383-1392, 2005. PMCID: PMC2718436.4. Kuperman DA, Lewis CC, Woodruff PG, Rodriguez MW, Yang YH, Dolganov GM,Fahy JV, Erle DJ. Dissecting asthma using focused transgenic modeling and functionalgenomics. J Allergy Clin Immunol 116:305-11, 2005. PMID: 16083784.5. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S,Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, Erle DJ, Yamamoto KR, Fahy JV.Genome-wide pr<strong>of</strong>iling identifies epithelial cell genes associated with asthma and withtreatment response to corticosteroids. Proc Natl Acad Sci USA 104:15858-63, 2007.PMCID: PMC2000427.6. Zhen G, Park SW, Nguyenvu LT, Rodriguez MW, Barbeau R, Paquet AC, Erle DJ.Interleukin-13 and EGF receptor have critical but distinct roles in epithelial cell mucinproduction. Am J Respir Cell Mol Biol 36(2):244-53, 2007. PMCID: PMC1899314.7. Lewis CC, Yang JY, Huang X, Banerjee SK, Blackburn MR, Baluk P, McDonald DM,Blackwell TS, Nagabhushanam V, Peters W, Voehringer D, Erle DJ. Disease-specificgene expression pr<strong>of</strong>iling in multiple models <strong>of</strong> lung disease. Am J Respir Crit Care Med177:376-87, 2008. PMCID: PMC2258439.8. Park SW, Verhaeghe C, Nguyenvu LT, Barbeau R, Eisley CJ, Nakagami Y, Huang X,Woodruff PG, Fahy JV, Erle DJ. Distinct roles <strong>of</strong> FOXA2 and FOXA3 in allergic airwaydisease and asthma. Am J Respir Crit Care Med 180:603-10, 2009. PMCID:PMC2753788.9. Park SW, Zhen G, Verhaeghe C, Nakagami Y, Nguyenvu LT, Barczak AJ, Killeen N,Erle DJ. The protein disulfide isomerase AGR2 is essential for production <strong>of</strong> intestinalmucus. Proc Natl Acad Sci USA 106:6950-52009, 2009. PMCID: PMC2678445.10. Wang BT, Ducker GS, Barczak AJ, Barbeau R, Erle DJ, Shokat KM. The mammaliantarget <strong>of</strong> rapamycin regulates cholesterol biosynthetic gene expression and exhibits arapamycin-resistant transcriptional pr<strong>of</strong>ile. Proc Natl Acad Sci USA 108:15201-6, 2011.PMCID: PMC3174577.11. Zhao W, Blagev D, Pollack J, Erle DJ. Toward a systematic understanding <strong>of</strong> mRNA 3’untranslated regions. Proc Am Thorac Soc 8:163-6, 2011. PMCID: PMC3131834.12. Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, RaymondWW, Erle DJ, Abrink M, Caughey GH, Huang X, Sheppard D. The αvβ6 integrinmodulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. JClin Invest 122:748-58, 2012. PMCID: PMC3266785.128
13. Diez D, Goto S, Fahy JV, Erle DJ, Woodruff PG, Wheelock ÅM, Wheelock CE.Network analysis identifies a putative role for the PPAR and type 1 interferon pathwaysin glucocorticoid actions in asthmatics. BMC Med Genomics 5:27, 2012. PMCID:PMC3408345.14. Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ.AGR2 is induced in asthma and promotes allergen-induced mucin overproduction. Am JRespir Cell Mol Biol 47:178-85, 2012. PMCID: PMC3423459.15. Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M,Nguyen C, Barczak AJ, Zlock LT, Blagev DP, Finkbeiner WE, Ansel KM, Arron JR,Erle DJ*, Woodruff PG*. Airway epithelial miRNA expression is altered in asthma. AmJ Respir Crit Care Med 2012 [Epub ahead <strong>of</strong> print] PubMed PMID: 22955319. * equalcontributions.<strong>Research</strong> SupportActiveR01 HL099101 (Erle) 01/01/2010-12/31/2013NIH/NHLBI $247,500Specialized Molecules with Essential Roles in Mucus ProductionThe major goals <strong>of</strong> this project are: 1) To analyze AGR2 and AGR3 substrate bindingspecificity. 2) To determine the in vivo roles <strong>of</strong> AGR2 and AGR3 in the mouse airway. 3) Toanalyze the functions <strong>of</strong> AGR2 and AGR3 in human airway epithelial cells.R21 HL108596 (Erle) 04/06/2011-03/31/2013NIH/NHLBI $125,000Micro-RNAs in airway epithelial differentiation and asthmaThe major goal <strong>of</strong> this project is to develop tools to determine how selected miRNAs affectairway epithelial cell differentiation and function.P01 HL108794 (PD: Sheppard) 08/09/2012-07/31/2017NIH/NHLBI $1,666,456Role: Co-InvestigatorProgram: Targeting Epithelial Cells to Treat Pulmonary FibrosisThis translational program project grant is designed to develop new effective therapies foridiopathic pulmonary fibrosis (IPF).PENDINGU19 AI 077439-06 (PD: Sheppard, Project 1 leader: Erle) 04/01/2013-03/31/2018NIH/NIAID Program: $1,086,097(priority score: 20, funding expected per NIH program <strong>of</strong>ficer) Project 1: $274,962Program: IL-13 and IL-17 dynamics in the asthmatic airwayProject 1: IL-13/17-regulated airway epithelial miRNAs in asthmaOverall project goal is to determine how immune cells producing IL-13 and IL-17 specificallymodulate contractile responses <strong>of</strong> airway smooth muscle and the relevance <strong>of</strong> these pathwaysto human asthma.129
CompletedR21 HG004665 (Erle) 04/01/08-03/31/10NIH/NHGRITools for high-throughput functional analysis <strong>of</strong> 3’ UTR cis-regulatory elementsThe major goals <strong>of</strong> this project were: 1) To optimize the design and implementation <strong>of</strong> the 3’UTR high-throughput reporter system. 2) To use the system to identify cis-regulatoryelements within a representative group <strong>of</strong> 3’ UTRs from ENCODE regions <strong>of</strong> the genome.UL1 RR024131 (PD: McCune) 09/30/06-06/30/11NIH/NCRRClinical and Translational Science InstituteRole on project: Co-investigatorThe overall mission <strong>of</strong> the CTSI is to create an integrated academic home that transformsresearch and education in clinical investigation and translational science at UCSF andthroughout the community. Dr. Erle is a member <strong>of</strong> the Bioinformatics unit <strong>of</strong> the CTSI,which supports clinical and translational research and training at UCSF.R01 HL085089 (Erle) 07/21/06-06/30/12NIH/NHLBIAirway Epithelial Responses to Allergic InflammationThe major goals <strong>of</strong> this project were: 1) To determine the role <strong>of</strong> the EGF receptor pathway inthe airway epithelial cell response to IL-13. 2) To determine the role <strong>of</strong> the transcription factorFOXA2 in the airway epithelial cell response to IL-13. 3) To determine how the IL-13-inducible epithelial anion exchanger SLC26A4 (Pendrin) contributes to allergic airwaydisease. 4) To determine how the IL-13-inducible goblet cell-specific secreted protein anteriorgradient 2 (AGR2) contributes to allergic airway disease.130
BIOGRAPHICAL SKETCHNAMEJohn Vincent Fahy, M.D., M.Sc.eRA COMMONS USER NAMEjohnfahyPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> Medicine in ResidenceEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDY<strong>University</strong> College Dublin MB BAO 1985 MedicineBCH<strong>University</strong> College Dublin Intern 1985-1986 Medicine and SurgeryTrinity College Dublin Resident 1986-1989 Internal Medicine<strong>University</strong> College Dublin Registrar 1988-1999 Respiratory Medicine<strong>University</strong> <strong>of</strong> California, San Francisco Fellow 1989-1993 Pulmonary/CriticalCare Medicine<strong>University</strong> College DublinTrinity College DublinPositionsInternship and ResidenciesM.D.(doctorate bythesis)M.Sc.1997 Respiratory Medicine2003(Sabbaticalyear)Molecular Medicine1985 - 1986 Medicine Intern, St Vincent’s Hospital, <strong>University</strong> College Dublin.1986 - 1988 Senior House Officer, St James’ Hospital, Trinity College Dublin.1988 - 1989 Medicine Registrar (pulmonary medicine), St Vincent’s Hospital, <strong>University</strong>College Dublin.1989 - 1993 Fellow, Pulmonary and Critical Care Medicine, UCSF.Academic Appointments1993 - 1998 Assistant Adjunct Pr<strong>of</strong>essor <strong>of</strong> Medicine, UCSF.1999 - 2005 Associate Pr<strong>of</strong>essor <strong>of</strong> Medicine in Residence, UCSF.2005 - Present Pr<strong>of</strong>essor <strong>of</strong> Medicine in Residence, UCSF.Other Experience and Pr<strong>of</strong>essional Memberships1993 - Present Member, Steering Committee, NHLBI’s <strong>Asthma</strong> Clinical <strong>Research</strong> Network2005 - 2006 Member, Executive Planning Committee, NHLBI Strategic Plan2006 - Present Director, Airway Clinical <strong>Research</strong> <strong>Center</strong>, UCSF.2007 - Present Chair, Data and Safety Monitoring Board for Division <strong>of</strong> Lung DiseasesSCCOR Program2004 - Present Member, Program Committee, <strong>Asthma</strong>, Inflammation, and ImmunologyAssembly, ATS.131
2009 - 2010 Chair (elected), Program Committee, <strong>Asthma</strong>, Inflammation, & ImmunologyAssembly, ATS.2009 - Present Member, Trans NIH <strong>Asthma</strong> Outcomes Working Group.2010 - Present Member, Scientific Committee, Transatlantic Airway Conference.Honors1984 Kirwan Gold Medal and Prize in Ophthalmology, <strong>University</strong> College Dublin (UCD)1985 Graduated fifth in class <strong>of</strong> 120 students, UCD Medical School (one <strong>of</strong> 10 to receive anhonors degree).1990 Travelling Studentship in Medicine, National <strong>University</strong> <strong>of</strong> Ireland1994 Physician Scientist Award, American College <strong>of</strong> Chest Physicians.2006 Michael S. Stulbarg Endowed Chair in Pulmonary Medicine, UCSF.Selected Peer-reviewed Publications1. Ordoñez CL, Khashayar, R, Wong HH, Ferrando R, Wu R, Hyde DM, Hotchkiss JA, ZhangY, Novikov A, Dolganov G, Fahy JV. Mild and moderate asthma is associated with gobletcell hyperplasia and abnormalities in mucin gene expression. Am J Resp Crit Care Med2001;163:517-523.2. Hays SR, Woodruff PG, Khashayar R, Ferrando RE, Liu J, Fung P, Zhao CQ, Wong HH,Fahy JV. Allergen challenge causes inflammation but not goblet cell degranulation inasthmatic subjects. J Allergy Clin Immunol 2001;108:784-790.3. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A,Sidhu S, Dao-Pick TP, Pantoja C, Erle DJ, Yamamoto KR, Fahy JV. Genome-wide pr<strong>of</strong>ilingidentifies epithelial cell genes associated with asthma and with treatment response tocorticosteroids. Proc Natl Acad Sci USA 2007; 104:15858-63.4. Seibold MA, Donnelly S, Solon M, Innes A, Woodruff PG, Boot R, Burchard EG, Fahy JV.Chitotriosidase is the primary active chitinase in the human lung and is modulated bygenotype and disease. J Allergy Clin Immunol. 2008;122:944-950. PMCID: PMC2666777.5. Innes AL. Carrington SD, David J. Thornton DJ, Kirkham S, Dougherty RH, Raymond WW,Caughey GH, Muller SJ, Fahy JV. Ex vivo sputum analysis reveals impairment <strong>of</strong> proteasedependentmucus degradation by plasma proteins in acute asthma. Am J Respir Crit CareMed. 2009;180:203-10. PMCID: PMC2724713.6. Woodruff PG, Modrek M, Choy DF, Guiquan J. Abbas AR, Ellwanger A, Koth LL, ArronJR, Fahy JV. TH2-driven inflammation defines major sub-phenotypes <strong>of</strong> asthma. Am JRespir Crit Care Med. 2009;180:388-95.7. Seibold MA, Reese TA, Choudhry S, Salam MT, Beckman K, Eng C, Atakilit A, Meade K,Lenoir M, Watson HG, Thyne S, Kumar R, Weiss KB, Grammer LC, Avila P, Schleimer RP,Fahy JV, Rodriguez-Santana J, Rodriguez-Cintron W, Boot RG, Sheppard D, Gilliland FD,Locksley RM, Burchard EG. Differential enzymatic activity <strong>of</strong> common haplotypic versions<strong>of</strong> the human acidic mammalian chitinase protein. J Biol Chem 2009;284:19650-8.8. Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, Woodruff PG,Fahy JV. Accumulation <strong>of</strong> Intra-epithelial Mast Cells with a Unique Protease Phenotype inTH2-high <strong>Asthma</strong>. J Allergy Clin Immunol. 2010;125:1046-1053.9. Sukhvinder SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Solon M, Hou L, Muller SL, FahyJV. Epithelial cell-derived periostin: roles in TGFβ activation, collagen production andcollagen elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170-5.132
10. Fahy JV, Dickey B. Airway Mucus - Function and Dysfunction. New Engl J <strong>of</strong> Med. 2010;2;363:2233-47.11. Innes AL, Wong McGrath K, Dougherty RH, McCulloch CE, Woodruff PG, Seibold MA,Okamoto KS, Kelsey J. Ingmundson KJ, Solon MC, Carrington SD, Fahy JV. The H Antigenat Epithelial Surfaces is Associated with Susceptibility to <strong>Asthma</strong> Exacerbation. Am J RespirCrit Care Med. 2011;183:189-94.12. Choy DF, Modrek B, Abbas AR, Kummerfeld S, Clark HF, Wu LC, Fedorowicz G,Modrusan Z, Fahy JV, Woodruff PG, Arron JR. Gene expression patterns <strong>of</strong> TH2inflammation and intercellular communication in asthmatic airways. J Immunol.2011;186:1861-9.13. Fahy JV, Locksley RM, The airway epithelium as a regulator <strong>of</strong> TH2 responses in asthma.American Journal <strong>of</strong> Respiratory and Critical Care Medicine 2011;184: 390-392.14. Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ, Huang X,Locksley RM, Fahy JV. A protective role for periostin and TGFβ in IgE-mediated allergyand airway hyperresponsiveness. Clinical and Experimental Allergy 2012;42:144-55.15. Wong McGrath K, Icitovic N, Boushey HA, Lazarus SC, Sutherland, ER, Chinchilli VM,Fahy JV. A large subgroup <strong>of</strong> mild moderate asthma is persistently non-eosinophilic.American Journal <strong>of</strong> Respiratory and Critical Care Medicine. 2012; 185(6):612-9.<strong>Research</strong> Support1P01HL107202 (Fahy, JV) 8/1/12 - 6/30/17Innate and Adaptive Immune Responses in Th2-high <strong>Asthma</strong>This PPG will comprehensively investigate the molecular underpinnings <strong>of</strong> the Th2-highmolecular subtype <strong>of</strong> asthmaRole: Overall PPG PI (Project leader for project 3 and Core leader for the Administrative Coreand the Human Subjects Core.1U10HL109146 (Fahy JV) 8/1/2011-7/31/2017Clinical and Molecular Phenotypes <strong>of</strong> Severe <strong>Asthma</strong>This grant was submitted in response to the RFA for Clinical <strong>Center</strong>s for the Severe <strong>Asthma</strong><strong>Research</strong> program) – a 6-center national program for research into mechanisms <strong>of</strong> severe asthma.Role: PI5 R01 HL080414 (Fahy, JV) 7/01/05-05/31/15Protein carbohydrate interactions in the pathophysiology <strong>of</strong> acute asthma exacerbationsThe major goals <strong>of</strong> this project are to investigate how lectins interact with mucin glycans tocause airway mucus obstruction in acute severe asthma.Role: PI1P50HL107191 (Fahy, JV) 5/01/11-04/30/13Preventing fucose-dependent binding <strong>of</strong> aspergillus and pseudomonas to lung mucinThis grant was submitted in response to the RFA for “<strong>Center</strong>s for Advanced Diagnostics andExperimental Therapeutics (CADET-1)”.Role: PI133
1 U19 A1077439 (Sheppard, D) 4/01/08-3/31/13NIH/NIAIDMechanisms <strong>of</strong> Initiation & Persistence <strong>of</strong> Allergic <strong>Asthma</strong>; Project 3: Chitinases and TGFβ inhuman asthma.The goal <strong>of</strong> this center grant is to examine the role <strong>of</strong> chitinases and TGFβ pathway genes inasthma. Project 3 specifically focuses on genetic and translational studies <strong>of</strong> chitinases & TGFβin asthma.Role: Co-Leader <strong>of</strong> Project 3 (with Esteban Burchard, M.D.)1 R01 HL097591 (Woodruff, PG) 7/01/09-06/30/13NIH/NHLBIRole <strong>of</strong> Th2 and non-Th2 Inflammation in Airway Smooth Muscle Remodeling in <strong>Asthma</strong>.This grant supports studies <strong>of</strong> airway smooth muscle in asthma.Role: Co-investigator134
BIOGRAPHICAL SKETCHNAMEXiaozhu Huang, M.D.POSITION TITLEAssociate Pr<strong>of</strong>essorEDUCATIONINSTITUTION AND LOCATION DEGREE YEAR FIELD OF STUDYTongji Medical <strong>University</strong>, Wuhan,People's Republic <strong>of</strong> ChinaTongji Medical <strong>University</strong>, Wuhan,People's Republic <strong>of</strong> ChinaM.D. 1983 MedicineM.S. 1988 PathologyPositions6/83 to 7/84 Teaching Assistant, Dept. <strong>of</strong> Pathology, Tongji Medical <strong>University</strong>, China8/84 to 8/85 Pathology Residence, The First Attached Hospital, Tongji Medical <strong>University</strong>,China9/88 to 1/92 <strong>Research</strong> Assistant, Dept. <strong>of</strong> Pathology, Tongji Medical <strong>University</strong>, China1/92 to 12/95 Postdoctoral Fellow, Dept. <strong>of</strong> Medicine, Lung Biology <strong>Center</strong><strong>University</strong> <strong>of</strong> California, San Francisco1/96 to 6/97 Postgraduate <strong>Research</strong>er, Dept. <strong>of</strong> Medicine, Lung Biology <strong>Center</strong><strong>University</strong> <strong>of</strong> California, San Francisco7/97 to 11/99 Assistant <strong>Research</strong> Molecular Biologist, Dept. <strong>of</strong> Medicine, Lung Biology<strong>Center</strong><strong>University</strong> <strong>of</strong> California, San Francisco12/99 to 6/2005 Assistant Pr<strong>of</strong>essor, Dept. <strong>of</strong> Medicine, Lung Biology <strong>Center</strong><strong>University</strong> <strong>of</strong> California, San Francisco07/2005 to present Associate Pr<strong>of</strong>essor, Dept. <strong>of</strong> Medicine, Lung Biology <strong>Center</strong><strong>University</strong> <strong>of</strong> California, San FranciscoHonors1989 "Outstanding Teacher" Honor, Department <strong>of</strong> Pathology,Tongji Medical <strong>University</strong>1/1992 to 12/1993 Cheng <strong>Research</strong> Scholar Award,135
Selected peer-reviewed publications1. Huang XZ, Chen A, Agrez M, Sheppard D. A point mutation in integrin b6 subunitabolishes both avb6 binding to fibronectin and receptor localization to focal adhesionplaques. Am. J. Respir. Cell Mol. Biol. 1995; 13:245-251.2. Huang XZ, Wu JF, Cass D, Erle DJ, Corry D, Young SG, Farese RV, Jr., SheppardD. Inactivation <strong>of</strong> the integrin b6 subunit gene reveals a role <strong>of</strong> epithelial integrins inregulating inflammation in the lungs and skin. J. Cell Biol. 1996; 133:921-928.3. Huang XZ, Wu JF, Zhu W, Pytela R, Sheppard D. Expression <strong>of</strong> the human integrinb6 subunit in alveolar type II cells and bronchiolar epithelial cells reverses lunginflammation in b6 knockout mice. Am. J. Respir. Cell Mol. Biol. 1998; 19:636-642.4. Huang XZ, Wu JF, Spong S, Sheppard D. The integrin avb6 is critical forkeratinocyte migration on both its known ligand, fibronectin, and on vitronectin. J.Cell Sci. 1998; 111:2189-2195.5. JS Munger, XZ Huang, H Kawakatsu, MJD Griffiths, SL Dalton, JF Wu, JF Pittet, NKaminiski, C Garat, MA Matthay, DB Rifkin, D Sheppard. The integrin avb6 bindsand activates latent TGFb1: a mechanism for regulating pulmonary inflammation andfibrosis. Cell 1999; 96:319-328.6. Milner R, Huang XZ, Wu J, Nishimura S, Pytela R, Sheppard D, ffrench-ConstantC. Distinct roles for astrocyte avb5 and avb8 integrins in adhesion and migration. J.Cell Sci. 1999; 112: 4271-4279.7. Huang XZ, Griffiths M, Wu JF, Farese RV, Sheppard D. Normal development,wound healing, and adenovirus susceptibility in b5-deficient mice. Mol. Cell Biol.2000; 20:755-759.8. Kaminski N, Allard J, Pittet J-F, Zuo F, Griffiths MJD, Morris D, Huang XZ,Sheppard D, Heller RA. Global analysis <strong>of</strong> gene expression in pulmonary fibrosisreveals distinct programs regulating lung inflammation and remodeling. Proc. Nat.Acad. Sci. 2000; 97:1778-1783.9. Huang XZ, Wu JF, Ferrando R, Wang YL, Lee JH, Farese RV, Sheppard D. FatalBilateral Chylothorax in Mice Lacking the Integrin a9b1. Mol. Cell Biol. 2000;20:5208-5215.10. Pittet JF, Griffiths MJ, Geiser T, Kaminski N, Dalton SL, Huang XZ, Brown LA,Gotwals PJ, Koteliansky VE, Matthay MA, Sheppard D. TGF-beta is a criticalmediator <strong>of</strong> acute lung injury. J Clin Invest. 2001, 107:1537-44.11. Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang XZ, SheppardD, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in micelacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002, 8:27-34.12. Eliceiri BP, Puente XS, Hood JD, Stupack DG, Schlaepfer DD, Huang XZ,Sheppard D, Cheresh DA. Src-mediated coupling <strong>of</strong> focal adhesion kinase to integrinalpha(v)beta5 in vascular endothelial growth factor signaling. J Cell Biol. 2002,157:149-60.13. Kuperman DA, Huang XZ, Koth LL, Chang GH, Dolganov GM, Zhu Z, Elias JA,Sheppard D, Erle DJ. Direct effects <strong>of</strong> interleukin-13 on epithelial cells cause airwayhyperreactivity and mucus overproduction in asthma. Nat Med. 2002, 8:885-9.14. Knight PA, Wright SH, Brown JK, Huang XZ, Sheppard D, Miller HR. Entericexpression <strong>of</strong> the integrin alpha(v)beta(6) is essential for nematode-induced mucosalmast cell hyperplasia and expression <strong>of</strong> the granule chymase, mouse mast cellprotease-1. Am J Pathol. 2002, 161:771-9.15. Morris DG, Huang XZ, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick A,Sheppard D. Loss <strong>of</strong> integrin-mediated TGF-b activation causes Mmp12-dependent136
pulmonary emphysema. Nature 2003, 422:169-73.16. Nandrot EF, Kim Y, Brodie SE, Huang XZ, Sheppard D, Finnemann SC. Loss <strong>of</strong>synchronized RPE phagocytosis and age-related blindness in mice lacking avb5integrin. J Exp Med. 2004, 200:1539-45.17. Lane NE, Yao W, Nakamura MC, Humphrey MB, Kimmel D, Huang XZ, Sheppard,Ross FP, Teitelbaum SL. Mice lacking the integrin beta5 subunit have acceleratedosteoclast maturation and increased activity in the estrogen-deficient state. J BoneMiner Res. 2005, 20:58-66.18. Kuperman DA, Huang XZ, Nguyenvu L, Hölscher C, Brombacher F, Erle DJ. IL-4receptor signaling in Clara cells is required for allergen-induced mucus production. J.Immunol. 2005, 175:3746-3752.19. Atabai K, Fernandez R, Huang X, Ueki I, Kline A, Li Y, Sadatmansoori S, Smith-Steinhart C, Zhu W, Pytela R, Werb Z, Sheppard D. Mfge8 Is Critical for MammaryGland Remodeling during Involution. Mol Biol Cell. 2005, 16:5528-37.20. Voehringer D, Reese TA, Huang X, Shinkai K, Locksley RM. Type 2 immunity iscontrolled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells <strong>of</strong> theinnate immune system. J Exp Med. 2006, 203:1435-46.21. Wang B, Huang X*, Wolters PJ, Sun J, Kitamoto S, Yang M, Riese R, Leng L,Chapman H A., Finn P W., David J R., Bucala R, and Shi GP. Deficiency <strong>of</strong>Macrophage Migration Inhibitory Factor Impairs Murine Airway Allergic Responses.J Immunol 2006, 177:5779-5784 (* Co-first author)22. Chen C, Huang X, Sheppard D. ADAM33 is not essential for growth anddevelopment and does not modulate allergic asthma in mice. Mol Cell Biol. 2006,26:6950-6.23. Chen C, Huang X, Atakilit A, Zhu QS, Corey SJ, Sheppard D. The Integrinalpha9beta1 Contributes to Granulopoiesis by Enhancing Granulocyte Colony-Stimulating Factor Receptor Signaling. Immunity. 2006, 25:895-906.24. Su G, Hodnett M, Wu N, Atakilit A, Kosinski C, Godzich M, Huang XZ, Kim JK,Frank JA, Matthay MA, Sheppard D, Pittet JF. Integrin {alpha}v{beta}5 RegulatesLung Vascular Permeability and Pulmonary Endothelial Barrier Function. Am JRespir Cell Mol Biol. 2007, 36:377-86.25. Travis MA, Reizis B, Melton AC Masteller E, Tang Q, Proctor J, Wang Y, BernsteinX, Huang X, Riechardt L, Bluestone J, Sheppard D. Loss <strong>of</strong> integrin avb8 ondendritic cells causes autoimmunity and colitis in mice. Nature 2007; 449:361-365.26. Horan GS, Wood S, Ona V, Li DJ, Lukashev ME, Weinreb PH, Simon KJ, Hahm K,Allaire NE, Rinaldi NJ, Goyal J, Feghali-Bostwick CA, Matteson EL, O'hara C,Lafyatis R, Davis GS, Huang X, Sheppard D, Violette SM. Partial Inhibition <strong>of</strong>Integrin {alpha}v{beta}6 Prevents Pulmonary Fibrosis Without ExacerbatingInflammation. Am J Respir Crit Care Med 2008; 177:56-65.27. Nakagami Y, Favoreto S Jr, Zhen G, Park SW, Nguyenvu LT, Kuperman DA,Dolganov GM, Huang X, Boushey HA, Avila PC, Erle DJ. The epithelial aniontransporter pendrin is induced by allergy and rhinovirus infection, regulates airwaysurface liquid, and increases airway reactivity and inflammation in an asthma model.J Immunol. 2008 Aug 1;181(3):2203-10.28. Song Y, Pittet JF, Huang X, He H, Lynch SV, Violette SM, Weinreb PH, Horan GS,Carmago A, Sawa Y, Bernstein XL, Wiener-Kronish JP. Role <strong>of</strong> integrin alphavbeta6 in acute lung injury induced by Pseudomonas aeruginosa. Infect Immun. 2008Jun;76(6):2325-3229. Caceres AI, Brackmann M, Elia MD, Bessac BF, del Camino D, D'Amours M, WitekJS, Fanger CM, Chong JA, Hayward NJ, Homer RJ, Cohn L, Huang X, Moran MM,Jordt SE.A sensory neuronal ion channel essential for airway inflammation and137
hyperreactivity in asthma.Proc Natl Acad Sci U S A. 2009 Jun 2;106(22):9099-104.30. Park SW, Verhaeghe C, Nguyenvu LT, Barbeau R, Eisley CJ, Nakagami Y, HuangX, Woodruff PG, Fahy JV, Erle DJ. Distinct Roles <strong>of</strong> FOXA2 and FOXA3 inAllergic Airway Disease and <strong>Asthma</strong>. Am J Respir Crit Care Med. 2009 Jul 23.31. Huang F, Rock JR, Harfe BD, Cheng T, Huang X, Jan YN, Jan LY. Studies onexpression and function <strong>of</strong> the TMEM16A calcium-activated chloride channel. ProcNatl Acad Sci U S A. 2009 Dec 15;106(50):21413-832. Frank Kirstein, Horsnell G William, Douglas A Kuperman, Xiaozhu Huang, David JErle, Andreas L Lopata, Frank Brombacher, Expression <strong>of</strong> IL-4 Receptor alpha onsmooth muscle cells is not necessary for development <strong>of</strong> experimental allergicasthma. J Allergy Clin Immunol. 2010 Aug;126(2):347-35433. Li, S. , Chen, Y. , Zhang, S. , More, S.S. , Huang, X. , Giacomini, K.M. Role <strong>of</strong>Organic Cation Transporter 1, OCT1 in the Pharmacokinetics and Toxicity <strong>of</strong> cisDiammine(pyridine)chloroplatinum(II) and Oxaliplatin in Mice. Pharm Res. 2010Nov 2334. Yu Hua Chow, Xiao Dong Zhu, Li Liu, Barbara Schwartz, Xiaozhu Huang, JohnHarlan, Lynn M Schnapp. Role <strong>of</strong> Cdk4 in lymphocyte function and allergenresponse. Cell Cycle. 2010 Dec 15;9(24):4922-3035. Masuno K, Haldar SM, Jeyaraj D, Mailloux C, Huang X, Panettieri Jr RA, Jain MK,Gerber AN. Expression Pr<strong>of</strong>iling Identifies Klf15 as a Glucocorticoid Target thatRegulates Airway Hyperresponsiveness. Am J Respir Cell Mol Biol. 2011 Jan 2136. Kudo M, Melton AC, Chen C, Engler MB, Huang KE, Ren X, Wang Y, Bernstein X,Li JT, Atabai K, Huang X*, Sheppard D. IL-17A produced by αβ T cells drivesairway hyper-responsiveness in mice and enhances mouse and human airway smoothmuscle contraction. Nat Med. 2012 Mar 4;18(4):547-54 (*co-authorship)37. Vijayanand P, Seumois G, Simpson LJ, Abdul-Wajid S, Baumjohann D, Panduro M,Huang X, Interlandi J, Djuretic IM, Brown DR, Sharpe AH, Rao A, Ansel KM.Interleukin-4 production by follicular helper T cells requires the conserved Il4enhancer hypersensitivity site V. Immunity. 2012 Feb 24;36(2):175-8738. Thornton E, Looney M, Sheppard D, Locksley R, Huang X, Krummel M. Dynamics<strong>of</strong> antigen capture and presentation revealed by two-photon live imaging in the lung.J Exp Med. 2012 Jun 4;209(6):1183-9939. Schroeder BW, Verhaeghe C, Park SW, Nguyenvu LT, Huang X, Zhen G, Erle DJ.AGR2 is Induced in <strong>Asthma</strong> and Promotes Allergen-Induced Mucin Overproduction.Am J Respir Cell Mol Biol. 2012 Aug;47(2):178-8540. Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H,Atakilit A, Burlingame AL, Matthay MA, Sheppard D. IQGAP1 is necessary forpulmonary vascular barrier protection in murine acute lung injury and pneumonia. AmJ Physiol Lung Cell Mol Physiol. 2012 Jul 1;303(1):L12-941. Chun Chen, Makoto Kudo, Florentine Rutaganira, Hiromi Takano, Candace Lee,Amha Atakilit, Toshimitsu Uede, Paul Wolters, Stephen Liggett, Kevan M. Shokat,Xiaozhu Huang and Dean Sheppard . Integrin α9β1 in airway smooth muscleregulates a novel brake on exaggerated murine and human airway narrowing. JCI2012 Aug 1;122(8):2916-2742. Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD,Donne ML, Huang X, Sheppard, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE, LiM, Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16Amodulates mucin secretion and airway smooth muscle contraction. Proc Natl AcadSci U S A. 2012 Oct 2;109(40):16354-9.43. Julia Freimuth, Frederic F. Clermont, Xiaozhu Huang, Angela De Sapio, TakuTokuyasu, Dean Sheppard, Rosemary J. Akhurst. Epistatic interactions between138
Tgfb1 and genetic loci, Tgfbm2 and Tgfbm3, determine susceptibility to an asthmaticstimulus. Proc Natl Acad Sci U S A. 2012 Oct 30;109(44):18042-744. Kudo M, Khalifeh Soltani SM, Sakuma SA, McKleroy W, Lee TH, Woodruff PG,Lee JW, Huang K, Chen C, Arjomandi M, Huang X, Atabai K. Mfge8 suppressesairway hyperresponsiveness in asthma by regulating smooth muscle contraction. ProcNatl Acad Sci U S A. 2013 Jan 8;110(2):660*Equal contribution139
BIOGRAPHICAL SKETCHNAMEMatthew Frederick Krummel, Ph.DeRA COMMONS USER NAMEKrummelPOSITION TITLEAssociate Pr<strong>of</strong>essorEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s)FIELD OFSTUDY<strong>University</strong> <strong>of</strong> Illinois at Champaign-Urbana B.S. 1989Biology andChemistry<strong>University</strong> <strong>of</strong> California at Berkeley Ph.D. 1995 ImmunologyWalter and Eliza Hall Institute, MelbourneAustralia1996-1997 ImmunologyStanford <strong>University</strong> 1997-2001 ImmunologyPositionsSummer 1987Summer 1988Summer Undergraduate <strong>Research</strong> Fellow, UTHSCDStagiare (Technician) Institut Pasteur, Paris,Unite de Genie Micro-Biologique.1989-1996 Graduate Student and Postdoctoral Fellow, <strong>University</strong> <strong>of</strong> California atBerkeley, Department <strong>of</strong> Molecular and Cell Biology1996-1997 Postdoctoral Fellow, Walter and Eliza Hall Institute, MelbourneAustralia1997-2001 Postdoctoral Fellow, Beckman Institute, Stanford <strong>University</strong>, Stanford,CA2001-2006 Assistant Pr<strong>of</strong>essor, Department <strong>of</strong> Pathology, UCSFJuly 2006-Present2006-Present2012-PresentHonorsAssociate Pr<strong>of</strong>essor, Department <strong>of</strong> Pathology, UCSFFaculty Director, Biological Imaging Development <strong>Center</strong>, UCSFPr<strong>of</strong>essor, Department <strong>of</strong> Pathology, <strong>University</strong> <strong>of</strong> California atSan Francisco2009-2012 Fellow <strong>of</strong> the American <strong>Asthma</strong> Foundation2005-2010 Leukemia and Lymphoma Foundation, Career Award2004-2007 Investigator Award, Cancer <strong>Research</strong> Institute.1997-2000 NRSA Postdoctoral Fellowship, National Institutes <strong>of</strong> Health1996-1997 Postdoctoral Fellowship, Juvenile Diabetes Foundation International140
1998 PATENT: J.P.Allison, D.R.Leach, and M.F. Krummel.Blockade <strong>of</strong> T Lymphocyte Down-Regulation Associated withCTLA-4 Signaling. US Patent 5,811,0971987 Summer Undergraduate <strong>Research</strong> Fellowship, Howard HughesMedical InstituteSelected Peer-reviewed Publications (out <strong>of</strong> 51)1. Krummel MF and Allison JP. 1995. CD28 and CTLA-4 have opposing signalswhich regulate the response <strong>of</strong> T cells to stimulation. J. Exp Med. 182, 459-465.PMCID: PMC21921272. Krummel MF, Sullivan TJ and Allison JP. 1995. Superantigen responses andcostimulation: CD28 and CTLA-4 have opposing effects on T cell expansion InVitro and In Vivo. Int.Immunol. 8, 101-105. PMCID: 86716383. Krummel MF and Allison JP. 1996. CTLA-4 Engagement Inhibits IL-2Accumulation and Cell Cycle Progression Upon Activation <strong>of</strong> Resting T cells. JExp Med 183, 2533-2540. PMCID: PMC21926134. Leach, D.R., Krummel MF and Allison JP. 1996. Enhancement <strong>of</strong> antitumorimmunity by CTLA-4 blockade. Science. 271, 1734-1736. PMID: 85969365. Krummel MF, Sjaastad MD, Wülfing C and Davis MM. 2000. Differentialclustering <strong>of</strong> CD4 and CD3z during T cell recognition. Science. 289, 1349-1352.PMID: 109587816. Jacobelli J, Chmura SA, Buxton DB, Davis MM and Krummel MF. 2004. Asingle class II myosin modulates T cell motility and stopping, but not synapseassembly. Nature Immunology 5, 531 – 538. PMCID: 150647617. Friedman RS Jacobelli J and Krummel MF. 2006. Surface-bound ChemokinesCapture and Prime T cells For Synapse Formation. Nature Immunology 7, 1101-8.PMID: 169642618. Sabatos CA, Doh J, Chakravarti S, Friedman RS, Pandurangi PG, Tooley AJ,Krummel, MF. 2008. A Synaptic Basis for Paracrine Interleukin-2 Signaling inActivating T cells. Immunity. 29(3): 238-248. PMID: 186749349. Tooley AJ, Gilden J, Jacobelli J, Trimble W, Kinoshita M and Krummel MF.2009. Amoeboid T lymphocytes require the septin cytoskeleton for corticalintegrity and persistent motility. Nature Cell Biology. Jan;11(1):17-26. Epub 2008Nov 30. PMID: 1904340810. Jacobelli J, Friedman RS, Conti MA, Lennon-Dumenil AM, Piel M, SorensenCM, Adelstein RS, Krummel MF. Confinement-optimized three-dimensional Tcell amoeboid motility is modulated via myosin IIA-regulated adhesions. NatImmunol. 11: 953-961. PMCID: PMC294356411. Friedman RS, Beemiller P, Sorensen CM, Jacobelli J, Krummel MF. 2010. Realtimeanalysis <strong>of</strong> T cell receptors in naive cells in vitro and in vivo revealsflexibility in synapse and signaling dynamics. J Exp Med. 11(10):953-61.PMCID: PMC298976612. Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF. 2011.Stabilized Imaging <strong>of</strong> immune surveillance in the mouse lung. Nat Methods.8(1):91-6. PMCID: PMC307600513. Englehardt JE, Boldajipour B, Beemiller P, Werb Z, Pandurangi P, Sorenson C,Egelblad M, Krummel MF. Marginating dendritic cells <strong>of</strong> the tumormicroenvironment cross-present tumor antigens and stably engage tumor-specificT cells. Cancer Cell. 2012 Mar 20;21(3):402-17. doi: 10.1016/j.ccr.2012.01.008.PMCID: PMC3311997
14. Thornton EE, Krummel MF, Looney, M.R. 2012. Live Imaging <strong>of</strong> the Lung. CurrProtoc Cytom. Apr; Chapter 12:Unit12.28.15. Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, Huang X,Krummel MF. 2012. Spatiotemporally Separated Antigen Uptake by Alveolar DendriticCells and Airway Presentation to T Cells in the Lung. J Exp Med., 209(6):1183-99.<strong>Research</strong> supportOngoing <strong>Research</strong> Support1U01HL111054-01 (Chapman, Chuang, 01/01/12-12/31/16Krummel, multi-PI) (co-PI)NIHEpithelial Progenitor Cells in Lung Repair and Regeneration: Note: Scored 20 at study sectionThe goal <strong>of</strong> this project is to image the process <strong>of</strong> lung repair and regeneration using 2-photonmicroscopy.Role: co-PI1U01CA141451 (PI: Krummel) 09/01/09-08/31/14NIHCollaborative Innate-Adaptive Immune Regulation <strong>of</strong> Tumor ProgressionThe major goals <strong>of</strong> this project areGoal 1: Visualize the progression in crosstalk between the innate and adaptive immune responseduring tumor development using mouse models <strong>of</strong> luminal and basal breast cancer.Goal 2: Define the specific attractants that regulate immune cell-cell interactions in the tumor.Goal 3: Use mouse models to determine mechanisms <strong>of</strong> existing and putative immuno- andcytotoxic anti-cancer regimens and to design and test combinatorial therapies based upon thisinformation.Role: co-PIP01 HL024136 (PI: Caughey) 05/11/10-03/31/14NIH/NHLBIEvolving Microenvironments in Airway InflammationThe aims <strong>of</strong> this proposal are to identify shifts in antigen-trafficking into APC, the temporalpairing <strong>of</strong> specific APC with T cell subsets, and the effects <strong>of</strong> Mycoplasma-mediatedinflammation and mast-cell-mediated regulation upon T cell-APC pairing in lungmicroenvironments.Role: co-PIP01 HL024136 (PI: Caughey) 05/11/10-03/31/14Core BThis Core supports the basic activities <strong>of</strong> the PPG.Role: co-PI142
1R21CA167601 (PI) 05/01/12-03/31/14NIH/NCIDefining the First Hours <strong>of</strong> Lung metastasis using Intravital Live-ImagingThis proposal will apply novel intravital imaging <strong>of</strong> the lung to define the first hours followingthe arrival <strong>of</strong> metastatic cells into the mouse lung. As we know very little about why metastatictumor cells survive in this environment, this represents a major undertaking in determining howto decrease their success.Role: PIR01AI052116 (PI: Krummel) 01/15/12-12/31/17NIHMyosin Motors in T cell Synapse Formation and ActivationThe major goals <strong>of</strong> this project are to analyze MyoIIA regulation during T cell motilityand synapse formation. This includes mutational analyses as well as generation andanalyses <strong>of</strong> knockout animals.Role: PIU54 CA163123-01 (Coussens, Krummel, 09/1/12-08/30/13Van't Veer: multi-PI) (PI (MPI))NIH/NCILeukocyte Biomarkers for Predicting Human Breast Cancer OutcomeThe goal <strong>of</strong> this project is to identify predictive biomarkers in human breast cancer, using genomicpr<strong>of</strong>iling <strong>of</strong> mouse and human breast cancer infiltrates and correlated analyses <strong>of</strong> outcome.Role: PI1R21CA167415-01 (PI) 05/01/12-04/30/14NIH/NCIPhage-Display Technology to Define and Manipulate Breast Tumor StromaThis proposal will utilize rapid phage-display screening methods to identify novel epitopeson tumor stromal cells. Through a rapid evolution and Fc-tagging method, both diagnosticand therapeutic uses <strong>of</strong> these reagents will be assessed. This will provide a rapid pipelinebetween identification <strong>of</strong> key cell types and their characterization and targeting.Role: PICompleted <strong>Research</strong> SupportR01 CA134622 ARRA (PI: Krummel) 07/17/09-06/30/11NIHRegulation <strong>of</strong> T cell Functions by the Breast Cancer MicroenvironmentThe major goals <strong>of</strong> this project areAim 1: Define the Nature <strong>of</strong> the CD8+ T cell response in the microenvironment <strong>of</strong> aSpontaneous Breast Tumor.143
Aim 2: Define the Nature <strong>of</strong> Tumor Sampling APCs (TSAPCs), the phenotype and capacity <strong>of</strong>these cells to activate T cells, and the changes in this population with addition <strong>of</strong> functionalCD8+ T cells.Aim 3: Define the effects <strong>of</strong> CD4 +T cell subsets, particularly regulatory cells, on CD8 activityin vivo and upon the quantity and phenotype <strong>of</strong> TSAPCs.Role: PIFellowship (PI: Krummel) 07/01/05-06/30/10Leukemia & Lymphoma SocietyTumor Suppressors in T cell Synapse Formation and SignalingThe major goals <strong>of</strong> this project areAim 1. We will determine the role <strong>of</strong> Septin9/MSF in T cell synapse development, signaling, andproliferative control.Aim 2. We will determine the role <strong>of</strong> lgl proteins in T cell synapse development, signaling, andproliferative control.Role: PIAAF Fellow (PI: Krummel) 07/01/09-06/30/12American <strong>Asthma</strong> FoundationDirecting Antigens to Specific APC and T cell subsets in the LungThe major goals <strong>of</strong> this project are to screen for conditions that bias antigens towards particularantigen presenting cell populations and then to read out, through imaging and functionalassays, the resulting T cell responses with the aim <strong>of</strong> optimizing regulatory interactionpathways.Role: PI144
BIOGRAPHICAL SKETCHNAMELimin Liu, Ph.D.eRA COMMONS USER NAMELIMINLIUPOSITION TITLEAssociate Pr<strong>of</strong>essorEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDY<strong>University</strong> <strong>of</strong> Science & Technology <strong>of</strong> China B.S. 1986 Biology<strong>University</strong> <strong>of</strong> Missouri, Columbia, MO Ph.D. 1995 Molecular BiologyDuke <strong>University</strong> Medical <strong>Center</strong>Nitric Oxide BiologyPositions1996- 1997 <strong>Research</strong> Associate, Department <strong>of</strong> Animal Sciences, <strong>University</strong> <strong>of</strong>Missouri-Columbia1997- 2004 <strong>Research</strong> Associate, Department <strong>of</strong> Medicine, Division <strong>of</strong> Pulmonary Medicine,Duke <strong>University</strong> Medical <strong>Center</strong>1998-2003 <strong>Research</strong> Associate, Howard Hughes Medical Institute, Duke <strong>University</strong> Medical<strong>Center</strong>2005-2012 Assistant Pr<strong>of</strong>essor, <strong>Sandler</strong> <strong>Center</strong> for <strong>Basic</strong> <strong>Research</strong> in <strong>Asthma</strong>, Department <strong>of</strong>Microbiology and Immunology, <strong>University</strong> <strong>of</strong> California San Francisco2012-Present Associate Pr<strong>of</strong>essor, <strong>Sandler</strong> <strong>Center</strong> for <strong>Basic</strong> <strong>Research</strong> in <strong>Asthma</strong>, Department <strong>of</strong>Microbiology and Immunology, <strong>University</strong> <strong>of</strong> California San FranciscoHonors1989-1990 Scholarship, Division <strong>of</strong> Biological Sciences, <strong>University</strong> <strong>of</strong> Missouri1989-1993 Predoctoral Fellowship, Molecular Biology Program, <strong>University</strong> <strong>of</strong> Missouri1994 New Investigator Award (First Place), Society for the Study <strong>of</strong> Reproduction(Biology <strong>of</strong> Reproduction, Vol. 52, pp. 261)2005 Stewart Trust Cancer <strong>Research</strong> Award2006 <strong>Sandler</strong> Innovative <strong>Research</strong> AwardSelected peer-reviewed publications (in chronological order)1. Wu WY, Zhao YG and Liu L (1989). Trophic effect <strong>of</strong> slow nerve on minced fast musclefrom newly hatched chicks transplanted into three-week chicks. Chin J Physiol Sci 5:95-97.145
2. Wu WY, Zhao YG, Liu L, Lu DX and Tian, WH (1990). The second stage commitment <strong>of</strong>chick muscle satellite cells and its relation to the state <strong>of</strong> innervation. Chin J Physiol Sci6:19-29.3. Roberts RM and Liu L (1996). Interferon-ω. In: Aggarawal BB and Gutterman U, eds.,Human Cytokines: Handbook for <strong>Basic</strong> and Clinical <strong>Research</strong> (II), pp.168-177.4. Liu L, Leaman DW, Bixby JA and Roberts RM (1996). A type 1 ovine interferon withlimited similarity to IFN-α, IFN-ω, and IFN-τ: gene structure, biological properties andunusual species specificity. Biochem Biophys Acta 1294:55-62.5. Liu L and Roberts RM (1996). Silencing <strong>of</strong> the gene for the β-subunit <strong>of</strong> human chorionicgonadotropin by the embryonic transcription factor Oct-3/4. J Biol Chem 271:16683-16689.6. Liu L, Leaman DW and Roberts RM (1996). The interferon-tau genes <strong>of</strong> the giraffe, anonbovid species. J Interferon Cytokine Res 16:949-951.7. Roberts RM, Liu L and Alexenko A (1997). New and atypical families <strong>of</strong> type I interferonsin mammals: comparative functions, structures and evolutionary relationships. Prog NucleicAcid Res Mol Biol 56:287-325.8. Liu L, Leaman DW, Villalta M and Roberts RM (1997). Silencing <strong>of</strong> the gene for thealpha-subunit <strong>of</strong> human chorionic gonadotropin by the embryonic transcription factorOct-3/4. Mol Endocrinol 11:1651-1658.9. Roberts RM, Liu L, Guo Q, Leaman D and Bixby J (1998) The evolution <strong>of</strong> type Iinterferons. J Interferon Cytokine Res 18:805-816.10. Liu L, Leaman DW and Roberts RM (1998). Possible role <strong>of</strong> the transcription factor Oct-3/4in control <strong>of</strong> human chorionic gonadotropin expression. In: D. Carson, ed., EmbryoImplantation: Molecular, Cellular and Clinical Aspects (Serono Symposium), pp.261-270.11. Mannick JB, Hausladen A, Liu L, Hess DT, Zeng M, Miao QX, Kane LS, Gow AJ, StamlerJS (1999). Fas-induced caspase denitrosylation. Science 284:651-654.12. Liu L, Stamler JS. (1999) NO: an inhibitor <strong>of</strong> cell death. Cell Death Differ 6:937-94213. Liu L, Zeng M, Stamler JS. (1999) Hemoglobin induction in mouse macrophages. Proc NatlAcad Sci USA 96:6643-6647.14. Eu JP, Liu L, Zeng M and Stamler JS (2000). An apoptotic model for nitrosative stress.Biochemistry 39:1040-1047.15. Liu L, Zeng M, Hausladen A, Heitman J, Stamler JS (2000). Protection from nitrosativestress by yeast flavohemoglobin. Proc Natl Acad Sci USA 97:4672-4676.16. Ealy AD*, Larson SF*, Liu L*, Winkelman GL, Kubisch HM, Alexnko AP, Bixby JA,Roberts RM. (2001) Polymorphic forms <strong>of</strong> expressed bovine interferon-tau genes: relativetranscript abundance during early placental development, promoter sequences <strong>of</strong> genes andbiological activity <strong>of</strong> protein products. Endocrinology 142:2906-2915. *These authorscontributed equally.17. Liu L, Hausladen A, Zeng M, Loretta Que, Heitman J, Stamler JS (2001). A metabolicenzyme for S-nitrosothiol conserved from bacteria to humans. Nature 410:490-494.18. Matsumoto A, Comatas KE, Liu L, Stamler JS (2003). Screening for nitricoxide-dependent protein-protein interactions. Science 301:657-661.19. Jesus-Berrios MD, Liu L, Nussbaum JC, Cox GM, Stamler JS, Heitman J (2003).Enzymes that counteract nitrosative stress promote fungal virulence. Current Biology13:1963-1968.146
20. Liu L, Yan Y, Zeng M, Zhang J, Hanes MA, Ahearn G, McMahon TJ, Dickfeld T,Marshall HE, Que LG, Stamler JS (2004). Essential roles <strong>of</strong> S-nitrosothiols in vascularhomeostasis and endotoxic shock. Cell 116:617-628.21. Que LG, Liu L, Yan Y, Whitehead GS, Gavett SH, Schwartz DA, Stamler JS (2005).Protection from experimental asthma by an endogenous bronchodilator. Science308:1618-1621.22. Bang IS, Liu L, Vazquez-Torres A, Crouch ML, Stamler JS, Fang FC (2006).Maintenance <strong>of</strong> nitric oxide and redox homeostasis by the Salmonella flavohemoglobinHmp. J Biol Chem 38:28039-28047.23. Foster MW*, Liu L*, Zeng M, Hess DT, Stamler JS (2009). A Genetic Analysis <strong>of</strong>Nitrosative Stress. Biochemistry 48:792–799. *Co-first author.24. Choudhry S, Que LG, Yang Z, Liu L, Eng C, Kim SO, Kumar G, Thyne S, Chapela R,Meade K, Watson HG, LeNoir M, Rodriguez-Santana JR, Rodriguez-Cintron W, Avila PC,Stamler JS, Burchard EG (2010). GSNO Reductase and β2 Adrenergic Receptor Gene-geneInteraction: Bronchodilator Responsiveness to Albuterol. Pharmacogenetics and Genomics20:351-8.25. Wei W, Li B, Hanes MA, Kakar S, Chen X, Liu L (2010). Deficiency <strong>of</strong>S-Nitrosoglutathione Reductase Impairs DNA Repair and Promotes Hepatocarcinogenesis.Science Translational Medicine 2: 19ra13 (PMC3085984).(This paper has been selected and rated by Faculty <strong>of</strong> 1000 as “Must Read”.)26. Yang Z, Wang Z, Doulias P, Wei W, Ischiropoulos H, Locksley RM, Liu L (2010).Lymphocyte development requires S-nitrosoglutathione reductase. J Immunol, 185:6664-6669(PMC3070165).27. Wei W, Yang Z, Tang CH, Liu L (2011). Targeted deletion <strong>of</strong> GSNOR in hepatocytes <strong>of</strong>mice causes nitrosative inactivation <strong>of</strong> O 6 -alkylguanine-DNA alkyltransferase and increasedsensitivity to genotoxic diethylnitrosamine. Carcinogenesis 32:973–977 (PMC3128557).28. Lagoda G, Xie Y, Sezen SF, Hurt KJ, Liu L, Musicki B, Burnett AL (2011). FK506Neuroprotection after Cavernous Nerve Injury is Mediated by Thioredoxin and GlutathioneRedox Systems. J Sexual Medicine 8:3325-3334.29. Na B, Huang ZM, Wang Q, Qi ZX, Tian YJ, Lu CC, Yu JW, Hanes MA, Sanjay Kakara S,Huanga EJ, Ou JHJ, Liu L ‡, *, and Yen TSB ‡,† (2011). Transgenic Expression <strong>of</strong> EntireHepatitis B Virus in Mice Induces Hepatocarcinogenesis Independent <strong>of</strong> Chronic LiverInjury. PLoS One 6:e26240 (PMC3192172).‡ co-senior author; *Corresponding author; † Deceased August 31, 2009.30. Khan O, Headley M, Gerard A, Wei W, Liu L, Krummel MF (2011). Regulation <strong>of</strong> T cellpriming by lymphoid-stromal inducible nitric oxidesynthase. PLoS ONE 6:e26138(PMC3215700).31. Tang CH, Wei W, Liu L (2012). Regulation <strong>of</strong> DNA Repair by S-Nitrosylation. BiochimBiophys Acta 1820:730–735 (PMC3170507; http://dx.doi.org/10.1016/j.bbagen.2011.04.014).32. Ozawa K*, Tsumoto H, Wei W, Tang CH, Komatsubara AT, Kawafune H, Shimizu K, LiuL*, Tsujimoto G (2012). Proteomic analysis <strong>of</strong> the role <strong>of</strong> S-nitrosoglutathione reductase inlipopolysaccharide-challenged mice. Proteomics 12, 2024-2035. *Corresponding author.33. Leung J, Wei W, Liu L (2013). S-nitrosoglutathione reductase deficiency increasesmutagenesis from alkylation in mouse liver. Carcinogenesis (in press).147
34. Tang CH, Wei W, Hanes MA, Liu L (2013). Hepatocarcinogenesis driven by GSNORdeficiency is prevented by iNOS inhibition. Cancer <strong>Research</strong> (in press).<strong>Research</strong> SupportOngoing <strong>Research</strong> Support1 R01 CA122359-01A2 NIH/NCI Liu (PI) 05/01/09-4/30/14Title: Role <strong>of</strong> S-nitroso-glutathione Reductase in Hepatocellular CarcinomaThe goal <strong>of</strong> this study is to determine whether GSNOR protects DNA repair proteins andplays a role in liver cancer.Role: PICompleted1. Innovative <strong>Research</strong> Award Liu (PI) 1/1/06-6/30/08<strong>Sandler</strong> <strong>Center</strong> for <strong>Basic</strong> <strong>Research</strong> in <strong>Asthma</strong>, UCSFTitle: Role <strong>of</strong> GSNOR in <strong>Asthma</strong> (PI)2. Pilot-feasibility funding Liu (PI) 6/1/06-5/31/07UCSF Liver <strong>Center</strong>Title: Potential Role <strong>of</strong> S-nitroso-glutathione Reductase in Liver Tumorigenesis (PI)3. <strong>Research</strong> Award Liu (PI) 7/1/2007-6/30/2008Cancer <strong>Research</strong> Coordinating Committee, UCTitle: Role <strong>of</strong> S-nitroso-glutathione reductase in protection against carcinogenicN-nitrosamines (PI)4. <strong>Research</strong> Contract, N30 Pharmaceuticals Liu (PI) 07/01/09-09/30/10Title: Haplosufficiency <strong>of</strong> S-nitroso-glutathione ReductaseThe goal <strong>of</strong> this study is to test if GSNOR is haplosufficient for protection against nitrosativestress.Role: PI5 R01 CA055578-14S1 NIH/NCI Pulliam & Liu (PI) 09/30/2009-09/29/2011Title: Role <strong>of</strong> PreS2 Mutants in Pathogenesis <strong>of</strong> Chronic Hepatitis BThe goal <strong>of</strong> this study is to test if GSNOR deficiency functions synergistically with PreS2Mutants in the pathogenesis <strong>of</strong> chronic hepatitis BRole: one PI <strong>of</strong> the double-PI team.6. 5P01CA123328 J. Ou (PI) 01/01/2010-05/31/2012Project 2 Title: Hepatic Carcinogenesis Induced By Hepatitis B Virus PreS2 MutantThis project (part <strong>of</strong> a program project based at the <strong>University</strong> <strong>of</strong> Southern California )examines how the preS2 mutant may interact with other HBV proteins and immunological148
factors in causing HCC and the role <strong>of</strong> the various mutant products <strong>of</strong> the preS2 mutant incarcinogenesisRole: PI <strong>of</strong> Project 2.149
BIOGRAPHICAL SKETCHNAMERichard M. Locksley, M.D.eRA COMMONS USER NAMELocksleyPOSITION TITLE<strong>Sandler</strong> Distinguished Pr<strong>of</strong>essor, Department<strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California, SanFranciscoEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYHarvard College, Cambridge, MA B.A. 1970 Biochemistry<strong>University</strong> <strong>of</strong> Rochester, Rochester, NY M.D. 1976 Medicine<strong>University</strong> <strong>of</strong> California, San Francisco, CA 1976-80Resident, ChiefResident<strong>University</strong> <strong>of</strong> Washington, Seattle, WA 1980-83Infectious DiseasesFellowPositions and Honors1986-2003 Chief, Division <strong>of</strong> Infectious Diseases, UCSF Medical <strong>Center</strong>, SanFrancisco, CA1988-93 Member and Chair (1991-93), Tropical Medicine and ParasitologyStudy Section, NIH1991-94 Co-Director, Immunology Section, Biology <strong>of</strong> Parasitism Course,Woods Hole, MA1994-99 Chair, Parasitology Pathogenesis Committee, WHO, Geneva1995-05 Council, Chair (1998), Midwinter Conference <strong>of</strong> Immunologists,Asilomar1995-01 Faculty, Association <strong>of</strong> American Immunology Annual Course,Advanced Immunology1997- Investigator, Howard Hughes Medical Institute, UCSF1998-01 Member, Chair (2000-01), US-Japan Immunology Board, NIH2002-05 Council, NIAID, National Institutes <strong>of</strong> Health2003- Director, Strategic <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>, UCSFEditorial Boards(1999-03) Immunity, J Clin Invest, Immunology & Cell Biology, Annu RevImmunol150
Honors1991 American Society for Clinical Investigation1994 Association <strong>of</strong> American Physicians1992-97 Burroughs Wellcome Fund Scholar in Molecular Parasitology1994 Bailey K Ashford Medal, American Society Tropical Medicine andHygiene2001-05 Ellison Medical Foundation Senior Scholar in Global InfectiousDiseases2005 American Academy <strong>of</strong> Arts & SciencesSelected peer-reviewed publications (in chronological order)1. Grunig G, M Warnock, AE Wakil, R Venkayya, F Brombacher, DM Rennick, DSheppard, M Mohrs, DD Donaldson, RM Locksley, DB Corry. 1998. Requirement forIL-13 independently <strong>of</strong> IL-4 in experimental asthma. Science 282:2261-63.2. Loots GG, RM Locksley, CM Blankespoor, Z-E Wang, W Miller, EM Rubin, KAFrazer. 2000. Identification <strong>of</strong> a coordinate regulator <strong>of</strong> interleukins 4, 13 and 5 bycross-species sequence comparisons. Science 288:136-40.3. Grogan JL, M Mohrs, B Harmon, DA Lacy, JW Sedat, RM Locksley. 2001. Earlytranscription and silencing <strong>of</strong> cytokine genes underlie polarization <strong>of</strong> T helper cellsubsets. Immunity 14:205-15.4. Mohrs M, CM Blankespoor, ZE Wang, GG Loots, V Afzal, H Hadeiba, K Shinkai, EMRubin, RM Locksley. Deletion <strong>of</strong> a coordinate regulator <strong>of</strong> type 2 cytokine expression inmice. Nature Immunol 2:842-7, 2001.5. Mohrs M, K Shinkai, K Mohrs, RM Locksley. Analysis <strong>of</strong> type 2 immunity in vivo witha biscistronic IL-4 reporter. Immunity 15:303-11, 2001.6. Shinkai K, M Mohrs, RM Locksley. 2002. Helper T cells regulate type 2 immunity invivo. Nature 420:825-9.7. Voehringer D, K Shinkai, RM Locksley. 2004. Type 2 immunity reflects orchestratedrecruitment <strong>of</strong> cells committed to IL-4 production. Immunity 20:267-77.8. Mohrs K, AE Wakil, N Killeen, RM Locksley, M Mohrs. 2005. A two-step process forcytokine production revealed by IL-4 dual-reporter mice. Immunity 23:419-29.PMC28263209. Scheu S, DB Stetson, RL Reinhardt, JH Leber, M Mohrs, RM Locksley. 2006.Activation <strong>of</strong> the integrated stress response during T helper cell differentiation. NatureImmunol 7:644-51. PMC262926910. Voehringer D, TA Reese, X Huang, K Shinkai, RM Locksley. 2006. Type 2 immunityis controlled by IL-4/IL-13 expression in hematopoietic non-eosinophil cells <strong>of</strong> the innateimmune system. J Exp Med 203:1435-46. PMC211830211. Reese TA, H-E Liang, AM Tager, AD Luster, N van Rooijen, D Voehringer, RMLocksley. 2007. Chitin induces accumulation in tissue <strong>of</strong> innate immune cellsassociated with allergy. Nature 447:92-6. PMC2527589151
12. Reinhardt RL, H-E Liang, RM Locksley. 2009. Cytokine-secreting follicular T cellsshape the antibody repertoire. Nature Immunol 10:385-93. PMC271405313. Price AE, H-E Liang, BM Sullivan, RL Reinhardt, CJ Eisley, DJ Erle, Grunig G, MWarnock, AE Wakil, R Venkayya, F Brombacher, DM Rennick, D Sheppard, M Mohrs,DD Donaldson, RM Locksley, DB Corry. 1998. Requirement for IL-13 independently<strong>of</strong> IL-4 in experimental asthma. Science 282:2261-63.14. Voehringer D, K Shinkai, RM Locksley. 2004. Type 2 immunity reflects orchestratedrecruitment <strong>of</strong> cells committed to IL-4 production. Immunity 20:267-77.15. Mohrs K, AE Wakil, N Killeen, RM Locksley, M Mohrs. 2005. A two-step process forcytokine production revealed by IL-4 dual-reporter mice. Immunity 23:419-29.MC282632016. Price AE, H-E Liang, BM Sullivan, RL Reinhardt, CJ Eisley, DJ Erle, RM Locksley.2010. Systemically dispersed innate IL-13-producing cells in type 2 immunity. ProcNatl Acad Sci USA 107:11489-94. PMC2895098.17. Ricardo-Gonzalez RR, A Red Eagle, JI Odegaard, H Jouihan, CR Morel, JE Heredia, LMukudan, D Wu, RM Locksley, A Chawla. 2010. IL-4/Stat6 immune axis regulatesperipheral nutrient metabolism and Insulin sensitivity. Proc Natl Acad Sci USA107:22617-22. PMC301250018. Locksley RM. 2010. <strong>Asthma</strong> and allergic inflammation. Cell 140:777-83.PMC313438819. Wu D, AB Mol<strong>of</strong>sky, H-E Liang, RR Ricardo-Gonzalez, HA Jouihan, JK Bando, AChawla, RM Locksley. 2011. Eosinophils sustain adipose alternatively activatedmacrophages associated with glucose homeostasis. Science 332:243-7. PMC314416020. Sullivan BM, H-E Liang, JK Bando, D Wu, LE Cheng, JK McKerrow, CDC Allen, RMLocksley. 2011. Genetic analysis <strong>of</strong> basophil function in vivo. Nat Immunol 12:527-35.PMC3271435Additional Publications (selected from >150 total)1. Amiri P, RM Locksley, TG Parslow, MD Sadick, E Rector, D Ritter, JH McKerrow.1992. Tumor necrosis factor a restores granulomas and induces parasite egg-laying inschistosome-infected SCID mice. Nature 356:604-607.2. Locksley RM, SL Reiner, F Hatam, DR Littman, N Killeen. 1993. Helper T cellswithout CD4: control <strong>of</strong> leishmaniasis in CD4-deficient mice. Science 261:1448-1451.3. Reiner SL, ZE Wang, F Hatam, P Scott, RM Locksley. 1993. Th1 and Th2 cell antigenreceptors in experimental leishmaniasis. Science 259:1457-60.4. Mougneau E, F Altare, AE Wakil, S Zheng, T Coppola, ZE Wang, R Waldmann, RMLocksley, N Glaichenhaus. 1995. Expression cloning <strong>of</strong> a protective Leishmaniaantigen. Science 268:563-566.5. Fowell DJ, J Magram, CW Turck, N Killeen, RM Locksley. 1997. Impaired Th2development in the absence <strong>of</strong> CD4. Immunity 6:559-69.6. Bix M, Locksley RM. 1998. Independent and epigenetic regulation <strong>of</strong> the interleukin 4alleles in CD4+ T cells. Science 281:1352-4.152
7. Bix M., ZE Wang, B Thiel, NJ Schork, RM Locksley. 1998. Genetic regulation <strong>of</strong>commitment to IL-4 production by a CD4 T cell-intrinsic mechanism. J Exp Med188:2289-99.8. Grunig G, M Warnock, AE Wakil, R Venkayya, F Brombacher, DM Rennick, DSheppard, M Mohrs, DD Symula DJ, KA Frazer, Y Ueda, P Denefle, ME Stevens, Z-EWang, RM Locksley, EM Rubin. 1999. Functional screening <strong>of</strong> an asthma QTL inYAC transgenic mice. Nature Genetics 23:241-44.9. Stetson DB, M Mohrs, V Mallet-Designe, L Teyton, RM Locksley. 2002. Rapidexpansion and IL-4 expression by Leishmania-specific naive helper T cells in vivo.Immunity 17:191-200.10. Fowell DJ, K Shinkai, XC Liao, AM Beebe, RL C<strong>of</strong>fman, DR Littman, RM Locksley.1999. Impaired NFATc translocation and failure <strong>of</strong> Th2 development in itk-deficientCD4 T cells. Immunity 11:399-409.11. Voehringer D, DB Rosen, LL Lanier, RM Locksley. 2004. CD200R family membersrepresent novel DAP12-associated activating receptors on basophils and mast cells. JBiol Chem 279:54117-23.12. Kang S-J, H-E Liang, B Reizis, RM Locksley. 2008. Regulation <strong>of</strong> hierarchicalclustering and activation <strong>of</strong> innate immune cells by dendritic cells. Immunity 29:819-33.NIHMS8042213. Voehringer D, D Wu, H-E Liang, RM Locksley. 2009. Efficient generation <strong>of</strong>long-distance conditional alleles using recombineering and a dual selection strategy inreplicate plates. BMC Biotechnol 9:69. PMCID: PMC2724507. 2010.14. Price AE, Liang, H-E, Sullivan BM, Reinhardt, RL, Eisley CJ, Erle, DJ, Locksley RM.2010. Systemically dispersed innate IL-13-producing cells in type 2 immunity. Proc NatlAcad Sci USA 107(25):11489-94. PMC2895098.15. Van Dyken SJ, D Garcia, P Porter, X Huang, PJ Quinlan, PD Blanc, DB Corry, RMLocksley. 2011. Fungal chitin from asthma-associated home environments induceseosinophilic lung infiltration. J Immunol 187:2261-2267. PMC315972516. Nguyen KD, Y Qui, X Cui, YP Sharon Goh, J Mwangi, T David, L Mukundan, FBrombacher, RM Locksley, A Chawla. 2011. Alternatively activated macrophagesproduce catecholamines to sustain adaptive thermogenesis. Nature 480:104-108.PMC337176117. Liang H-E, RL Reinhardt, JK Bando, BM Sullivan, I-C Ho, RM Locksley. 2011.Divergent expression patterns <strong>of</strong> IL-4 and IL-13 define unique functions in allergicimmunity. Nat Immunol 13:58-66. PMC324293818. Gordon E, S Sidhu, Z-E Wang, P Woodruff, S Yuan, M Solon, S Conway, X Huang, RMLocksley, J Fahy. 2012. A protective role for periostin and TGF-b in IgE-mediatedallergy and airway hyperresponsiveness. Clin Exp Allergy 42:144-155. PMC327179219. Thornton EE, MR Looney, O Bose, D Sen, D Sheppard, RM Locksley, X Huang, MFKrummel. 2012. Spatiotemporally separated antigen uptake by alveolar dendritic cellsand airway presentation to T cells in the lung. J Exp Med 209:1183-1199. PMC337173020. Price AE, RL Reinhardt, H-E Liang, RM Locksley. 2012. Marking and quantifyingIL-17A-producing cells in vivo. PLoS ONE 7:e39750. PMC3387253153
21. Dickgreber N, KJ Farrand, N van Panhuys, DA Knight, ML Chong, SMiranda-Hernandez, AG Baxter, RM Locksley, G Le Gros, IF Hermans. 2012.Immature murine NKT cells pass through a stage <strong>of</strong> developmentally programmed innateIL-4 secretion. J Leukoc Biol (in press)22. Cheng LE, K Hartmann, A Roers, MF Krummel, RM Locksley. 2013. Perivascularmast cells dynamically probe cutaneous blood vessels to capture IgE. Immunity38:166-175. PMC in process23. Oghumu S, R Dong, S Varikuti, T Shawler, T Kampfrath, CA Terrazas, CLezama-Davila, BMM Ahmer, CC Whitacre, S Rajagopalan, R Locksley, AH Sharpe,AR Sataskar. 2013. Distinct populations <strong>of</strong> innate CD8 T cells revealed in a CXCR3reporter mouse. J Immunol (in press).24. Mol<strong>of</strong>sky AB, JC Nussbaum H-E Liang, SJ Van Dyken, LE Cheng, A Mohapatra, AChawla, RM Locksley. 2013. Innate lymphoid type 2 cells ILC2) sustain visceraladipose tissue eosinophils and alternatively activated macrophages. J Exp Med (inpress). PMC in process25. Van Dyken SJ, RM Locksley. 2013. Interleukin-4- and interleukin-13-mediatedalternatively activated macrophages: roles in homeostasis and disease. Annu RevImmunol 31:317-43. PMC in process26. Spits H, D Artis, M Colonna, A Diefenbach, JP Di Santo, G Eberl, S Koyasu, RMLocksley, ANJ McKenzie, RE Mebius, F Powrie, E Vivier. 2013. Innate lymphoid cells– a proposal for a uniform nomenclature. Nature Rev Immunol 13:145-149. PMC inprocess27. Heredia JE, L mukundan, FM Chen, AA Mueller, R Deo, RM Locksley, TA Radno, AChawla. 2013. Type 2 innate signals regulate functionality <strong>of</strong> fibro/adipogenicprogenitors to facilitate muscle regeneration. Cell (in press).<strong>Research</strong> SupportActiveNot assigned Locksley (PI) 10/97-9/12 (budgeted annually)Howard Hughes Medical InstituteActivation <strong>of</strong> ImmunityThe major goals <strong>of</strong> this project are to provide new strategies to optimize host defense andvaccines and to treat pathologic immune responses associated with autoimmunity and allergy.Support from HHMI pays Dr. Locksley's salary.R37 AI26918 Locksley (PI) 7/88-3/16NIH/NIAIDParasite immunity orchestrated by Th2 cellsThe major goal <strong>of</strong> this project is to identify the role <strong>of</strong> basophils and eosinophils required forimmunity to parasitic helminths.154
RO1 AI30663 Locksley (PI) 6/08-5/13NIH/NIAIDInitiation <strong>of</strong> allergic immunity by parasitesThe major goals <strong>of</strong> this grant are to understand the mechanism by which chitin in helminthescontributes to eosinophilic inflammation in tissues in response to migrating organisms and eggs.U19 AI077439 Sheppard (PI) 3/08-2/13NIH/NIAIDMechanisms <strong>of</strong> initiation and persistence <strong>of</strong> allergic asthmaThe major goals <strong>of</strong> this grant are to understand mechanisms <strong>of</strong> allergic lung inflammationinduced by fungi in murine models and human studies.Role: PI Subproject 1P01 AI078869 DeFranco (PI) 7/08-6/13NIH/NIAIDCross-talk between innate and adaptive immune cells in inflammation and autoimmunityThe major goals are to assess the role <strong>of</strong> innate signaling pathways in the induction <strong>of</strong> mucosalresponses to pathogens.Role: PI Subproject 4P01 AI119944 Fahy (PI) 7/12-6/12NIH/NHLBIInnate and Adaptive Immune Responses in TH2-High <strong>Asthma</strong>Project 1: Innate Helper Type-2 Cells in Allergic LungThe goal <strong>of</strong> this project is to focus on the role <strong>of</strong> iH2 cells as proximal regulators <strong>of</strong> TH2inflammation in the airway. This project proposes to characterize markers for these cells,delineate their role in allergic airway responses and collaborate with investigators in Project 3 toadvance understanding <strong>of</strong> iH2 cells in human asthma.Larry L. Hillblom <strong>Center</strong> for the immunobiology <strong>of</strong> type 2 diabetes(Chawla PI, Locksley co-PI) 1/09-12/12The goal <strong>of</strong> this project is to understand the inferface between immune cell activation andmetabolic disorders.Completed5-2008-214 Locksley (PI) 12/07-12/08Juvenile Diabetes <strong>Research</strong> Foundation Innovative GrantsFunctional immune cell activation in type 1 diabetesThe major goal <strong>of</strong> this project was to use mice with marker alleles in informative cytokine genesto identify evidence for functionally important cytokine elaboration in mediating peripheralinsulin sensitivity.155
BIOGRAPHICAL SKETCHNAMEWilliam E. Seaman, M.D.eRA COMMONS USER NAMEBSEAMANPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> Medicine and <strong>of</strong> Microbiology andImmunology, UCSFEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYPrinceton <strong>University</strong>, Princeton, NJ A.B. 1964 EnglishHarvard Medical School, Boston, MA M.D. 1969 MedicineMassachusetts General Hospital, Boston, MA Resident 1969-1971 Internal MedicineArthritis and Rheumatism Branch, NIAMDD,NIH Bethesda, MDFellow 1971-1974Immunology andRheumatologyMassachusetts General Hospital, Boston, MAChiefResident1974-1975 MedicineMassachusetts General Hospital, Boston, MA Fellow 1976 RheumatologyPositions and HonorsAcademic Positions1976 - 1984 Assistant Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California San Francisco1978 - Present Staff Physician, San Francisco VA Medical <strong>Center</strong>1981 - 1992 Chief, Arthritis/Immunology Section, San Francisco VA Medical <strong>Center</strong>1984 - 1988 Associate Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California, San Francisco1988 - Present Pr<strong>of</strong>essor <strong>of</strong> Medicine and <strong>of</strong> Microbiology and Immunology,<strong>University</strong> <strong>of</strong> California San Francisco1992 - 1999 Chief, Medical Service, San Francisco VA Medical <strong>Center</strong>1999 - Present Chief, Immunology Section, San Francisco VA Medical <strong>Center</strong>Other Recent Positions1999 - Present <strong>Research</strong> Director, American <strong>Asthma</strong> Foundation1999 - 2003 NIH Study Section, Experimental Immunology2000 - 2008 Director, Macrophage Biology Laboratory, Alliance for Cellular Signaling2002 - 2005 President, Society for Natural Immunity2011 – Present Associate Chair <strong>of</strong> Medicine for <strong>Research</strong>, UCSF156
Honors1964 AB cum laude1969 MD cum laude2007 Master, American College <strong>of</strong> RheumatologyMedical and <strong>Research</strong> Society Memberships and Board Certifications1973 to Present American College <strong>of</strong> Rheumatology1974 American Board <strong>of</strong> Internal Medicine1978 American Board <strong>of</strong> Rheumatology1979 to Present American Federation for Clinical <strong>Research</strong>1980 to Present American Association <strong>of</strong> Immunologist1984 to Present American Society for Clinical Investigation1994 to Present American Association <strong>of</strong> Physicians1998 to Present Society for Natural Immunity2001 to Present American Association for Cancer <strong>Research</strong>2007 to Present International Bioiron Society2007 to Present International Society <strong>of</strong> NeuroimmunologyEditorships1985-1989 Associate Editor, Journal <strong>of</strong> Immunology1989-1993 Section Editor, Journal <strong>of</strong> Immunology1992-1997 Consulting Editor, Journal <strong>of</strong> Clinical Investigation2005 to Present Faculty <strong>of</strong> 100015 Selected Peer-Reviewed Publications (<strong>of</strong> 93)1. W<strong>of</strong>sy D, Seaman WE: Successful treatment <strong>of</strong> autoimmunity in NZB/NZW mice withmonoclonal antibody to L3T4. J Exp Med 161:378-391, 1985.2. Seaman WE, Eriksson E, Dobrow R, Imboden JB: Inositol trisphosphate is generated by arat natural killer cell tumor in response to target cells or to cross-linked monoclonalantibody OX-34: possible signaling role for the OX-34 determinant during activation bytarget cells. Proc Natl Acad Sci USA 84:4239-4243, 1987.3. Seaman WE, Niemi EC, Stark MR, Goldfien RD, Pollock AS, Imboden JB: Molecularcloning <strong>of</strong> gp42, a cell-surface molecule that is selectively induced on rat natural killer cellsby interleukin-2: Glycolipid membrane anchoring and capacity for transmembranesignaling, J Exp Med. 173:251-260, 1991.4. Yokoyama WM, Ryan JC, Hunter JJ, Smith HMC, Stark M, Seaman WE. cDNA cloning<strong>of</strong> mouse NKR-P1 and genetic linkage with Ly-49: Identification <strong>of</strong> a natural killer genecomplex on mouse chromosome VI. J Immunol 147:3229, 1991.157
5. Ryan JC, Turck J, Niemi EC, Yokoyama WM, Seaman WE. Molecular cloning <strong>of</strong> theNK1.1 antigen, a member <strong>of</strong> the NKR-P1 family <strong>of</strong> natural killer cell activation molecules.J Immunol. 149:1631-1635, 1992.6. Ryan JC, Niemi EC, Nakamura MC, Seaman WE. NKR-P1A is a target-specific receptorthat activates natural killer cell cytotoxicity. J Exp Med 181:1911-1915, 1995.7. Nakamura, MC, Linnemeyer, PA, Niemi EC, Mason L, Ortaldo JR, Ryan JC, Seaman WE.Mouse Ly-49D recognizes H-2Dd and activates natural killer cell cytotoxicity. J Exp Med,189:493-500, 1999.8. Nakamura MC, Hayashi S, Niemi EC, Ryan JC, Seaman, WE. Activating Ly-49D andinhibitory Ly-49A NK cell receptors demonstrate distinct requirements for interaction withH2-Dd. J Exp Med 7:192:447-54, 2000.9. Daws MR, Sullam PM, Niemi EC, Chen TT, Seaman WE. Pattern recognition byTREM-2: binding <strong>of</strong> anionic ligands. J Immunol. 171:594-9, 2003.10. Chen TT, Li L, Chung D-H, Allen CDC, Torti SV, Torti FM, Cyster JG, Chen C, Brodsky,FM, Niemi EC, Nakamura MC, Seaman WE, Daws MR. TIM-2 is expressed on B cellsand in liver and kidney and it is a receptor for H ferritin endocytosis. J Exp Med.202:955-65, 2005.11. Roach TI, Rebres RA, Fraser ID, Decamp DL, Lin KM, Sternweis PC, Simon MI, SeamanWE. Signaling and cross-talk by C5a and UDP in macrophages selectively use PLCbeta3 toregulate intracellular free calcium. J Biol Chem 283(25):17351-61, 2008.12. N'Diaye EN, Branda CS, Branda SS, Nevarez L, Colonna M, Lowell C, Hamerman JA,Seaman WE. TREM-2 (triggering receptor expressed on myeloid cells 2) is a phagocyticreceptor for bacteria. J Cell Biol 184(2):215-23, 2009.13. Hsieh CL, Koike M, Spusta SC, Niemi E, Yenari M, Nakamura MC, and Seaman WE. Arole for TREM2 ligands in the phagocytosis <strong>of</strong> apoptotic neuronal cells by microglia. JNeurochem 109(4):1144-1156, 2009.14. Li L, Fang CJ, Ryan JC, Niemi EC, Lebrón JA, Björkman PJ, Arase H, Torti FM, Torti SV,Nakamura MC, Seaman WE. Binding and uptake <strong>of</strong> H-ferritin is mediated by humantransferrin receptor-1. Proc Natl Acad Sci USA. Proc Natl Acad Sci USA 107(8):3505-10,2010.15. Rebres, RA, Roach TIA, Fraser IDC, Philip F, Moon C, Lin KM, Liu J, Santat L, Cheadle L,Ross EM, Simon MI, Seaman WE. Synergistic Ca 2+ responses by Ga i - and Gaq-coupledG-protein-coupled receptors require a single PLCb is<strong>of</strong>orm that is sensitive to both Gbg andGaq. J Biol Chem 286:1-10, 2011<strong>Research</strong> SupportOngoing <strong>Research</strong> SupportTitle: The Role <strong>of</strong> Microglial Subsets in Regulating Brain InjuryAgency/Type: Department <strong>of</strong> Defense PT075679 PI: WE Seaman 7/1/06 to 6/30/2012Role: Principal InvestigatorProgram: These studies examine the role <strong>of</strong> microglial subsets in the response to TBI. Theyexamine the hypothesis that microglia, like macrophages may be divided into pro-inflammatory158
vs. reparative subsets, and that injury following TBI may be improved by driving microglia fromthe former to the latter. Key persons are Christine Hsieh, a postdoctoral fellow, my colleague,Mary Nakamura, MD, and Jialing Liu, PhD, a colleague in Neurosurgery who is expert in TBI.Ongoing Mentored GrantTitle: The role <strong>of</strong> CCR2 and Macrophages in Traumatic Brain InjuryAgency/Type: Department <strong>of</strong> Veterans Affairs PI: Christine Hsieh 1/1/2011 to 12/31/2013Role: MentorProgram: This is a VA Career Development Award to Christine Hsieh, PhD, a postdoctoralfellow in my laboratory for whom I am the mentor on this award. Dr. Hsieh has been studyingtraumatic brain injury as part <strong>of</strong> my DoD grant (above). As part <strong>of</strong> this work, she showed thatTBI results in an influx <strong>of</strong> macrophage to the brain, and that this influx is primarily dependent onthe chemokine receptor, CCR2. This grant has allowed her to study the functional consequences<strong>of</strong> this response and to develop as an independent investigator. Key persons in addition to Dr.Hsieh and myself include our colleague, Mary Nakamura.Completed support (last 3 years)Title: Role <strong>of</strong> the Tim-2 Receptor in Immunity and AutoimmunityAgency/Type: NIH RO1 AI061164-01A1 Seaman (PI) 7/1/05 to 3/31/11Role: Principal InvestigatorProgram: These studies examined the role in immunity and autoimmunity <strong>of</strong> TIM-2, a receptorexpressed on mouse lymphocytes and on liver cells and renal tubule cells159
BIOGRAPHICAL SKETCHNAMEDean Sheppard, M.D.eRA COMMONS USER NAMEsheppardPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> MedicineEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYHarvard College, Cambridge, MA AB 6/72SUNY at Stony Brook, Stony Brook, NY MD 6/75 Medicine<strong>University</strong> <strong>of</strong> Washington, Seattle, WA Resident 7/75-6/78 Internal Medicine<strong>University</strong> <strong>of</strong> California, San Francisco, San Fellow 7/78-6/81 PulmonaryFranciscoPositions2009-Present Chief, Pulmonary, Critical Care, Allergy and Sleep Division, UCSF1986-Present Director, Lung Biology <strong>Center</strong>, <strong>University</strong> <strong>of</strong> California, San Francisco1999-2004 Acting Director, <strong>Sandler</strong> <strong>Basic</strong> <strong>Asthma</strong> <strong>Research</strong> <strong>Center</strong>, UCSF1981-1987 Assistant Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California, San Francisco1987-1992 Associate Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California, San Francisco1992-Present Pr<strong>of</strong>essor <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California, San Francisco1997-2009 Associate Chair for Biomedical <strong>Research</strong>, Department <strong>of</strong> Medicine, UCSFOther ExperienceMember, NHLBI Program Project Review Committee, 1998-2002, Chair 2000-2002Member, Lung Injury and Repair Study Section, 2004-2008, Chair 2006-2008Scientific Advisory Board, Parker B. Francis Foundation 2006-2009Editorial Board, Journal <strong>of</strong> Clinical Investigation 2003-presentEditorial Board, Clinical and Translational Science 2008-presentAssociate Editor, American Journal <strong>of</strong> Respiratory Cell and Molecular Biology 1995-2002Editorial Board, American Journal <strong>of</strong> Physiology; Lung Cell and Molecular Biology 1996-2007Chair, Oversight Committee, NHLBI Lung Tissue Consortium, 2004-presentHonors and AwardsElected Member, American Society for Clinical Investigation, 1992Elected Member, Association <strong>of</strong> American Physicians, 1995160
Clean Air Award, American Lung Association <strong>of</strong> California, 1995Parker B. Francis Lecturer, Aspen Lung Conference, 1996Lifetime Scientific Achievement Award, American Thoracic Society, 1998Jerome I. Flance Visiting Pr<strong>of</strong>essor, Washington <strong>University</strong>, 2000Roger Mitchell Lecturer, Aspen Lung Conference, 2001NIH Merit Award, 2004-2014Robert Johnston Lecturer, Drexel <strong>University</strong>, 2005McClement Lecturer, New York <strong>University</strong>, 2006Kass Medal, <strong>University</strong> <strong>of</strong> Nebraska, 2007Amberson Lecturer, American Thoracic Society, 2010Selected Relevant Publications (from a total 252)1. Grunig G, Warnock M, Wakil AE, Venkayya R, Brombacher F, Rennick DM, SheppardD, Mohrs M, Donaldson DD, Locksley RM, Corry DB. Requirement for IL-13independently <strong>of</strong> IL-4 in experimental asthma. Science 1998; 282:2261-3.PMID:98569502. Munger JS, Huang XZ , Kawakatsu H , Griffiths MJD, Dalton SL, Wu JF, Pittet JF,Kaminiski N, Garat C, Matthay MA, Rifkin DB, Sheppard D. The integrin avb6 bindsand activates latent TGFb1: a mechanism for regulating pulmonary inflammation andfibrosis. Cell 1999; 96:319-328.PMID:100253983. Kaminski N, Allard J, Pittet J-F, Zuo F, Griffiths MJD, Morris D, Huang XZ, SheppardD, Heller RA. Global analysis <strong>of</strong> gene expression in pulmonary fibrosis reveals distinctprograms regulating lung inflammation and remodeling. Proc Nat Acad Sci 2000;97:1778-1783.PMID:106775344. Pittet J-F, Griffiths MJD, Geiser T, Kaminski N, Dalton SL, Huang X Brown LAS,Gotwals PJ, Koetiansky VE, Matthay MA, Sheppard D. TGFβ is a critical mediator <strong>of</strong>acute lung injury. J. Clin Invest 2001; 107:1529-1536.5. Morris DG, Huang X, Kaminski N, Wang Y, Shapiro SD, Dolganov G, Glick, A,Sheppard D. Loss <strong>of</strong> integrin avb6-mediated TGFb activation causes Mmp12-dependentemphysema. Nature 2003 422:169-173. PMID: 126347876. Chen C, Young BA, Coleman CS, Pegg AE, Sheppard D. Spermidine/SpermineN 1 -Acetyltransferase specifically binds to the integrin α9 subunit cytoplasmic domainand enhances cell migration J Cell Biol 2004 167:161-170. PMID: 154797427. Vlahakis NE, Young BA, Atakilit A, Hawkridge AE, Issaka RB, Boudreau N, SheppardD. Integrin alpha 9beta 1 directly binds to vascular endothelial growth factor (VEGF)-Aand contributes to VEGF-A induced angiogenesis. J Biol Chem. 2007 282:15187-15196.PMID: 173633778. Jenkins RG, SuX, Su G, Scotton CJ, Camerer E, Laurent GJ, Davis JE, Chambers RC,Matthay MA, Sheppard D. Ligation <strong>of</strong> the protease-activated receptor-1 induces αvβ6integrin-dependent TGFβ activation and promotes acute lung injury. J. Clin Invest 2006116:1606-1614.9. Travis MA, Reizis B, Melton AC Masteller E, Tang Q, Proctor J, Wang Y, Bernstein X,Huang X, Riechardt L, Bluestone J, Sheppard D. Loss <strong>of</strong> integrin αvβ8 on dendritic161
cells causes autoimmunity and colitis in mice. Nature 2007 449:361-365.PMID:1769404710. Su G, Hodnett M, Wu N, Atakilit A, Kosinski C, Godzich M, Huang XZ, Kim K, FrankJA, Matthay MA, Sheppard D*, Pittet J-F*. Integrin avb5 regulates lung vascularpermeability and pulmonary endothelial barrier function Am J Respir Cell Mol Biol 200736:377-86 (*denotes equal contributions) PMID:1707977911. Ganter MT, Roux J, Miyazawa B, Howard M, Frank JA, Su G, Sheppard D, VioletteSM, Weinreb PH, Horan GS, Matthay MA, Pittet JF. Interleukin-1β causes acute lunginjury via αvβ5 and αvβ6 integrin-dependent mechanism Circ Res 2008 102:804-12.12. Atabai K, Jame S, Kuo A, Lam M, Dehart G, Xia DD, Azhar N, Werb Z, Sheppard D.Mfge8-mediated collagen endocytosis limits the severity <strong>of</strong> fibrotic disease J Clin Invest2009 219:3713-22. PMCID: PMC278680413. Melton A, Bailey-Bucktrout SL, Travis MA, Fife BT, Bluestone JA, Sheppard D. avb8integrin on dendritic cells regulates Th17 cell development and experimental autoimmuneencephalomyelitis in mice. J Clin Invest 2010. 120:4436-44. PMCID: PMC299359514. Hogmalm A, Sheppard D, Lappalainen U, Bry K. Beta6 integrin subunit deficiencyalleviates lung injury in a mouse model <strong>of</strong> bronchopulmonary dysplasia. Am J Respir CellMol Biol 2010 43:88-98. PMCID: PMC291157315. Spassov DS, Wong CH, Sergina N, Ahuja D, Fried M, Sheppard D, Moasser MM. Asrc-driven phosphotyrosine signaling switch determines the anchorage state <strong>of</strong> epithelialcells. Mol Cell Biol 2011. 31:776-82. PMCID: PMC302865316. Su G, Atakilit A, Li T. J, Wu N, Bhattacharya M, Zhu J, Li E, Sun S, Chen R, Su C,Sheppard D. Integrin αvβ3 prevents vascular leak in mice by regulating cortical actinformation in endothelial cells. Am J Resp Crit Care Med 2012 (in press)17. Kudo M, Melton AC, Chen C, Engler M, Huang KE, Ren X, Wang Y, Bernstein X, Li J,Atabai K, Huang X, Sheppard D. IL-17A produced by ab T cells drives airway smoothmuscle contraction. Nature Medicine 2012 (in press).18. Giacomini M, Travis M, Sheppard D. Epithelial cells utilize cortical actin/myosin toactivate latent TGF-β through integrin αvβ6-dependent physical force. Exp Cell Res 2012318:716-22. PMID: 22309779.19. Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, Wang Y, RaymondWW, Erle D, Abrink M, Caughey GH, Huang X, Sheppard D. The avb6 integrinmodulates airway hyperresponsiveness by regulating intra-epithelial mast cells. J ClinInvest 2012 (in press).20. Chen C, Kudo M, Rutaganira F, Takano H, Lee C, Atakilit A, Robinett, KS, Uede T,Wolters P, Shokat KM, Huang X, Sheppard D. Integrin alpha9beta1 in airway smoothmuscle regulates a novel brake on exaggerated murine and human airway narrowing JClin Invest 2012 122:2916-27 PMID 22772469.21. Bhattacharya M, Su G, Su X, Oses-Prieto JA, Li JT, Huang X, Hernandez H, Atakilit A,Burlingame AL, Matthay M, Sheppard D. IQGAP1 is necessary for pulmonary vascularbarrier protection in murine acute lung injury and pneumonia Am J Physiol: Lung Celland Mol Biol 2012 303:L12-19 PMID: 22561460.162
<strong>Research</strong> SupportOngoingR01 HL102292 (Sheppard) 12/03/10-11/30/14NIHIntegrin-mediated Regulation <strong>of</strong> Airway Smooth MuscleThe major goals <strong>of</strong> this project are to determine the mechanisms by which the alpha9beta1integrin inhibits the sensitivity <strong>of</strong> airway smooth muscle to contraction induced by agonists <strong>of</strong> Gprotein coupled receptors.R37 HL53949 (Sheppard)4/1/04-3/31/14 (merit award)NIHIn Vivo Functions <strong>of</strong> Pulmonary IntegrinsThe major goals <strong>of</strong> this project are to determine the roles <strong>of</strong> TGFβ1 activation in the induction <strong>of</strong>lung inflammation and protection from pulmonary fibrosis in integrin β6 subunit null mice.U19 AI077439 (Sheppard) 3/1/08-2/28/13NIH/NIAID-Project 1 and Core AMechanisms <strong>of</strong> Initiation and Pesistence <strong>of</strong> Allergic <strong>Asthma</strong>The major goals <strong>of</strong> this project are to determine the roles <strong>of</strong> integrin-mediated TGFβactivation in regulating auto-immunity, regulatory T cells and airway hyperresponsivenessafter chronic allergen challenge in mice.Scleroderma <strong>Research</strong> Foundation (Sheppard) 5/1/12-4/30/13Role <strong>of</strong> αv integrins in SclerodermaThe goals <strong>of</strong> this project are to determine whether specific integrins expressed on epithelialcells or my<strong>of</strong>ibroblasts could be effective therapeutic targets for treatment <strong>of</strong> SclerodermaP01 HL108794 (Sheppard) 07/01/2012–6/30/17NIH/NHLBI- Project 2 and Core ATargeting Epithelial Cells to Treat Pulmonary FibrosisOverall project goal: This grant will address two critical needs - developing effective treatmentsfor pulmonary fibrosis and better ways to determine if drugs are actually hitting their targets. Bytargeting specific well-defined pathways, modifying drugs for delivery into the airways, andidentifying markers <strong>of</strong> drug efficacy from readily available sites, we hope to dramaticallyimprove current approaches to treatment <strong>of</strong> pulmonary fibrosis.T32 HL007185 (Sheppard) 07/01/2012–6/30/17NIH/NHLBIMultidisciplinary training program in lung diseaseRole: Program Co-PI163
Overall project goal: This is a training grant to train future leaders in basic, clinical andtranslational pulmonary scienceRecently completedR01 HL083950 (Sheppard) 4/1/06-3/31/11NIH/NHLBIRegulations <strong>of</strong> Pulmonary Vascular Permeability by Integrin AlphaVbeta5The major goals <strong>of</strong> this project were: 1) To identify the pathways by which αvβ5 facilitatedRhoA activation and contributed to pulmonary vascular permeability. 2) To determine howthe integrin β5 subunit contributed to the formation <strong>of</strong> multi-protein signaling complexesthat regulated endothelial permeability. 3) To determine whether the pathways examined inaims 1 and 2 were broadly important in in vivo models <strong>of</strong> non-cardiogenic pulmonaryedema.U19 AI077439-02S1 (Sheppard) 8/15/09-7/31/11NIH/NIAID (Administrative Supplement Award)Mechanisms <strong>of</strong> Initiation and Persistence <strong>of</strong> Allergic <strong>Asthma</strong>This administrative supplement to our U19 grant had three goals – to develop an assay formeasurement <strong>of</strong> environmental chitin, to replace our outdated tissue processor and processour backlog <strong>of</strong> fixed murine and human tissues, and to develop multi-plexed, bead basedassays for measurements <strong>of</strong> multiple secreted proteins in small volume samples from humanand murine airways.R01 AI024674 (Sheppard) 3/1/06-2/28/10NIH/NIAIDNovel Leukocyte IntegrinsThe major goal <strong>of</strong> this project was to understand how glycan phosphoatidly inositolanchored proteins influenced the function <strong>of</strong> Fc and complement receptors on macrophagesand dendritic cells.(Sheppard) 6/8/09-12/31/11<strong>University</strong> <strong>of</strong> Edinburgh (Subcontract)The Role <strong>of</strong> the Alphavbeta8 Integrin in Hepatic Inflammation and FibrosisThe subcontract is to support a postdoctoral fellow, Neil Henderson, to work in Dr.Sheppard’s laboratory. Dr. Henderson was working on the mechanisms <strong>of</strong> activation <strong>of</strong>TGF beta in the liver.The major goals <strong>of</strong> this project are to determine the mechanisms by which the alpha9beta1integrin inhibits the sensitivity <strong>of</strong> airway smooth muscle to contraction induced by agonists <strong>of</strong> Gprotein coupled receptors.164
BIOGRAPHICAL SKETCHNAMEJeoung-Sook Shin, Ph.D.eRA COMMONS USER NAMESHINJSPOSITION TITLEAssistant Pr<strong>of</strong>essorEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYSeoul National <strong>University</strong>, Seoul, Korea BS 1988-1993 ChemistrySeoul National <strong>University</strong>, Seoul, Korea MS 1993-1995 BiochemistryDuke <strong>University</strong>, Durham, NC Ph.D. 1997-2002 PathologyDuke <strong>University</strong>, Durham, NC Postdoc 2002-2003 PathologyYale <strong>University</strong>, New Haven, CT Postdoc 2003-2008 Cell BiologyPr<strong>of</strong>essional Positions1996 <strong>Research</strong> Associate, Cheong-Am Biotech, Seoul, Korea2008 Assistant Pr<strong>of</strong>essor, <strong>University</strong> <strong>of</strong> California San Francisco, Dept. <strong>of</strong>Microbiology Immunology & <strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> <strong>Research</strong> <strong>Center</strong>Honors and Awards1999 The Best <strong>Research</strong> Student Award in the Department <strong>of</strong> Pathology9 th Graduate Student Symposium, Duke <strong>University</strong>2004 The Jane C<strong>of</strong>fin Childs Memorial Fund <strong>Research</strong> Fellowship Award2009 Strategic Innovative Award in <strong>Asthma</strong> <strong>Research</strong>2009 Cancer <strong>Research</strong> Institute Investigator AwardPr<strong>of</strong>essional Memberships2008 - 2009 American Thoracic Society, member2010 American Society <strong>of</strong> Cell Biology, member2011 - Present American Association <strong>of</strong> Immunologists, member165
4. Locksley, R.M., Reiner, S.J., Sadick, M.D., Wang, Z., Heinzel, H.P. andHoladay, B.J.: Evidence for restricted V-D-Jß T cell receptor usage in the Th2response to Leishmania major. 1991 FASEB J. 5:A1369.5. Reiner SL, S Zheng, Z Wang, L Stowring, RM Locksley. 1994. Leishmaniapromastigotes evade IL-12 induction by macrophages and stimulate a broad range <strong>of</strong>cytokines from CD4 cells during initiation <strong>of</strong> infection. J Exp Med 179:447-56.6. Loh, E., Wang, M., Wang, Z., Hyjek, E., and Kozbor, D.: Expression functionalg/d T cell receptor recognize tetanus toxin. J <strong>of</strong> Cellular Biochemistry. 1992 165(D):67.7. Kozbor, D., Hyjek, E., Wiaderkiewicz, R., Wang, Z., Wang, M. and Loh, E.:Competitor mRNA fragments for quantitation <strong>of</strong> cytokine specific transcripts in celllysates. Molecular Immunology, 1993. 30(1):1.8. Reiner SL, Z Wang, F Hatam, P Scott, RM Locksley. 1993. Th1 and Th2 cellantigen receptors in experimental leishmaniasis. Science 259:1457-60.9. Wang Z, SL Reiner, F Hatam, FP Heinzel, J Bouvier, CW Turck, RMLocksley. 1993. Targeted activation <strong>of</strong> CD8 cells and infection <strong>of</strong>b2-microglobulin-deficient mice fail to confirm a primary protective role forCD8 cells in experimental leishmaniasis . J Immunol 151:2077-86.10. Reiner SL, S Zheng, Z Wang, L Stowring, RM Locksley. 1994. Leishmaniapromastigotes evade IL-12 induction by macrophages and stimulate a broad range <strong>of</strong>cytokines from CD4 cells during initiation <strong>of</strong> infection. J Exp Med 179:447-56.11. Wang ZE, SL Reiner, S Zheng, D Dalton, RM Locksley. 1994. CD4+ effector cellsdefault to the Th2 pathway in IFN-g-deficient mice. J Exp Med 179:1367-71.12. Wang, Z., Zheng S., Corry D.B., Dalton, D.K., Seder, R.A., Reiner, S.L., andLocksley, R.M.: Interferon-g-dependent effects <strong>of</strong> interleukin-12 administered duringacute or established infection due to Leishmania major. Proc. Natl. Acad. Sci. USA.1994 91(24):12932-6.13. Mougneau E, F Altare, AE Wakil, S Zheng, T Coppola, ZE Wang, R Waldmann,RM Locksley, N Glaichenhaus, N. 1995. Expression cloning <strong>of</strong> a protectiveLeishmania antigen. Science 268:563-6.14. Wakil AE, ZE Wang, RM Locksley. 1996. Leishmania major: targeting IL-4 insuccessful immunomodulation <strong>of</strong> murine infection. Exp Parasitol 84:214-22.15. Pingel S, ZE Wang, RM Locksley. 1998. Distribution <strong>of</strong> protein kinase C is<strong>of</strong>ormsafter infection <strong>of</strong> macrophages with Leishmania major. Infect Immun 66:1795-9.16. Wakil AE, ZE Wang, JC Ryan, DJ Fowell, RM Locksley. 1998. Interferon gammaderived from CD4(+) T cells is sufficient to mediate helper cell type 1 development.J Exp Med 188:1651-6.17. Bix, M, ZE Wang, B Thiel, NJ Schork, RM Locksley. 1998. Genetic regulation <strong>of</strong>commitment to interleukin 4 production by a CD4(+) T cell-intrinsic mechanism. JExp Med 188:2289-99.18. Symula DJ, KA Frazer, Y Ueda, P Denefle, ME Stevens, ZE Wang, RM Locksley,EM Rubin. 1999. Functional screening <strong>of</strong> an asthma QTL in YAC transgenic mice.Nat Genet 23:241-4.169
19. Cretu G, RM Locksley, ZE Wang, EM Rubin, KA Frazer. 2000. Functional analysis<strong>of</strong> CNS-1 in YAC transgenic mice. Science 288:136-9.20. Lacy DA, ZE Wang, DJ Symola, C McArthur, EM Rubin, KA Frazer, RM Locksley.2000. Faithful expression <strong>of</strong> the human 5q31 cytokine cluster in transgenic mice. JImmunol 164:4569-75.21. Loots GG, RM Locksley, CM Blankespoor, ZE Wang, W Miller, EM Rubin, KAFrazer. 2000. Identification <strong>of</strong> a coordinate regulator <strong>of</strong> interleukins 4, 13, and 5 bycross-species sequence comparisons. Science 288:136-140.22. Mohrs M, CM Blankespoor, Z Wang, GG Loots, V Afzal, H Hadeiba, K Shinkai,EM Rubin, RM Locksley. 2001. Deletion <strong>of</strong> a coordinate regulator <strong>of</strong> type 2cytokine expression in mice. Nat Immunol 2, 842-7.23. Grogan JL, ZE Wang, S Stanley, B Harmon, GG Loots, EM Rubin, RM Locksley.2003. Basal chromatin modification at the IL-4 gene in helper T cells. J Immunol171:6672-9.24. Xu M, ZE Wang, RM Locksley. 2004. Innate immune responses in peptidoglycanrecognition protein L-deficient mice. Mol Cell Biol 24:7949-57.25. Reinhardt RL, S Hong, SJ Kang, ZE Wang, RM Locksley. 2006. Visualization <strong>of</strong>IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promoteTh1 differentiation. J Immunol 177:1618-27.26. Cheng LE, ZE Wang, RM Locksley. 2010. Murine B cells regulate serum IgElevels in a CD23-dependent manner. J Immunol 185:5040-7.27. Yang Z, ZE Wang, PT Doulias, W Wei, H Ischiropoulos, RM Locksley, L Liu.2010. Lymphocyte development requires S-nitrosoglutathione reductase. J Immunol185:6664-9.28. Gordon E, S Sidhu, Z-E Wang, P Woodruff, S Yuan, M Solonm S Conway, XHuang, RM Locksley, J Fahy. 2012. A protective role for periostin and TGF-β inIgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy 42: 144-155.PMC3271792170
BIOGRAPHICAL SKETCHNAMEArthur Weiss, M.D., Ph.D.eRA COMMONS USER NAMEweissaPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> Medicine and <strong>of</strong> Microbiology andImmunologyEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDY<strong>University</strong> <strong>of</strong> Chicago Ph.D. 1978 Immunology<strong>University</strong> <strong>of</strong> Chicago M.D. 1979 MedicinePositions and Employment1979-1980 Postdoctoral Fellow, Swiss Institute for Experimental Cancer <strong>Research</strong>,Lausanne, Switzerland1980-1982 Resident, Department <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong> California, San Francisco(UCSF)1982-1984 Fellow in Rheumatology/Clinical Immunology, UCSF1982-1985 Associate, Howard Hughes Medical Institute, UCSF1984-1985 Instructor, Department <strong>of</strong> Medicine, Division <strong>of</strong> Rheumatology/ClinicalImmunology, UCSF1985-1989 Assistant Investigator, Howard Hughes Medical Institute, UCSF1985-1989 Assistant Pr<strong>of</strong>essor <strong>of</strong> Medicine, Microbiology and Immunology, UCSF1987- Chief, Division <strong>of</strong> Rheumatology/Clinical Immunology, Department <strong>of</strong> Medicine,<strong>University</strong> <strong>of</strong> California, San Francisco1989-1993 Associate Pr<strong>of</strong>essor or Medicine, Microbiology and Immunology, UCSF1989-1994 Associate Investigator, Howard Hughes Medical Institute, UCSF1991- Ephraim P. Engleman Distinguished Pr<strong>of</strong>essor <strong>of</strong> Rheumatology, UCSF1992- Pr<strong>of</strong>essor <strong>of</strong> Medicine, Microbiology and Immunology, UCSF1993- Investigator, Howard Hughes Medical Institute, UCSF1998-2005 Associate Director, The Rosalind Russell Medical <strong>Research</strong> <strong>Center</strong> for Arthritis,UCSF2002-2006 Director, Medical Scientist Training Program (MSTP), UCSF2007-2010 Co-Director, Institute for Molecular Medicine, UCSF171
Other Experience and Pr<strong>of</strong>essional Memberships1986-1991 Councilor, American Federation for Clinical <strong>Research</strong>1991 President, Western Region <strong>of</strong> the American College <strong>of</strong> Rheumatology1998-2002 Member, Allergy and Immunology Study Section (NIH)1999- Chair, Scientific Advisory Board, American <strong>Asthma</strong> Foundation2000-2002 Chair, Allergy and Immunology Study Section (NIH)2003-2010 Council, American Association <strong>of</strong> Immunologists2008-2009 President, American Association <strong>of</strong> Immunologists2005- Advisory Council, RIKEN <strong>Research</strong> <strong>Center</strong> for Allergy & ImmunologyYokohama, JapanHonors1990 Young Investigator Award, Western Society for Clinical Investigation1990 Henry Kunkel Young Investigator Award, American College <strong>of</strong> Rheumatology1993 Junior Investigator Award, American Association <strong>of</strong> Immunologists1997 Lee C. Howley Prize, Arthritis Foundation1998 Forty-First Faculty <strong>Research</strong> Lecturer, <strong>University</strong> <strong>of</strong> California, San Francisco2001 American Association <strong>of</strong> Immunologist-Huang Foundation Meritorious Career Award2003 Fellow, American Academy <strong>of</strong> Arts and Sciences2004 Member, National Academy <strong>of</strong> Sciences2004 Fellow, American Academy <strong>of</strong> Microbiology2004 Member, Institute <strong>of</strong> Medicine2004 Distinguished Investigator Award, American College <strong>of</strong> Rheumatology2004 Walter Bauer Visiting Pr<strong>of</strong>essor in Rheumatology, Massachusetts GeneralHospital2004 Bridget Ogilvie Lecture, <strong>University</strong> <strong>of</strong> Dundee, Scotland2004 Sue Kim Hansen Lecture, Boston <strong>University</strong> School <strong>of</strong> Medicine2005 Dan H. Campbell Memorial Lecture, Midwinter Conference <strong>of</strong> Immunologists,Asilomar, CA2005 Visiting Pr<strong>of</strong>essor, Harvard Medical School Rheumatology Division2005 Beirne B. Carter Lecture in Immunology, <strong>University</strong> <strong>of</strong> Virginia2005 Dan H. Campbell Memorial Lecture, Midwinter Conference <strong>of</strong> Immunologists,Asilomar, CA2006 Keynote Speaker, American Association <strong>of</strong> Immunologists, AdvancedImmunology Course2009 Ishizaka Lecture, La Jolla Institute for Allergy and Immunology2009 46 th Charles A. Stuart Memorial Lecture, Brown <strong>University</strong>2010 Dorothy Baugh Harmon Endowed Lectureship, Oklahoma Medical<strong>Research</strong> Foundation2012 Lifetime Achievement Award, American Association <strong>of</strong> Immunologists2012 UCSF Lifetime Achievement in Mentoring Award172
Selected Peer-reviewed Publications (from a total <strong>of</strong> 207)1. Deindl S, Kadlecek TA, Brdicka T, Cao X, Weiss A, and Kuriyan. Structuralbasis for the inhibition <strong>of</strong> the tyrosine kinase activity <strong>of</strong> ZAP-70. Cell, 129:735-746, 2007. PMID: 175124072. Palacios E, Weiss A. Distinct roles for Syk and ZAP-70 during thymicdevelopment. J Exp Med. 2007; 204: 1703-15. PMID: 17606633. PMVIF:PMV2118636.3. Zhu JW, Brdicka T, Katsumoto TR, Lin J, and Weiss A. Structurally distinctphosphatases CD45 and CD148 both regulate B cell and macrophageimmunoreceptor signaling. Immunity. 28:183-196. 2008. PMC22651064. Hsu L-Y, Tan YX, Xiao Z, Malissen M, and Weiss A. A hypomorphic allele <strong>of</strong>ZAP-70 reveals a distinct thymic threshold for autoimmune disease versusautoimmune reactivity. J. Exp. Med., 206:2527-2541, 2009. PMC27688605. Das J, Ho M, Zikherman J, Govern C, Yang M, Weiss A, Chakraborty AK, RooseJP. Digital signaling and hysteresis characterize Ras activation in lymphoid cells.Cell. 2009; 136337-51. PMCID: PMC2662698.6. Zikherman J, Hermiston M, Steiner D, Hasegawa K, Chan A and Weiss A.PTPN22 deficiency cooperates with the CD45 E613R allele to break tolerance ona non-autoimmune background. J. Immunol., 182:4093-4106. 2009.PMC27659787. Deindl S, Kadlecek TA, Cao X, Kuriyan J, and Weiss A. Stability <strong>of</strong> anautoinhibitory interface in the structure <strong>of</strong> the tyrosine kinase ZAP-70 impacts Tcell receptor response. Proc. Natl. Acad. Sci. USA 106:20699-20704, 2009.PMC27785808. Zikherman J, Jenne C, Watson S, Doan K, Raschke W, Goodnow CC, and WeissA. The balance between positive and negative regulatory roles <strong>of</strong> CD45 during TCR signaling varies throughout T cell development. Immunity, 32:342-354.2010. PMC28651989. Phee H, Dzhagalov I, Mollenauer M, Wang Y, Irvine DJ, Robey ., and Weiss A.Regulation <strong>of</strong> thymocyte positive selection and motility by GIT2. Nat. Immunol.,11:503-511. 2010. PMID: 2043162110. Au-Yeung BB, Levin SE, Zhang C, Hsu, L-Y, Cheng DA, Killeen N, Shokat Kand Weiss A. A genetically selective inhibitor demonstrates a function for thekinase Zap70 in regulatory T cells independent <strong>of</strong> its catalytic activity. Nat.Immunol., 11:1085-1093. 2010. PMID: 21037511. Limnander A, Depeille P, Freedman TS, Liou J, Leitges M, Kurosaki T, RooseJP, and Weiss A. Stim1, PKCd, and RasGRP proteins set a threshold for proapoptoticErk signaling during B cell development. Nat. Immunol., 12:425-433.2011. PMID: 2144193412. Zhu JW, Doan K., Park J, Chau AH, Zhang H, Lowell CA, and Weiss A. Distinctfunctions <strong>of</strong> receptor-like tyrosine phosphatases CD45 and CD148 inchemoattractant-mediated neutrophil migration and response to S. aureusinfection. Immunity. 35:757-769. 2011. PMID: 22078799173
13. Zikherman J, Doan K, Parameswaran R, Raschke W, and Weiss A. Quantitativedifferences in CD45 expression unmask functions for CD45 in B-celldevelopment, tolerance and survival. Proc. Natl. Acad. Sci. USA. ePub ahead <strong>of</strong>print. 2011. PMID: 2213546514. Wei P, Wong WW, Corcoran EE, Peisajovich SG, Weiss A, and Lim WA.Bacterial virulence proteins as tools to synthetically rewire kinase pathways inyeast and immune cells. Nature. 488:384-388. 2012. PMCID: PMC342241315. Zikherman, J., Parameswaran, R., and Weiss. A. Endogenous antigen tunesresponsiveness <strong>of</strong> antigen recepor signaling in B cells but not T cells. Nature.489:160-164. 2012. PMID: 2290250316. Mukherjee S, Zhu J, Zikherman J, Parameswaran R, Kadlecek TA, Wang Q, Au-Yeung B, Ploegh H, Kuriyan J, Das J*, and Weiss, A* Monovalent andmultivalent ligation <strong>of</strong> the B cell receptor exhibit differential dependence uponSyk and Src family kinases. Sci. Signal. 2013. Jan 1;6(256):ra1 PMID:23281368 *co-corresponding authors17. Zikherman J, Parameswaran R, Hermiston M, and Weiss A. The wedge domain<strong>of</strong> the receptor-like tyrosine phosphatase CD45 enforces B cell tolerance byregulating substrate specificity. J. Immunol., in press. 2013.<strong>Research</strong> SupportOngoing <strong>Research</strong> SupportHoward Hughes Medical Institute Weiss (PI) 07/01/85-08/31/12Cell surface molecules and molecular events involved in human T cell activationThe goal is to study cell surface molecules and molecular events involved in T cellactivation. HHMI personnel (3 postdocs and 4 technicians) focus on structure <strong>of</strong> theTCR and the ZAP-70 protein tyrosine kinase.Role: Principal Investigator1 RC2 AR058947-01 (A.Weiss) 09/25/09-08/31/12 NoCost ExtensionNIH/NIAMSAn allosteric inhibitor <strong>of</strong> ZAP-70 as a novel therapeutic for autoimmune diseaseThe goals <strong>of</strong> this project are develop and allosteric inhibitor <strong>of</strong> ZAP-70 and determinethe preclinical utility <strong>of</strong> a model catalytic inhibitor <strong>of</strong> ZAP-70 in preclinical models <strong>of</strong>disease.Role: Principal Investigator1PO1 AI091580-0107/15/11-06/30/16NIH/NIAID (Program Leader A. Weiss)Deconstructing and Reconstructing the T Cell Signaling NetworkThe goals <strong>of</strong> this project are to understand the molecular mechanims that operate atthe plasma membrane to control the specificity, activity and regulation <strong>of</strong> the TCRsignaling mechanisms that lead to protein tyrosine phosphorylation and Ras activity.174
A119632Weiss (PI)07/01/12-06/30/14American College <strong>of</strong> RheumatologyIdentifying antigen-specific T cells in mouse and human arthritisThe goals <strong>of</strong> this grant are to understand how antigen specific T cells are stimulatedand to identify and characterize the T cell antigen receptors in mouse and humanarthritis.Role: Principal InvestigatorCompleted <strong>Research</strong>RO1 AI066120 Weiss (PI)02/15/06-01/31/12 No CostExtensionNIH/NIAIDFunction <strong>of</strong> the RPTP CD148 in the Hematopoietic LineageThe goals in this research 1) characterize the developmental, functional, andbiochemical consequences <strong>of</strong> CD148 loss on the T cell lineage, with emphasis onTCR signaling pathways; 2) characterize the developmental, functional andbiochemical consequences <strong>of</strong> the loss CD148 on the B cell lineage, with emphasis onthe antigen receptor signaling pathways; and, 3) characterize the biochemical,functional and immunological consequences <strong>of</strong> inactivating the CD148 gene inmyeloid cells on integrin and Fc-receptor dependent pathways.Role: Principal InvestigatorPO1 AI35297 Abbas (PI) 08/01/06-07/31/11NIH/NIAIDPeripheral Tolerance and AutoimmunityProject #3Regulation <strong>of</strong> CD45 signaling in tolerance and autoimmunityThe goals <strong>of</strong> this project are to exploit a newly discovered model <strong>of</strong> autoimmunitycaused by a genetic disruption that prevents CD45 dimerization as a model for defectsin signaling receptors that interfere with self-tolerance. T cell lineages responsiblefor autoimmunity and the role <strong>of</strong> antigen will be defined, and the molecular basis <strong>of</strong>CD45 regulation <strong>of</strong> lymphocyte responses and self-tolerance will be examined.Role: Co-InvestigatorPN2 EY016546 Lim (PI) 09/01/08-08/30/09NIHCellular Control: Synthetic Signaling/Motility (RMI)The goals <strong>of</strong> this project are to engineer cells or cell-like molecular assemblies thatperform “smart” therapeutic functions: tasks such as tissue repair or “search anddeliver” actions to treat microscopic tumors or cardiovascular lesions.Role: Project Principal Investigator175
RO1 AI52127 Goodnow (PI) 09/15/02-06/30/07NIH/NIAIDGenes for Tolerance and Immunity ConsortiumA consortium <strong>of</strong> investigators will develop and validate screening tests to identifymice with altered immune regulation, and then define the mutant gene, molecularpathways, and cellular processes revealed by each new mouse strain.Role: Co-Investigator176
BIOGRAPHICAL SKETCHNAMEJonathan S. Weissman, Ph.D.eRA COMMONS USER NAMEWEISSMANPOSITION TITLEPr<strong>of</strong>essor, <strong>University</strong> <strong>of</strong> California San FranciscoInvestigator, Howard Hughes Medical InstituteEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYHarvard <strong>University</strong> A.B. 1984-1988 PhysicsMassachusetts Institute <strong>of</strong> Technology Ph.D. 1988-1993 PhysicsPositions and Honors1993 - 1996 Postdoctoral Fellow, Yale <strong>University</strong>, Structural and Biochemical Studies <strong>of</strong>GroEL1996 - 2000 Assistant Pr<strong>of</strong>essor, <strong>University</strong> <strong>of</strong> California San Francisco, Departments <strong>of</strong>Cellular & Molecular Pharmacology, and Biochemistry & Biophysics2000 - 2005 Assistant Investigator, Howard Hughes Medical Institute2000 - 2003 Associate Pr<strong>of</strong>essor, <strong>University</strong> <strong>of</strong> California San Francisco, Departments <strong>of</strong>Cellular & Molecular Pharmacology, and Biochemistry & Biophysics2003 - Present Pr<strong>of</strong>essor, <strong>University</strong> <strong>of</strong> California San Francisco, Departments <strong>of</strong> Cellular &Molecular Pharmacology, and Biochemistry & Biophysics2005 - Present Investigator, Howard Hughes Medical InstituteOther Experience and Pr<strong>of</strong>essional Memberships1999 Keynote Speaker at Scripps <strong>Research</strong> Institute Society <strong>of</strong> Fellows AnnualSymposium2001 to Present Associate Editor, Molecular Cell2001 - 2003 Ad hoc reviewer, CDF-2 NIH study section2002 Organizer, Second International Yeast Prion Symposium2002 to Present Organizer, Cold Spring Harbor Heat Shock Meeting2003 to Present Editorial Board, BMC Cell Biology2003 Burroughs Wellcome Visiting Scholar, <strong>University</strong> <strong>of</strong> Chicago2003 to Present Editorial Board, Public Library <strong>of</strong> Science (PLoS) Biology2004 Organizer, FASEB meeting on Amyloid and Diseases <strong>of</strong> Misfolding20052004 - 2008Co-organizer, The Protein Society 19 th Annual MeetingPermanent Member, NIH Molecular Biology & Protein Processing StudySection2005 - 2008 Editorial Board, Molecular Biology <strong>of</strong> the Cell2005 External Reviewer, Lawrence Berkeley National Lab Physical BiosciencesDivision177
2005 - 2007 Advisory Board NIH on Amyloid Diseases2005 Organizer, Mini-symposium <strong>of</strong> Quality Control for the Annual AmericanSociety <strong>of</strong> Cell Biology (ASCB) Meeting2006 to Present Member, Yeast Genetics & Molecular Biology Meeting Program Committee2006 to Present Editorial Board, Journal <strong>of</strong> Molecular Biology2007 to Present Board <strong>of</strong> Reviewing Editors, Science2007 to Present Scientific Advisory Board, GlycoFi (Merck & Company, Inc.)2008 to Present Scientific Advisor, Merck <strong>Research</strong> Labs2008 to Present Editorial Board, Cell2009 Program Committee Member, ASCB2009 to Present Editorial Board, Current Opinion in Cell Biology2009 Keynote address at the annual retreat <strong>of</strong> the Genentech <strong>Research</strong>Organization2009 Keynote lecture at the annual International Conference on Systems Biology2009 to Present Scientific Advisory Board, Proteostasis Therapeutics2009 Member, NAKFI Steering Committee on Synthetic Biology2010 Member, NIH College <strong>of</strong> CSR ReviewersHonors and Awards1987 Phi Beta Kappa, Harvard <strong>University</strong>1988 Summa cum laude in Physics, Harvard <strong>University</strong>1988 Karl Taylor Compton Pre-doctoral Fellowship1988 National Science Foundation Pre-doctoral Fellowship1996 David and Lucile Packard Fellowship1997 Searle Scholars Program Fellowship2000 Assistant Investigator, Howard Hughes Medical Institute2004 Protein Society’s Irving Sigal Young Investigator’s Award2008 Raymond & Beverly Sackler International Prize in Biophysics2009 Alexander M. Cruikshank Lecturer, Gordon <strong>Research</strong> Conference on Stress2009 Elected to the National Academy <strong>of</strong> Sciences2010 David Perlman Award Lecturer <strong>of</strong> the ACS Division <strong>of</strong> Biochemical Technology(BIOT)2010 Fellow, American Academy <strong>of</strong> MicrobiologySelected Peer-reviewed Publications (In chronological order; 13 selected <strong>of</strong> 119 publications)1. Breslow DK, Cameron DM, Collins SR, Schuldiner M, Stewart-Ornstein J, NewmanHW, Braun S, Madhani HD, Krogan NJ, Weissman JS. (2008) A comprehensivestrategy enabling high-resolution functional analysis <strong>of</strong> the yeast genome. NatMethods, 5:711-8. PMC27560932. Breslow DK, Collins SR, Bodenmiller B, Aebersold R, Simons K, Shevchenko A,Ejsing CS, Weissman JS. (2010) ORM family proteins mediate sphingolipidhomeostasis. Nature, 463:1048-53. PMC2877384178
3. Ingolia NT, Ghaemmaghami S, Newman JRS, Weissman JS. (2009) Genome-wideanalysis in vivo <strong>of</strong> translation with nucleotide resolution using ribosome pr<strong>of</strong>iling.Science, 324(5924)218-23. PMC27464834. Breslow DK, Weissman JS. (2010) Membranes in Balance: Mechanisms <strong>of</strong>Sphinogolipid Homeostasis. Mol Cell, 40(2): 267-79. PMC29876445. Churchman LS, Weissman JS. (2011) Nascent transcript sequencing visualizestranscription at nucleotide resolution. Nature, 469: 368-73. PMID 212488446. Ingolia NT, Lareau LF, Weissman JS. (2011) Ribosome pr<strong>of</strong>iling <strong>of</strong> mouseembryonic stem cells reveals the complexity and dynamics <strong>of</strong> Mammalian proteomes.Cell, 147: 789-802. PMC32252887. Oh E, Becker AH, Sandikci A, Huber D, Chaba R, Gloge F, Nichols RJ, Typas A,Gross CA, Kramer G, Weissman JS, Bukau B. (2011) Selective ribosome pr<strong>of</strong>ilingreveals the co-translational chaperone action <strong>of</strong> trigger factor in vivo. Cell, 147:1295-1308. PMC32778508. Brar GA, Yassour M, Friedman N, Regev A, Ingolia NT, Weissman JS. (2012)High-resolution view <strong>of</strong> the yeast meiotic program revealed by ribosome pr<strong>of</strong>iling.Science, 335: 552-7. PMID 221944139. Li GW, Oh E, Weissman JS. (2012) The anti-Shine-Dalgamo sequence drivestranslational pausing and codon choice in bacteria. Nature, 484: 538-41.PMC333887510. Ingolia NT, Brar GA, Rouskin S, McGeachy AM, Weissman JS. (2012) Theribosome pr<strong>of</strong>iling strategy for monitoring translation in vivo by deep sequencing <strong>of</strong>ribosome-protected mRNA fragments. Nature Protocols, 7: 1534-50. PMID2283613511. Carvunis AR, Rolland T, Wapinski I, Calderwood MA, Yildirim MA, Simonis N,Charloteaux B, Hidalgo CA, Barbette J, Santhanam B, Brar GA, Weissman JS,Regev A, Thierry-Mieg N, Cusick ME, Vidal M. (2012) Proto-genes and de novogene birth. Nature, 487: 370-4. PMC340136212. Brandman O, Stewart-Ornstein J, Wong D, Larson A, Williams CC, Li GW, Zhou S,King D, Shen PS, Weibezahn J, Dunn JG, Rouskin S, Inada T, Frost A, WeissmanJS. (2012) A ribosome-bound quality control complex triggers degradation <strong>of</strong> nascentpeptides and signals translation stress. Cell, 151: 1042-54. PMID 2317812313. Stern-Ginossar N, Weisburd B, Michalski A, Le VT, Hein MY, Huang SX, Ma M,Shen B, Qian SB, Hengel H, Mann M, Ingolia NT, Weissman JS. (2012) Decodinghuman cytomegalovirus. Science, 338: 1088-93. PMID 23180859<strong>Research</strong> SupportOngoing <strong>Research</strong> SupportNo Project Number (Weissman) 10/01/2000-8/31/2017Howard Hughes Medical InstitutePrion-Based Inheritance, Protein Folding, and Analysis <strong>of</strong> Cellular Systems179
This grant supports our studies <strong>of</strong> how cells insure that proteins fold into their correct shape, aswell as the role <strong>of</strong> protein misfolding in disease and normal physiology. We are also developingexperimental and analytical approaches for exploring the organizational principles <strong>of</strong> biologicalsystems.Hughes Collaborative Investigator Award -- No Project Number (Weissman) 09/18/2012 -08/17/2017Howard Hughes Medical InstituteA combined chemical and genetic approach to explore how chaperone and stress networksmaintain the integrity <strong>of</strong> oncogene-addicted cancer cellsP01 AG70770-15 (Prusiner; Weissman Project 2) 04/01/11 - 3/31/16NIHMolecular Pathogenesis <strong>of</strong> Age-Dependent CNS DegenerationSpecific aims <strong>of</strong> this project are to 1) identify a novel prion domain in the yeast protein New1p;2) identify novel prion phenomena; and 3) establish a genetic screen in yeast to studypropagation <strong>of</strong> the mammalian prion protein (PrP).U01 CA168370-01 (McManus) 05/01/12 – 04/30/17NIHBay Area Cancer Target Discovery and Development NetworkThe overarching goal <strong>of</strong> the BACTDDN is to discover and characterize new cancer targets andtheir modulators, placing them into druggable pathways. Specific aims: 1) develop EXPANDlibraries targeting cancer-specific genetic alterations; 2) identify novel drivers that showtransforming potential in immortalized primary cells; and 3) produce genetic EMAPs to uncoverpathway relationships between candidate drivers.P50 GM073210 (Stroud; Weissman - Key Personnel) 09/01/09 – 08/31/2014NIH/NIGMS<strong>Center</strong>s for Innovation in Membrane Protein Production for Structure DeterminationThe goals are to develop approaches that will make the solution <strong>of</strong> simple membrane proteinstructures routinely achievable and develop novel methods that can be applied to morecomplicated membrane proteins containing multiple subunits <strong>of</strong> the same (homo-oligomers) anddifferent (hetero-oligomers) structure; and to produce and determine structures for complexesthat are formed between membrane proteins and their soluble protein partners, small ligandsand/or macromolecules.U01 GM098254 (Agard/Walter) 09/2012 - 08/2017NIHStructural Basis <strong>of</strong> Protein HomeostasisTo obtain structural insights into the mechanism by which unfolded and non-native states arerecognized by the cytosolic (Hsp70, TRiC chaperones) and ER (UPR and ERAD pathways)protein homeostasis machineries.180
P50 GM102706-01 (Cate) 09/2012 - 08/2017NIH<strong>Center</strong> for RNA Systems BiologyThis <strong>Center</strong> aims to use systems biological methods to discover the regulation <strong>of</strong> mRNA fatecontrolled by RNA structural elements in pre-mRNAs and mRNAs.No Project Number (Bivona & Weissman) 02/01/2012 - 1/30/2014California Institute for Quantitative BiosciencesDiscovery <strong>of</strong> Rational Companion Targets in Human Lung CancerThe goal is to define rational polytherapies for lung cancer patients through genome-wide RNAiscreening. Specific aims <strong>of</strong> this Rogers Foundation Bridging the Gap Award project are: 1)determine which genes act together to make cancer tumor cells more vulnerable; 2) test drugcombinations against these targets; 3) identify additional drug combinations to tackle othersubtypes <strong>of</strong> lung cancers, and expand studies to include different categories <strong>of</strong> cancer.Completed <strong>Research</strong> Support<strong>Sandler</strong> <strong>Center</strong> for Drug Discovery (Weissman) 11/01/11 - 10/31/2012Identifying and Characterizing Asiatic Cholera Host FactorsSpecific aims <strong>of</strong> this project are: 1) Develop a quantitative functional reporter assay for action <strong>of</strong>cholera toxin in cell culture; 2) Conduct a comprehensive genome-wide screen for factorsaffecting cholera toxin action; 3) Validate and characterize the targets in physiological assays forcholera activity.SABRE <strong>Center</strong> (Weissman) 01/01/09-06/30/11<strong>Sandler</strong> <strong>Asthma</strong> <strong>Basic</strong> REsearch <strong>Center</strong>Defining the Function <strong>of</strong> ORMDL3 and Exploring its Potential Role in <strong>Asthma</strong>Specific aims <strong>of</strong> this project are: 1) Define the function <strong>of</strong> ORM genes in yeast; 2) Characterizethe function <strong>of</strong> the human ORM homolog ORMDL3; 3) Examine the potential role <strong>of</strong> ORMDL3in asthma.P0032295 (Weissman) 12/01/09 - 11/30/2010AFAR (Glenn Foundation for Medical <strong>Research</strong>)Monitoring the Effects <strong>of</strong> Aging on Protein TranslationUsing a ribosome pr<strong>of</strong>iling approach pioneered in our lab, we will investigate the impact <strong>of</strong>aging and the accumulation <strong>of</strong> misfolded proteins on protein synthesis in a yeast model system.<strong>Sandler</strong> <strong>Center</strong> (Weissman and F 01/15/09-01/14/10<strong>Sandler</strong> Program in <strong>Basic</strong> SciencesDefining Roles on N-Glycans in Endopasmic Reticulum-Mediated Quality ControlSpecific aims <strong>of</strong> this project are: 1) Biochemical characterization <strong>of</strong> the putative mannosidaseHtm1; and 2) Elucidation <strong>of</strong> contributions <strong>of</strong> glycan and misfolded protein components tosubstrate recognition in late stages <strong>of</strong> ERAD-L pathway.181
Fight for Mike Program (Weissman) 07/23/07-07/22/09California Institute for Quantitative Biosciences (QB3)Identification <strong>of</strong> Factors Important for the Folding <strong>of</strong> Mammalian Prion ProteinThe broad goal <strong>of</strong> our studies is to identify key factors required for folding <strong>of</strong> the mammalianprion protein.182
BIOGRAPHICAL SKETCHNAMEZena Werb, Ph.D.eRA COMMONS USER NAMEwerbzenaPOSITION TITLEPr<strong>of</strong>essor <strong>of</strong> AnatomyEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDY<strong>University</strong> <strong>of</strong> Toronto, Toronto, Canada B.Sc. 06/1966 BiochemistryRockefeller <strong>University</strong>, New York Ph.D. 06/1971 Cell BiologyStrangeways <strong>Research</strong> Laboratory, Cambridge, Postdoc. 1971-73 Protein ChemistryPositions1973-1975 <strong>Research</strong> Scientist, Strangeways Res. Lab., Cambridge, United Kingdom1975-1976 Visiting Assistant Pr<strong>of</strong>essor <strong>of</strong> Medicine,Dartmouth Medical School, Hanover, NH1976-1980 Assistant Pr<strong>of</strong>essor Radiobiology, Radiology<strong>University</strong> <strong>of</strong> California, San Francisco1979-1980 Assistant Pr<strong>of</strong>essor Anatomy, <strong>University</strong> <strong>of</strong> California San Francisco1980-1983 Associate Pr<strong>of</strong>essor <strong>of</strong> Anatomy and Radiology<strong>University</strong> <strong>of</strong> California, San Francisco1983-Present Pr<strong>of</strong>essor Anatomy, UCSF1985-1986 Visiting Pr<strong>of</strong>essor, Sir William Dunn School <strong>of</strong> Pathology<strong>University</strong> <strong>of</strong> Oxford, United Kingdom1998 Visiting Pr<strong>of</strong>essor, Institut Curie, Paris1999-Present Vice-chair, Dept. <strong>of</strong> Anatomy, <strong>University</strong> <strong>of</strong> California, San Francisco2006-2008 Visiting Pr<strong>of</strong>essor, Max-Planck Institute for BiochemistryMartinsried, GermanyOther Experience and Pr<strong>of</strong>essional MembershipsEditorial Boards1983-1985 Journal <strong>of</strong> Cell Biology1982-1987 American Journal <strong>of</strong> Physiology1985-2004 Journal <strong>of</strong> Experimental Medicine1990-2001 Science1999-Present Matrix Biolog1999-Present Neoplasia2000-2009 Cell2001-Present Developmental Cell2001-Present Cancer Cell183
2002-2006 Molecular Biology <strong>of</strong> the Cell2007-2009 Genes & Development2009-Present Current Opinion in Cell Biology2010-Present Guest Editor, Proc. National Academy Science, USA2010-Present Member, Editorial Board, Disease Models and MechanismsPr<strong>of</strong>essional Memberships1976-present American Society for Cell Biology1979-present American Society for Biochemistry and Molecular Biology1967-71 & American Association for the Advancement <strong>of</strong> Science1979-present1988-present Society for Developmental Biology2001-present American Association for Cancer <strong>Research</strong>2001-present American Society for Matrix Biology2004-present International Society for DifferentiationScientific Leadership1990-1992 Member, Cell and Molecular Biology Panel, National Cancer Institute <strong>of</strong>Canada1991-1995 Member, Board <strong>of</strong> Scientific Counselors, NIAMS1992-1995 Council Member, American Society for Cell Biology1993-1995 Council Delegate, Am. Assoc. for the Advancement <strong>of</strong> Science1994-2001 Member, Scientific Advisory Board, Keystone Symposia2001-2003 Council Member, American Society for Matrix Biology2001 NIH Oncological SS Boundaries Team2002 NIH Biochem SS, ad hoc2003-2005 Council Member, International Society for Matrix Biology2003-2006 Board <strong>of</strong> Directors, AACR2005 President, American Society for Cell Biology2007-2009 Nominating Committee, AACR2007 Member, NIH ZRG1 ICI–D012008 Reviewer, NIH Pioneer Awards2008 Chair, NIH ZRG1 MOSS-A (02)2008-2010 Chair, NIH ICI Study Section2009-2012 Chair, American Academy <strong>of</strong> Arts and Sciences, Membership SelectionCommittee Class II, section2010 Co-organizer, CNIO Cancer Symposium on Frontiers in Invasion andMetastasis, Madrid2011-Present Member, Steering Committee, AACR Council <strong>of</strong> Scientific Advisors2011-2016 Member, Scientific Advisory Board, Max Planck Institute for Biology <strong>of</strong>Ageing, Cologne, GermanyHonors1971-1973 Fellow, Medical <strong>Research</strong> Council, Canada1982 R.R. Bensley Memorial Award, American Association <strong>of</strong> Anatomists1985-1986 Fellow, John Simon Guggenheim Foundation1992 Elected Fellow, American Association for the Advancement <strong>of</strong> Science1996 FASEB Excellence in Science Award184
1998 Rotschild/Mayent Fellowship, Institut Curie2002 Elected Member, Institute <strong>of</strong> Medicine2003 Elected Fellow, American Academy <strong>of</strong> Arts and Sciences2003 Doctor <strong>of</strong> Medicine (honoris causa), <strong>University</strong> <strong>of</strong> Copenhagen2006-2007 Alexander von Humboldt Foundation (Germany) <strong>Research</strong> Award2007 E.B. Wilson Medal, American Society for Cell Biology2009 Colin Thomson Memorial Medal, AICR2010 Elected Member, National Academy <strong>of</strong> Sciences2010 American Society for Cell Biology, Women in Cell Biology Senior Award2011 Zero Breast Cancer 2011 Community Breast Cancer <strong>Research</strong> AwardNamed Lectureships (selected)2001 44th Faculty <strong>Research</strong> Lecture Award, <strong>University</strong> <strong>of</strong> California, San Francico2001 H.B. Parker Lecture, Pacific Northwest National Lab.2003 Pharmacology Founders’ Seminar, Wayne State <strong>University</strong>, Detroit, Michigan2004 Schlessinger Lecturer, BIDMC, Harvard Medical School2004 A. S. Wiener Lecture, NY Blood <strong>Center</strong>2005 Maud L. Menten Lecture, <strong>University</strong> <strong>of</strong> Pittsburgh2005 17th Otto Herz Memorial Lecture, Tel Aviv <strong>University</strong>, Israel2008 Whitley Lecture, Northwest Developmental Biology Society2009 Suzanne M. Bernier Lecture in Skeletal Biology, <strong>University</strong> <strong>of</strong> Western Ontario,London, Canada2011 John H. Blaffer Lecture, M.D. Anderson Cancer <strong>Center</strong>, <strong>University</strong> <strong>of</strong> Texas,Houston TX2011 McAllister Lecture, Pathology Grand Rounds, Yale Medical School, New Haven CT2012 Keynote Lecture, 28 th International Association for Breast Cancer <strong>Research</strong>Breakthrough Breast Cancer Conference, Manchester, UKSelected Publications 15 Selected Publications (>450 total full publications)1. Sternlicht MD, A. Lochter, CJ Sympson, B Huey, JP Rougier, JW Gray, D Pinkel, MJBissell & Z Werb (1999). The stromal proteinase MMP-3/stromelysin-1 promotesmammary carcinogenesis. Cell. 98: 137-146. PMCID: PMC28532552. Coussens LM & Z Werb (2002). Inflammation and cancer. Nature 420:860-867.PMID: 12490959; PMCID: PMC28030353. Egeblad MA, J Ewald, HA Askautrud, BE Welm, M Truitt, E Bainbridge, G Peeters,M Krummell & Z Werb (2008). Visualizing stromal cell dynamics in differenttumor microenvironments by spinning disk confocal microscopy. Dis. Model. Mech.1:155-167. PMCID: PMC2562195.4. Ewald AJ, A Brenot, M Duong, BS Chan & Z Werb (2008). Collective epithelialmigration and cell rearrangements drive mammary branching morphogenesis. Dev.Cell. 14:570-581. PMCID: PMC2773823.5. Kouros-Mehr H, SK Bechis, EM Slorach, LE Littlepage, M Egeblad, AJ Ewald, SYPai, IC Ho & Z Werb (2008). GATA-3 links tumor differentiation and disseminationin a luminal breast cancer model. Cancer Cell. 13:141-152. PMCID: PMC2262951.185
6. Welm BE, GJP Dijkgraaf, AS Bledau, AL Welm & Z Werb (2008). Lentiviraltransduction <strong>of</strong> stem cells for genetic analysis <strong>of</strong> mammary development and breastcancer. Cell Stem Cell. 2:90-102. PMCID: PMC2276651.7. Kessenbrock K, V Plaks & Z Werb (2010). Matrix metalloproteinases: Regulators <strong>of</strong>the tumor microenvironment. Cell. 141:52-67. PMCID: PMC286205.8. Littlepage LE, MD Sternlicht, N Rougier, J Phillips, E Gallo, Y Yu, K Williams, ABrenot, JI Gordon & Z Werb (2010). Matrix metalloproteinases contribute distinctroles in neuroendocrine prostate carcinogenesis, metastasis, and angiogenesisprogression. Cancer Res. 70:2224-2234 PMID: 20215503; PMCID: PMC2840052.9. Slorach EM, J Chou & Z Werb (2011). Zeppo1 is a novel metastasis promoter thatrepresses E-cadherin expression and regulates p120-catenin is<strong>of</strong>orm expression andlocalization. Genes Dev. 25: 471-484. [Epub ahead <strong>of</strong> print February 11, 2011].PMCID: PMC3049288.10. Caudrillier A, K Kessenbrock, BM Gilliss, JX Nguyen, MB Marques, M Monestier, PToy, Z Werb & MR Looney (2012). Platelets induce neutrophil extracellular traps intransfusion-related acute lung injury. J. Clin. Invest. 122(7):2661–2671. [Epub ahead<strong>of</strong> print, Jun 11]. PMID: 22684106; PMCID: PMC3386815.11. Engelhardt J, B Boldajipour, P Beemiller, P Pandurangi, C Sorenson, Z Werb, MEgeblad & MF Krummel (2012). Marginating dendritic cells <strong>of</strong> the tumormicroenvironment cross-present antigens and stably engage tumor-specific T cells.Cancer Cell. 21:402-417. PMID: 22439936; PMCID: PMC3311997.12. Littlepage LE, AS Adler, H Kouros-Mehr, G Huang, J Chou, SR Krig, OL Griffith,JE Korkola, K Qu, DA Lawson, Q Xue, MD Sternlicht, GJP Dijkgraaf, P Yaswen,Hope S Rugo, CA Sweeney, CC Collins, JW Gray, HY Chang & Z Werb (2012).The transcription factor ZNF217 is a prognostic biomarker and therapeutic targetduring breast cancer progression. Cancer Discov. 2:638-651 [Epub June 22]. PMID:22728437.13. Nakasone E, HA Askautrud, T Kees, V Plaks, AJ Ewald, MG Rasch, YX Tan, J Qin,M Fein, J Park, P Sinha, MJ Bissell, E Frengen, Z Werb & M Egeblad (2012).Imaging tumor-stroma interactions during chemotherapy reveals microenvironmentalcontributions to chemoresistance. Cancer Cell. 21:488-503. PMCID: PMC3332002.14. Phillips JJ, A Ward, E Huillard, A Robinson, DH Lum, MY Polley, SD Rosen, DHRowitch & Z Werb (2012). Heparan sulfate sulfatase SULF2 regulates PDGFRasignaling and growth in malignant glioblastoma. J. Clin. Invest. 122:911–922 [EpubFeb. 1]. PMID: 22293178; PMCID: PMC3287216.15. Chou J, JH Lin, A Brenot, JW Kim, S Provot & Z Werb (2013). GATA3 suppressesmetastasis and modulates the tumor microenvironment by regulating miR-29expression. Nat. Cell Biol. In press.186
<strong>Research</strong> SupportOngoingNIH/NCI R01 CA129523-05 Werb (PI) 07/01/08 - 04/30/13Transcriptional Regulation <strong>of</strong> Breast Cancer MetastasisThis study addresses how GATA-3 regulates the differentiated state <strong>of</strong> breast tumors.NCI R01 CA057621-20 Werb (PI) 04/15/93 - 05/31/13Role <strong>of</strong> Metalloproteinases in Mammary Gland RemodelingThis consortium grant between UCSF and LBNL determines functions <strong>of</strong> ECM-degradingproteinases and inhibitors in mammary epithelium during development.NIH/NIAID P01 AI053194-10 Mostov (PI) 09/30/02 - 08/31/13Mucosal Immune Barrier in Infection and InflammationThis project studies the role <strong>of</strong> the inflammatory response in mucosal epithelia.Role: Leader, Project 4 and Core CNIEHS/NCI 5U01 ES019458-03 (Werb, PI) 09/01/10-04/30/15Environmental Effect on The Mammary Gland Across The LifespanThe major goal <strong>of</strong> this program is to determine the susceptible times in breast developments andhow they are affected by environmental stressors.17UB-8705-02 California Breast Cancer <strong>Research</strong> Program (Werb, PI) 09/01/11-08/31/14Biomarkers for environmental exposures in breast cancerThe major goal <strong>of</strong> this project is to find glycan biomarkers that are induced by exposure toenvironmental stressors.CompletedNIH/NIAMS R01 AR046238-10 Werb (PI) 09/01/99 - 01/31/11Extracellular Remodeling in Bone Development And RepairThis project studied the role <strong>of</strong> extracellular remodeling in bone development and repair.NCI P01 CA072006-10 Werb (PI) 07/07/03 – 06/30/10Proteases in Cancer: Biology and Drug DevelopmentThis program tested new animal models <strong>of</strong> invasive cancer with antiprotease therapy.Role: Project Leader, Project 3 and Core ANIH/NCI U01 CA105379-05 Hanahan (PI) 09/27/04 - 03/31/09Immune Enhancement and Therapy in Cancer/ Mouse Models <strong>of</strong> Human Cancer ConsortiumThis project addressed the immunobiology <strong>of</strong> carcinogenesis in genetically engineered mousemodelsRole: Co-INIH/NIEHS U01 ES012801-07 Hiatt (PI), Werb (PI) 09/29/03 - 07/31/11This project studied environmental effects on the molecular architecture and function <strong>of</strong> themammary gland.Role: PI, Collaborative Project 1187
SU2C-AACR-DT0409-03 Gray, Slamon (Dream team leaders) 10/01/09 - 09/30/12Stand Up to Cancer-American Association for Cancer <strong>Research</strong> Dream Team TranslationalCancer <strong>Research</strong> GrantPersonalizing Treatment <strong>of</strong> Metastatic Breast CancerThe goal <strong>of</strong> this project was to develop xenograft models <strong>of</strong> human <strong>of</strong> drug-resistant, metastaticbreast cancers to then test therapies selected based on the “omic” features <strong>of</strong> the metastatictumors.Role: Dream Team Member188
BIOGRAPHICAL SKETCHNAMEPrescott Gurney Woodruff, M.D., M.P.H.eRA COMMONS USER NAMEwoodruffpPOSITION TITLEAssociate Pr<strong>of</strong>essor <strong>of</strong> Medicine in ResidenceEDUCATION/TRAININGINSTITUTION AND LOCATION DEGREE YEAR(s) FIELD OF STUDYWesleyan <strong>University</strong>, Middletown, CT B.A. 6/1989 LettersColumbia College <strong>of</strong> Physicians & Surgeons, NY M.D. 6/1993 MedicineMassachusetts General Hospital Resident 1993-1996 Internal MedicineHarvard School <strong>of</strong> Public Health M.P.H. 6/1998 EpidemiologyBrigham and Women’s Hospital Fellow 1997-1998 Resp Epidemiology<strong>University</strong> <strong>of</strong> California, San Francisco Fellow 1998-2002 Pulmonary/CriticalCarePositions and Honors1993-1994 Intern in Internal Medicine; Massachusetts General Hospital, Boston, MA1994-1996 Resident in Internal Medicine; Massachusetts General Hospital, Boston, MA1996-1998 <strong>Research</strong> Fellow, Department <strong>of</strong> Emergency Medicine; Massachusetts GeneralHospital, Boston, MA1997-1998 Clinical and <strong>Research</strong> Fellow, Channing Laboratory, Department <strong>of</strong> MedicineBrigham and Women’s Hospital, Boston, MA1998-2002 Clinical and <strong>Research</strong> Fellow, Pulmonary/Critical Care Medicine &Cardiovascular <strong>Research</strong> Institute, Department <strong>of</strong> Medicine, <strong>University</strong> <strong>of</strong>California San Francisco, San Francisco, CA2002-2005 Assistant Adjunct Pr<strong>of</strong>essor; <strong>University</strong> <strong>of</strong> California San Francisco2005- 2010 Assistant Pr<strong>of</strong>essor in Residence, Pulmonary/Critical Care Medicine, Department<strong>of</strong> Medicine and CVRI, <strong>University</strong> <strong>of</strong> California San Francisco2010- Associate Pr<strong>of</strong>essor in Residence, Division <strong>of</strong> Pulmonary and Critical CareMedicine, Department <strong>of</strong> Medicine and CVRI, <strong>University</strong> <strong>of</strong> CaliforniaSan Francisco2012- Vice Chief for <strong>Research</strong>, Division <strong>of</strong> Pulmonary, Critical Care, Sleep and Allergy,<strong>University</strong> <strong>of</strong> California, San Francisco189
Other Experience and Pr<strong>of</strong>essional Memberships2004- Steering Committee, NIH NHLBI COPD Clinical <strong>Research</strong> Network2009- Steering Committee, NIH NHLBI Spiromics Network2011- Steering Committee, NIH NHLBI Severe <strong>Asthma</strong> <strong>Research</strong> ProgramHonors1993 Alpha Omega Alpha, Columbia College <strong>of</strong> Physicians and Surgeons, NY, NY2012 Elected to membership, American Society <strong>of</strong> Clinical InvestigationSelected peer-reviewed publications (Selected from 58 peer-reviewed publications)1. Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S,Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, Erle DJ, Yamamoto KR, Fahy JV.Genome-Wide Pr<strong>of</strong>iling Identifies Epithelial Cell Genes Associated with <strong>Asthma</strong> andwith Treatment Response to Corticosteroids. Proc Natl Acad Sci U S A. 2007 Oct2;104(40):15858-63. PMCID: PMC20004272. Goswami S, Angkasekwinaim P, Shan M, Greenlee KJ, Barranco WT, PolikepahadS, Seryshev A, Redding D, Singh B, Sur S, Woodruff PG, Dong C, Corry DB,Kheradmand F. Divergent Roles for Airway Epithelial MMP7 and Retinoic Acid inExperimental <strong>Asthma</strong>, Nature Immunology, 2009 May;10(5):496-503. PMID:19329997 PMC Journal – In Process3. Woodruff PG, Modrek B, Choy DF, Jia G, Abbas AR, Ellwanger A, Koth LL, ArronJR, and Fahy JV. Th2-driven inflammation defines major sub-phenotypes <strong>of</strong> asthma.Am J Respir Crit Care Med Sep 1 2009;180(5):388-95. PMCID: PMC27427574. Park SW, Verhaeghe C, Nguyenvu LT, Barbeau R, Eisley CJ, Nakagami Y, Huang X,Woodruff PG, Fahy JV, Erle DJ. Roles <strong>of</strong> FOXA2 and FOXA3 in Allergic AirwayDisease and <strong>Asthma</strong>. Am J Respir Crit Care Med. Jul 23 2009 Oct 1;180(7):603-10.PMCID: PMC27537885. Dougherty RH, Sidhu SS, Raman K, Solon M, Solberg OD, Caughey GH, WoodruffPG, Fahy JV. Accumulation <strong>of</strong> intraepithelial mast cells with a unique proteasephenotype in T(H)2-high asthma. J Allergy Clin Immunol. 2010May;125(5):1046-1053.e8. PMC Journal – In Process6. Koth LL, Cambier CJ, Ellwanger A, Solon M, Hou L, Lanier LL, Abram CL,Hamerman JA, Woodruff PG. DAP12 is required for macrophage recruitment to thelung in response to cigarette smoke and chemotaxis toward CCL2. J Immunol. 2010Jun 1;184(11):6522-8. Epub 2010 Apr 26. PMC Journal – In Process7. Sidhu SS, Yuan S, Innes AL, Woodruff PG, Solon M, Hou L, Muller SJ, and FahyJV.Epithelial cell-derived periostin: roles in TGFbeta activation, collagen productionand collagen gel elasticity in asthma. Proc Natl Acad Sci U S A. 2010 Aug10;107(32):14170-5. PMCID: PMC2922596190
8. Choy DF, Modrek B, Abbas AR, Kummerfeld S, Clark HF, Wu LC, Fedorowicz G,Modrusan Z, Fahy JV, Woodruff PG, Arron JR. Gene expression patterns <strong>of</strong> th2inflammation and intercellular communication in asthmatic airways. J Immunol. 2011Feb 1;186(3):1861-9. PMC Journal – In Process9. Gordon ED, Sidhu SS, Wang ZE, Woodruff PG, Yuan S, Solon MC, Conway SJ,Huang X, Locksley RM, Fahy JV. A protective role for periostin and TGF-β inIgE-mediated allergy and airway hyper-responsiveness. Clin Exp Allergy. 2012Jan;42(1):144-55.10. Eri1 regulates microRNA homeostasis and mouse lymphocyte development andantiviral function. Thomas MF, Abdul-Wajid S, Panduro M, Babiarz JE, Rajaram M,Woodruff P, Lanier LL, Heissmeyer V, Ansel KM. Blood. 2012 Jul 5;120(1):130-42.Epub 2012 May 21.11. Jia G, Erickson RW, Choy DF, Mosesova S, Wu LC, Solberg OD, Shikotra A, CarterR, Audusseau S, Hamid Q, Bradding P, Fahy JV, Woodruff PG, Harris JM, ArronJR; Bronchoscopic Exploratory <strong>Research</strong> Study <strong>of</strong> Biomarkers inCorticosteroid-refractory <strong>Asthma</strong> (BOBCAT) Study Group. Periostin is a systemicbiomarker <strong>of</strong> eosinophilic airway inflammation in asthmatic patients. J Allergy ClinImmunol. 2012 Sep;130(3):647-65412. Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M,Nguyen C, Barczak AJ, Zlock LT, Blagev DP, Finkbeiner WE, Ansel KM, Arron JR,Erle DJ, Woodruff PG. Airway Epithelial miRNA Expression is Altered in <strong>Asthma</strong>.Am J Respir Crit Care Med. 2012 Sep 6. [Epub ahead <strong>of</strong> print]13. Huang F, Zhang H, Wu M, Yang H, Kudo M, Peters CJ, Woodruff PG, Solberg OD,Donne ML, Huang X, Sheppard D, Fahy JV, Wolters PJ, Hogan BL, Finkbeiner WE,Li M, Jan YN, Jan LY, Rock JR. Calcium-activated chloride channel TMEM16Amodulates mucin secretion and airway smooth muscle contraction. Proc Natl AcadSci U S A. 2012 Sep 17. [Epub ahead <strong>of</strong> print]<strong>Research</strong> SupportACTIVER01 HL097591 (Woodruff PG)7/1/2009 - 6/30/2013NIH/NHLBIRole <strong>of</strong> Th2 and non-Th2 Inflammation inAirway Smooth Muscle Remodeling in <strong>Asthma</strong>The goal <strong>of</strong> this study is to identify pathways linking Th2-driven inflammation to smooth muscleremodeling.R01 HL095372 (Woodruff PG)NIH/NHLBIMolecular Phenotyping <strong>of</strong> <strong>Asthma</strong>9/24/2008 - 7/31/2012No cost extension through 7/2013The goal <strong>of</strong> this study is to identify and characterize molecular phenotypes <strong>of</strong> asthma usinggene expression pr<strong>of</strong>iling, quantitative morphometry and related imaging techniques.191
HHSN268200900014C (Woodruff PG)2/1/2009 - 1/31/2016NIH/NHLBIThe Spiromics Project: Clinical <strong>Center</strong>The goal <strong>of</strong> this study is to identify sub-populations and intermediate outcome measuresin COPD through a large multi-center longitudinal study.P01 HL107202 (Fahy JV, Woodruff Core leader) 7/1/12-6/30/17NIH/NHLBIInnate and Adaptive Immune Responses in Th2 High <strong>Asthma</strong>To identify the roles <strong>of</strong> iH2 cells, IL-33 and miRNAs in local immune responses in the lung inasthma.2U19AI077439-06 (Sheppard D, Woodruff Core Leader, Project Co-I) 4/1/2013-3/31/18NIH/NIAIDIL-13 and IL-17 dynamics in the asthmatic airwayTo identify the cellular sources, tissue localization and local effects <strong>of</strong> IL-13 and IL-17 in thelung in asthma.R01 HL114447 (PI: Martinez, subcontract PI: Woodruff) 4/1/2012 - 3/31/2016NIH/NHLBIPulmonary bacterial microbiome-epithelial cell interactions in COPDThe goal <strong>of</strong> this study is to establish the lung microbiome in COPD and identify epithelial mucinresponses associated with selected pathogens.R21 HL108596 (Erle DJ, Woodruff Co-I)4/6/2011 - 3/31/2013NIH/NHLBIMicro-RNAs in Airway Epithelial Differentiationand <strong>Asthma</strong>The goal <strong>of</strong> this project is to understand how airway epithelial cell micro-RNAs functionand determine how these miRNAs contribute to asthma.U10 HL109146 (Fahy JV, Woodruff Co-I)7/1/2011 - 6/30/2017NIH/NHLBISevere <strong>Asthma</strong> <strong>Research</strong> ProgramThe goal <strong>of</strong> this project is to investigate molecular phenotypes and lectins that regulatemucus viscosity in severe asthma.U10 HL098107 (Boushey HA, Woodruff Co-I) 9/30/2009 - 6/30/2016NIH/NHLBIUCSF <strong>Asthma</strong>Net Clinical <strong>Center</strong>Clinical <strong>Research</strong> Skills Development Core194
The major goals are to serve as a clinical center participating in the conduct <strong>of</strong> NHLBI-supportedmulti-center clinical trials <strong>of</strong> asthma therapies in children and adults with asthma, Role: Co-CoreLeaderR01HL110883 ((Kheradmand F, Woodruff sub Co-I) 2/15/2012-1/31/2016NIH/NHLBIAncillary T-Cell Based Studies in SPIROMICSThe major goals are to determine whether T-cell autoreactivity in COPD is associated withemphysema in cross-sectional and longitudinal human studies (using the Spiromics cohort).U10 RFA-HL-12-013 (Koth LL, Woodruff Co-I) 4/012012 – 3/31/15NIHI/NHLBIGenomic Phenotyping and Mechanisms in sarcoidosis and AATTo perform genomic and microbiomic human studies to define disease heterogeneity insarcoidosis and AATN01 AI90052 (Busse W, Woodruff subcontract) 9/30/2009- 9/29/14NIH/NIAIDInner City <strong>Asthma</strong> Consortium (ICAC): Immunologic Approaches to Reduce <strong>Asthma</strong>”To identify immunological approaches to reduction in asthma prevalence and severity in innercity populations.195