ACCORD SOP INDEX PAGE
ACCORD SOP INDEX PAGE
ACCORD SOP INDEX PAGE
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
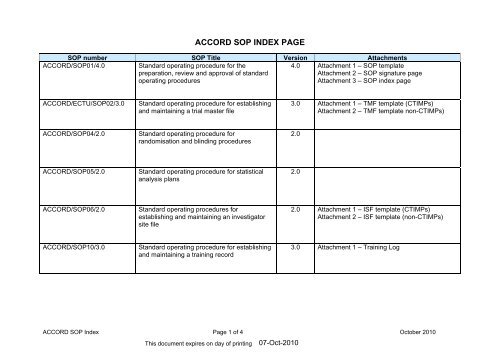
<strong>ACCORD</strong> <strong>SOP</strong> <strong>INDEX</strong> <strong>PAGE</strong><strong>SOP</strong> number <strong>SOP</strong> Title Version Attachments<strong>ACCORD</strong>/<strong>SOP</strong>01/4.0Standard operating procedure for thepreparation, review and approval of standardoperating procedures4.0 Attachment 1 – <strong>SOP</strong> templateAttachment 2 – <strong>SOP</strong> signature pageAttachment 3 – <strong>SOP</strong> index page<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>02/3.0Standard operating procedure for establishingand maintaining a trial master file3.0 Attachment 1 – TMF template (CTIMPs)Attachment 2 – TMF template non-CTIMPs)<strong>ACCORD</strong>/<strong>SOP</strong>04/2.0Standard operating procedure forrandomisation and blinding procedures2.0<strong>ACCORD</strong>/<strong>SOP</strong>05/2.0Standard operating procedure for statisticalanalysis plans2.0<strong>ACCORD</strong>/<strong>SOP</strong>06/2.0Standard operating procedures forestablishing and maintaining an investigatorsite file2.0 Attachment 1 – ISF template (CTIMPs)Attachment 2 – ISF template (non-CTIMPs)<strong>ACCORD</strong>/<strong>SOP</strong>10/3.0Standard operating procedure for establishingand maintaining a training record3.0 Attachment 1 – Training Log<strong>ACCORD</strong> <strong>SOP</strong> Index Page 1 of 4 October 2010
<strong>SOP</strong> number <strong>SOP</strong> Title Version Attachments<strong>ACCORD</strong>/<strong>SOP</strong>11/5.0Standard operating procedure for identifying,recording and reporting adverse events forclinical trials of investigational medicinalproducts5.0 Attachment 1 – SAE report templateAttachment 2 – SUSAR report templateAttachment 3 – Fax cover sheetAttachment 4 – <strong>ACCORD</strong> fax receipt formAttachment 5 – MREC safety report formAttachment 6 – Pregnancy notificationformAttachment 7 – AE reporting flowchartAttachment 8 – Identifying AEs flowchart<strong>ACCORD</strong>/<strong>SOP</strong>12/2.0<strong>ACCORD</strong>/<strong>SOP</strong>13/3.0<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>14/2.0<strong>ACCORD</strong>/<strong>SOP</strong>15/2.0Standard operating procedure for identifying,recording and reporting adverse events forresearch other than clinical trials ofinvestigational medicinal productsStandard operating procedure for archivingclinical research dataStandard operating procedure for writing aprotocol to good clinical practiceStandard operating procedure for preparingand submitting progress and safety reports2.0 Attachment 1 – REC reporting form3.02.0 Attachment 1 – Protocol template for CTIMPsAttachment 2 – Protocol template for CTIMPswith explanatory text2.0 Attachment 1 – Annual Progress Report (non-CTIMPs)Attachment 2 – Annual Progress Report(CTIMPs)Attachment 3 – <strong>ACCORD</strong> annual safety reporttemplateAttachment 4 – <strong>ACCORD</strong> annual safety reporttemplate (explanatory text)Attachment 5 – NRES safety report coveringformAttachment 6 – NRES Declaration of the End of<strong>ACCORD</strong> <strong>SOP</strong> Index Page 2 of 4 October 2010
<strong>SOP</strong> number <strong>SOP</strong> Title Version Attachmentsa StudyAttachment 7 - Declaration of the End of TrialNotification Form<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>16/3.0<strong>ACCORD</strong>/<strong>SOP</strong>18/2.0<strong>ACCORD</strong>/<strong>SOP</strong>/19/2.0<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>21/2.0<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>22/2.0<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>23/2.0<strong>ACCORD</strong>/ECTU/<strong>SOP</strong>24/2.0Standard operating procedure for studyclosureStandard operating procedure for study startupStandard operating procedure for recordingand managing research study data on casereport forms and other source documentsStandard operating procedure for validationand functional testing of study databasesStandard operating procedure for datacollection and handling on study databasesStandard operating procedure for studydatabase security measuresStandard operating procedure for alternativedata recording methods3.0 Attachment 1 – End of trial notification formAttachment 2 – Declaration of the end of astudy form2.02.02.02.02.02.0<strong>ACCORD</strong> <strong>SOP</strong> Index Page 3 of 4 October 2010