4.05 Microbiological Examination of Non-sterile Products
4.05 Microbiological Examination of Non-sterile Products
4.05 Microbiological Examination of Non-sterile Products
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
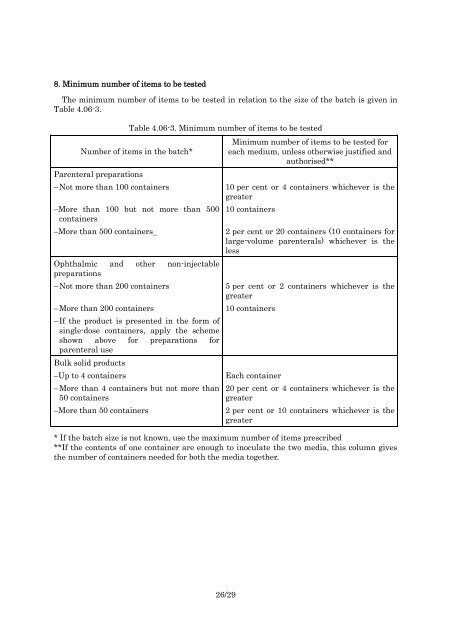
8. Minimum number <strong>of</strong> items to be testedThe minimum number <strong>of</strong> items to be tested in relation to the size <strong>of</strong> the batch is given inTable 4.06-3.Table 4.06-3. Minimum number <strong>of</strong> items to be testedNumber <strong>of</strong> items in the batch*Parenteral preparationsMinimum number <strong>of</strong> items to be tested foreach medium, unless otherwise justified andauthorised**– Not more than 100 containers 10 per cent or 4 containers whichever is thegreater–More than 100 but not more than 500containers–More than 500 containersOphthalmic and other non-injectablepreparations10 containers2 per cent or 20 containers (10 containers forlarge-volume parenterals) whichever is theless– Not more than 200 containers 5 per cent or 2 containers whichever is thegreater– More than 200 containers 10 containers– If the product is presented in the form <strong>of</strong>single-dose containers, apply the schemeshown above for preparations forparenteral useBulk solid products–Up to 4 containers– More than 4 containers but not more than50 containers–More than 50 containersEach container20 per cent or 4 containers whichever is thegreater2 per cent or 10 containers whichever is thegreater* If the batch size is not known, use the maximum number <strong>of</strong> items prescribed**If the contents <strong>of</strong> one container are enough to inoculate the two media, this column givesthe number <strong>of</strong> containers needed for both the media together.26/29