micro-oxygenation in contemporary winemaking - Cape Wine ...
micro-oxygenation in contemporary winemaking - Cape Wine ...
micro-oxygenation in contemporary winemaking - Cape Wine ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
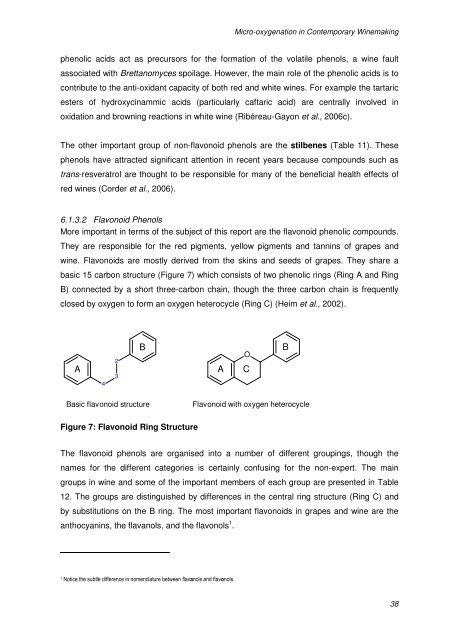
Micro-<strong>oxygenation</strong> <strong>in</strong> Contemporary W<strong>in</strong>emak<strong>in</strong>gphenolic acids act as precursors for the formation of the volatile phenols, a w<strong>in</strong>e faultassociated with Brettanomyces spoilage. However, the ma<strong>in</strong> role of the phenolic acids is tocontribute to the anti-oxidant capacity of both red and white w<strong>in</strong>es. For example the tartaricesters of hydroxyc<strong>in</strong>ammic acids (particularly caftaric acid) are centrally <strong>in</strong>volved <strong>in</strong>oxidation and brown<strong>in</strong>g reactions <strong>in</strong> white w<strong>in</strong>e (Ribéreau-Gayon et al., 2006c).The other important group of non-flavonoid phenols are the stilbenes (Table 11). Thesephenols have attracted significant attention <strong>in</strong> recent years because compounds such astrans-resveratrol are thought to be responsible for many of the beneficial health effects ofred w<strong>in</strong>es (Corder et al., 2006).6.1.3.2 Flavonoid PhenolsMore important <strong>in</strong> terms of the subject of this report are the flavonoid phenolic compounds.They are responsible for the red pigments, yellow pigments and tann<strong>in</strong>s of grapes andw<strong>in</strong>e. Flavonoids are mostly derived from the sk<strong>in</strong>s and seeds of grapes. They share abasic 15 carbon structure (Figure 7) which consists of two phenolic r<strong>in</strong>gs (R<strong>in</strong>g A and R<strong>in</strong>gB) connected by a short three-carbon cha<strong>in</strong>, though the three carbon cha<strong>in</strong> is frequentlyclosed by oxygen to form an oxygen heterocycle (R<strong>in</strong>g C) (Heim et al., 2002).A423BAOCBBasic flavonoid structureFlavonoid with oxygen heterocycleFigure 7: Flavonoid R<strong>in</strong>g StructureThe flavonoid phenols are organised <strong>in</strong>to a number of different group<strong>in</strong>gs, though thenames for the different categories is certa<strong>in</strong>ly confus<strong>in</strong>g for the non-expert. The ma<strong>in</strong>groups <strong>in</strong> w<strong>in</strong>e and some of the important members of each group are presented <strong>in</strong> Table12. The groups are dist<strong>in</strong>guished by differences <strong>in</strong> the central r<strong>in</strong>g structure (R<strong>in</strong>g C) andby substitutions on the B r<strong>in</strong>g. The most important flavonoids <strong>in</strong> grapes and w<strong>in</strong>e are theanthocyan<strong>in</strong>s, the flavanols, and the flavonols 1 .1Notice the subtle difference <strong>in</strong> nomenclature between flavanols and flavonols.38