Atomic Term Symbols
Atomic Term Symbols
Atomic Term Symbols
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
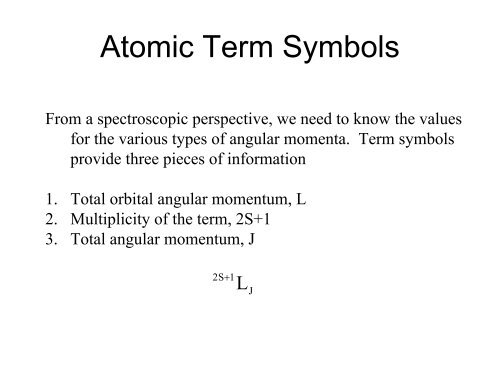
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>From a spectroscopic perspective, we need to know the valuesfor the various types of angular momenta. <strong>Term</strong> symbolsprovide three pieces of information1. Total orbital angular momentum, L2. Multiplicity of the term, 2S+13. Total angular momentum, J2S+1LJ
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>L v TotalL vL v 21Imagine then summing these vectors together. We can do it,but it is far easier to consider z-components as these addtogether as scalars!
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>In terms of z-components …M = m m ml l l2l3+ + +1....Or in terms of magnitudesL = l l l 2 3+ + +1....The total # or series of possible values for all orientations isgiven by the Clebsch-Gordon Series. For an atom withtwo electrons,L= l1 + l 2,l1+ l 2 −1,l1+ l 2 − 2,...l1−l2
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>L=l1 + l 2,l1+ l 2 −1,l1+ l 2 − 2,...Maximum L corresponds tol1l1+ l− l22Minimum L corresponds tol1−l2Quantum Number L designations areL: 0 1 2 3 4S P D F G
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>MultiplicityCan also determine the multiplicity for the term using aClebsch-Gordon seriesS = ½ + ½ , ½ - ½= 1, 0for two electron systemMultiplicity given by 2S + 1For S =1: 2S+1 = 3For S=0: 2S+1 = 1Called a triplet stateCalled a singlet state
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>Total Angular MomentumPermitted values of J again, given by a Clebsch-Gordon seriesJ = L + S, L + S - 1, L + S - 2 …. |L - S|
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>As a first example, lets consider the hydrogen atomFor case with L = 1, S = ½J = 1 + ½ and 1 - ½term symbols are 2P 3/2 and 2 P 1/2
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>A portion of the hydrogen atom transition level diagram foroptical spectra then, will look like2D 5/22P 3/2 3d2S 23p21/2 PD 3/23s1/23p3d2P 3/22p2S 21/2 P2s1/22p∆J=∆l2S 1/2∆s=1sSelection rules for opticaltransitions0, ± 1= ± 10}spin-orbitcoupling
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>What is the term symbol for helium with a 1s 1 2s 1configuration?L = 0 (why?)S = ½+ ½, ½-½= 1, 0Thus there are two possibilities: 3 S and 1 S
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>Energy level diagram for helium then is∞parahelium1S 1P 1D∞orthohelium3S 3P 3D44332211
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>What about an atom with the configuration 1s 2 ,2s 2 , 2p 1 ,3p 1(corresponds to an excited state for carbon)L=l1+ l2,l1+ l2−1,l1+ l2− 2,...l1−l2L = 2, 1, 0D, P, and SS can have S = 1, 0Given these values for L and S we have3D, 1 D, 3 P, 1 P, 3 S, and 1 S
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>Including total orbital angular momentum, we need to considerthe following3D 3 , 3 D 2 , 3 D 1 , 1 D 2 , 3 P 2 , 3 P 1 , 3 P 0 , 1 P 1 , 3 S 1 , and 1 S oQuestion is, which of these terms corresponds to the lowestenergy state??Use Hund’s Rules to determine!
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>Hund’s Rules1. State with the largest value of S is most stable and stabilitydecreases with decreasing S.2. For states with same values of S, the state with the largestvalue of L is the most stable.3. If states have same values of L and S then, for a subshellthat is less than half filled, state with smallest J is moststable; for subshells that are more than half filled, state withlargest value of J is most stable.
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>Here we are considering the terms 3 D, 3 P, 3 S, 1 D, 1 P, 1 S. Interms of stability we can rank these terms as1S1P1D3S3P3D Most stableGiven that the 3 D states are most stable, which of these termscorrespond to the most stable state?
<strong>Atomic</strong> <strong>Term</strong> <strong>Symbols</strong>Since the two p subshells are less than half filled, we wouldpredict that the 3 D 1 term corresponds to the most stablestate!Simple approach for finding the ground state term symbol forany atom:1. Find maximum value of S consistent with the PauliExclusion Principle – S = S max .2. For S = S max , find the maximum value of L consistent withthe Pauli Exclusion Principle – L = L max .3. Apply Hund’s Rules to find J for most stable state.