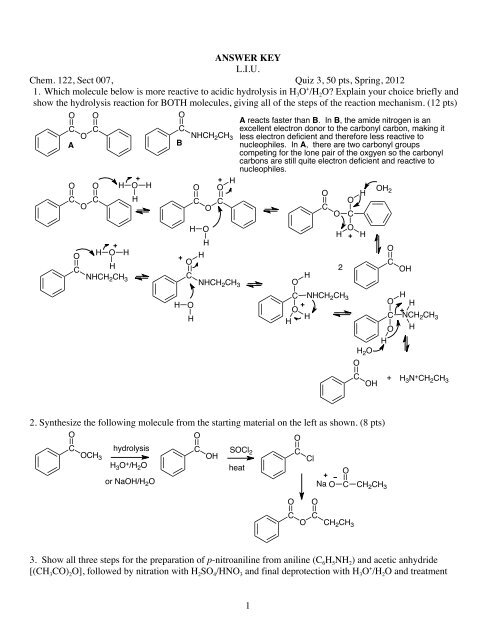
1 ANSWER KEY L.I.U. Chem. 122, Sect 007, Quiz 3, 50 pts ... - myweb
1 ANSWER KEY L.I.U. Chem. 122, Sect 007, Quiz 3, 50 pts ... - myweb
1 ANSWER KEY L.I.U. Chem. 122, Sect 007, Quiz 3, 50 pts ... - myweb
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
with NH 4 OH. For step one and step three you must the complete reaction mechanism, including the reactionthat occurs when NH 4 OH is added. (10 <strong>pts</strong>)(1)O OC CNH 2 CH 3 O CH3heatHNHOC OCH 3OCCH 3NHHOC CH 3OO CCH 3NHOCOCH 3 + HO CCH 3(2) NHOCCH 3HNO 3H 2 SO 4NHOC CH 3+ orthoO H O H(3)N C CH 3 HHNONO 22NHOCHH 2 OCH 3NO 2NOCH OHHOH 2NH + H OH- H H O H4 OHHHNN C CHNN H3HH OHHNO HNONONO 2 2224. A graduate student doing research had the following TLC plate. Lane one is starting material, lane two is thedesired product and lane three is the reaction mixture that the student isolated. (a) Did the reaction go tocompletion? Explain briefly. (b) Was the desired product formed as desired? Explain. (c) Did the reactionproduce one pure compound? Explain briefly. (10 <strong>pts</strong>)1 2 3HCH 3H(a) The reaction did NOT go to completion because there is still a spot in the product(lane 3) that corresponds to the starting material.(b) The desired product was formed, since there is a spot in lane 3 that correspondsto the desired product in lane 2.(c) The product was not pure. Besides the remaining starting material, there was oneother compound produced, an impurity.2
5. In the unknown amine experiment, an unknown amine formed one layer when treated with benzenesulfonylchloride (C 6 H 5 SO 2 Cl) in aqueous KOH solution and gave off tiny bubbles when treated with sodium nitrite andhydrochloric acid. Was the amine primary, secondary or tertiary? Explain your answer by showing all of thereactions that took place. (10 <strong>pts</strong>)The amine was primary.R NH 2 + Cl SOOKOHH 2 ORH O ONHClRHNHOSOHO - N SOHO H OR N SROOsoluble in water insoluble in water3