ACCORD SOP FORM INDEX Attachment/Template SOP Title SOP ...
ACCORD SOP FORM INDEX Attachment/Template SOP Title SOP ...
ACCORD SOP FORM INDEX Attachment/Template SOP Title SOP ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
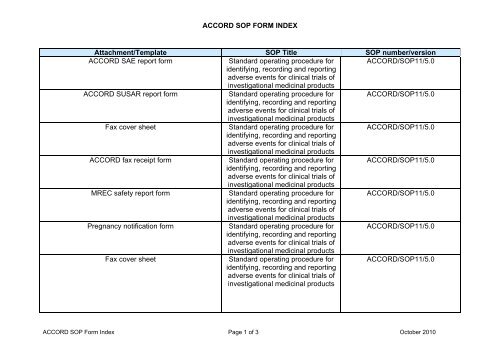
<strong>ACCORD</strong> <strong>SOP</strong> <strong>FORM</strong> <strong>INDEX</strong><strong>Attachment</strong>/<strong>Template</strong> <strong>SOP</strong> <strong>Title</strong> <strong>SOP</strong> number/version<strong>ACCORD</strong> SAE report formStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>11/5.0identifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal products<strong>ACCORD</strong> SUSAR report formStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>11/5.0identifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal productsFax cover sheetStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>11/5.0identifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal products<strong>ACCORD</strong> fax receipt formStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>11/5.0identifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal productsMREC safety report formStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>11/5.0identifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal productsPregnancy notification formStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>11/5.0identifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal productsFax cover sheetStandard operating procedure foridentifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal products<strong>ACCORD</strong>/<strong>SOP</strong>11/5.0<strong>ACCORD</strong> <strong>SOP</strong> Form Index Page 1 of 3 October 2010
<strong>Attachment</strong>/<strong>Template</strong> <strong>SOP</strong> <strong>Title</strong> <strong>SOP</strong> number/versionAE reporting flowchartStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>11/5.0identifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal productsIdentifying AEs flowchartStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>11/5.0identifying, recording and reportingadverse events for clinical trials ofinvestigational medicinal productsNRES safety reporting formStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>12/2.0identifying, recording and reportingadverse events for research otherthan clinical trials of investigationalmedicinal productsAnnual progress report form (non-CTIMPs) Standard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>15/2.0preparing and submitting progressand safety reportsAnnual progress report form (CTIMPs)Standard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>15/2.0preparing and submitting progressand safety reports<strong>ACCORD</strong> annual safety report formStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>15/2.0preparing and submitting progressand safety reports<strong>ACCORD</strong> annual safety report form (explanatory text) Standard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>15/2.0preparing and submitting progressand safety reportsNRES safety report covering formStandard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>15/2.0preparing and submitting progressand safety reportsNRES declaration of the End of a Study form Standard operating procedure forpreparing and submitting progressand safety reports<strong>ACCORD</strong>/<strong>SOP</strong>15/2.0<strong>ACCORD</strong> <strong>SOP</strong> Form Index Page 2 of 3 October 2010
<strong>Attachment</strong>/<strong>Template</strong> <strong>SOP</strong> <strong>Title</strong> <strong>SOP</strong> number/versionDeclaration of the end of trial notification form Standard operating procedure for <strong>ACCORD</strong>/<strong>SOP</strong>15/2.0preparing and submitting progressand safety reportsEnd of trial notification formStandard operating procedure for <strong>ACCORD</strong>/ECTU/<strong>SOP</strong>16/3.0study closureNRES declaration of the end of a study form Standard operating procedure for <strong>ACCORD</strong>/ECTU/<strong>SOP</strong>16/3.0study closureMHRA notification of serious breaches of GCP or trialprotocol formStandard operating procedure forescalation and notification ofserious breaches of GCP or the<strong>ACCORD</strong>/<strong>SOP</strong>25/2.0<strong>ACCORD</strong> log of protocol deviations,violations, serious breaches and urgent safetymeasures<strong>ACCORD</strong> protocol violation formNotification of amendment formUrgent safety measure formtrial protocolStandard operating procedure formanagement of protocol deviations,violations and urgent safetymeasuresStandard operating procedure formanagement of protocol deviations,violations and urgent safetymeasuresStandard operating procedure formanagement of protocol deviations,violations and urgent safetymeasuresStandard operating procedure formanagement of protocol deviations,violations and urgent safetymeasures<strong>ACCORD</strong>/<strong>SOP</strong>53/2.0<strong>ACCORD</strong>/<strong>SOP</strong>53/2.0<strong>ACCORD</strong>/<strong>SOP</strong>53/2.0<strong>ACCORD</strong>/<strong>SOP</strong>53/2.0<strong>ACCORD</strong> <strong>SOP</strong> Form Index Page 3 of 3 October 2010