Intermolecular forces - CRM2
Intermolecular forces - CRM2
Intermolecular forces - CRM2
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
can be expressed using the Madelung constant, α = 1.7476,U C = −Q 2 2α a2 · 1.7476= −1389.9 × kJ/mol = −861.3kJ/mol (2.7)5.64The Born-Mayer potential has two unknown parameters, B and ρ.◦ Repulsion energyU R = U expcoh − U C = −764.4 + 861.3 = 96.9kJ/mol (2.8)◦ Condition of equilibrium in the minimum of the lattice energy∂U coh∂a2α= −Q2a − 6B2 2ρ e−a/2ρ = − U ca − U R2ρ = 0 (2.9)Effective ”ion radius” is obtained from the repulsion and Madelung energies◦ The B parameter of the Born-Mayer potential isB =ρ = − 1 U R· a = 0.3164 (2.10)2 U CU R6e −a/2ρ = 1.168 · 105 kJ/mol (2.11)The quality of this potential can be checked by calculating the bulk modulusK = V ∂2 U coh∂V 2 (2.12)where the volume of 1 mole NaCl is V = N A a 3 /4. The volume derivative can becalculated as lattice-parameter derivative:( ) −1∂ ∂V∂V = ∂(2.13)∂a ∂aBulk modulus for the NaCl structureK = N Aa 34Second derivative of the cohesion energy∂ 2 U coh∂a 2Theoretical bulk modulus= − ∂ ∂aK = 49N A a( ) 2 4 ∂ 2 U coh= 4 ∂ 2 U coh(2.14)3N A a 2 ∂a 2 9N A a ∂a 2( ) Q 2 2α− 1 ∂U Ra 2 2ρ ∂a = 2 a U 2 C + 14ρ U 2 R (2.15)( 2UCConversion to gigapascal 1 kJ/mol/Å 3 = 1.667 GPa, i.e.a 2K theor = 24.57 GPa+ U R4ρ 2 )= 14.8016 kJ/mol/Å 3 (2.16)16K exp = 24 GPa
2.4.2 Rare gas crystalCohesion energy is the sum of pair potentialsU coh = N 2◦ Ar crystallizes in fcc lattice◦ lattice parameter a = 5.3109Å◦ ions are in (0,0,0) and ( 1 2 , 1 2 , 1 2 )◦ lattice energy U latt = −8.4732 kJ/mol(after zpe correction)∑U LJ (r ij ) (2.17)ijwhere N = 4, number of atoms in the unit cell, and U LJpotential:[ (σ ) 12 ( σ) ] 6U LJ (r) = 4ε −r ris the Lennard-Jones(2.18)Lattice sums can be calculated from the number of neighbours at the multiples ofthe nearest-neighbour distance d = a/ √ 2:General form of the lattice sumsshells∑( ) n σm i =f i dishell 1√2√3 4√5 ir d 2d 3d 2d 5d fi · dm i 12 6 24 12 24 m ishells∑Lattice sum for the 6- and 12-potentialsim i f −ni( σd) n= pn( σdi 1 5 fcc(∞) hcp (∞)p 12 = ∑ i m if −12i 12 12.13114 12.13188 12.13229p 6 = ∑ i m if −12i 12 14.01839 14.45392 14.45489Cohesion energy of the rare-gas lattice in terms of lattice sumsU coh = Nε [ ( σ) 12 ( σ) ] 6p 12 − p62 d dStability condition) n(2.19)(2.20)∂U(cohσ) 12∂d = −12p 1( σ) 612d d + 6p 16d d = 0 (2.21)17
yields the σ parameter in the function of d, nearest-neighbour distanced = 6 √2p12p 6· σ = 1.09026σ (2.22)Lattice constant a = 1.54186σ.Cohesion energy can be expressed in the function of ε.[U coh = Nε ( ) 2 ( ) ]p6p6p 12 − p 6 (2.23)2 2p 12 2p 12can be expressed in the function of εU coh = − N 8p 2 6p 12ε = −2.1525 N ε (2.24)Experimental lattice parameters from ”best” Lennard-Jones potentials:NeArKrXeε σ a U coh a U coh2.4.3 Three-body <strong>forces</strong> and the structure of rare gas solids2.5 Equation of state – virial coeffiicients2.5.1 van der Waals equation of stateEquation of state for an ideal gas (noninteracting point masses)P V = RT (2.25)where P is the pressure, V is molar volume, and R = 8.31J K −1 mol −1 is the gasconstant.18
The standard molar volume V s (1atm, T=273.15 K o of real gasesare different from the ideal value(22414 cm 3 ). Considerable deviationscan be observed in the behaviourof the compression factor,z = P V . RTThese deviations can be accounted for by the van der Waals equation of state, byintroducing two empirical constants, a and b(P + a )(V − b) = RT (2.26)V◦ excluded volume in a binary collision 4 3 πσ3b = 2 3 πN Aσ 3 (2.27)◦ reduction of pressure due to intermolecular attraction: the number of binaryinteractions is proportional to the square of the density, (a/V 2 )Virial equation of state – power series of (1/V )z = 1 + B 2V + B 3V 2 + B 4V 3 + . . . (2.28)2.5.2 Equations of state - generalitiesThe total energy is a function of x α external parametersE = E(x 1 , x 2 , . . . x n ) (2.29)Define generalized <strong>forces</strong> corresponding to external parametersX α = − ∂E∂x α(2.30)19
In statistical mechanics the equations of state describe the relationship of the externalparameters, the generalized <strong>forces</strong> and the temperature:X α = kT ∂ ln Z∂x α(2.31)where Z is the partition function. For the special case of volume (x α = V ) andpressure (X α = P )P = kT ∂ ln Z(2.32)∂VThe semi-classical partition function∫∫ ∫1Z =N!h 3N . . . e −(K+U)/kT d 3 p 1 d 3 p 2 . . . d 3 p N d 3 r 1 d 3 r 2 . . . d 3 r N (2.33)is a product of two terms, depending on the kinetic and the potential energy, respectively:K = 1 ∑p22m j U = 1 ∑u jk (2.34)2Z =∫∫1N!h 3N∫. . .∫∫ ∫e −K/kT d 3 p 1 d 3 p 2 . . .d 3 p N . . . e −U/kT d 3 r 1 d 3 r 2 . . .d 3 r N (2.35)} {{ }Z U} {{ }(2πmkT ) 3N/2jkPartition functionZ = 1 N!( 2πmkTh 2 ) 3N/2· Z U (2.36)2.5.3 Equations of state - ideal gasFor an ideal gas u ij → 0 (or if T is high kT → ∞)e −U/kT → 1 Z U = V N (2.37)and the corresponding partition functionln Z id = ln 1 N! + N [ln V + 3 2 ln kT + 3 2 ln ( 2πmh 2 )](2.38)Equation of state for an ideal gasorP = kT ∂ ln Z id∂V= NkTV(2.39)P V = NkT (2.40)20
2.5.4 Equations of state - real gasFor a real gas with low number density (n = N/V small) an approximate expressioncan be obtained for the configurational partition function.Take the average potential energy with β = 1/kT , which satisfies the followingrelationship ∫∫∫Ue −βU d 3 r 1 d 3 r 2 . . . d 3 r NU = ∫∫∫ = − ∂e −βU d 3 r 1 d 3 r 2 . . . d 3 r N ∂β ln Z U (2.41)The logarithm of the partition function isln Z U (β) = N ln V −∫ β0U(β ′ )dβ ′ (2.42)In a low-density system the U, the average potential energy of 1/2N(N −1) moleculepairs is equal N 2 /2-fold of the average potential energy of an arbitrary pair ofmolecules:U = 1 2 N(N − 1) ≈ 1 2 N 2 u (2.43)The average potential energy of a pair of molecules∫u(R)e −βu(R) d 3 Ru = ∫e −βu(R) d 3 R= − ∂ ∫∂β lne −βu(R) d 3 R (2.44)Since u(R) = 0 almost everywhere, excepted the small distances, it is worthwhile totransform the integral as∫e −βu(R) d 3 R =∫ [1+(e −βu(R) − 1 )] d 3 R = V + I(β) (2.45)The quantity in parentheses is the Mayer-function f(R) = e −βu(R) − 1, and itsintegral over the intermolecular separation, R isI(β) = 4π∫ ∞0(e −βu(R) − 1 ) R 2 dR (2.46)Calculate the average potential energy for a pair of moleculesu = ∂[ (∂ln [V + I(β)] = ln V + ln 1 + I(β) )]≈ − 1 ∂β ∂βV V · ∂I(β)∂βwhich is substituted in the expression of the configurational partition functionThe equation of state is(2.47)ln Z U (β) = N ln V + 1 N 2[I(0) − I(β)] (2.48)2 VPkT = ∂ ln Z∂V= ∂ ln Z = N ∂V U V − 1 N 2I(β) (2.49)2 V221
orP VRT = P VNkT = 1 − 4π 2NV∫ ∞This is the first term of the virial equation of state:and the virial coefficient isB 2 (T ) = −2πNz = P VRT = 1 + ∞∑∫ ∞00n=1(e −βu(R) − 1 ) R 2 dR (2.50)B n+1V n (2.51)(e −u(R)/kT − 1 ) R 2 dR (2.52)2.5.5 Temperature dependence of the virial coefficientT \R small (u > 0) large (u < 0)low+ – – B 2 < 0kT < u 0highkT > u 0+ – B 2 > 022
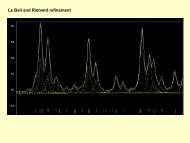
2.5.6 Third virial coefficientFor a strictly additive potentialB add3 = − N 3 8π3 ∫∫∫B 3 (T ) = B add3 + ∆B 3 (2.53)f 12 f 13 f 23 r 12 r 13 r 23 dr 12 dr 12 dr 12 (2.54)The non-additivity correction depends on the 3-body potential ∆U 3∆B 3 = − N 3 8π3 ∫∫∫ (e−∆U 3 /kT − 1 ) e −U 3/kT r 12 r 13 r 23 dr 12 dr 12 dr 12 ?? (2.55)2.6 VRT spectroscopy - water dimerCalculated splitting due to tunneling is very sensitive to the height and shape of thebarrier – stringent test of the PES.23
2.6.1 VRT spectroscopy - water dimerExperimental vs. calculated VRT splittings with different water-water potentials.None of the 14 potentials was found to be fully satisfactory.Fellers, Braly, Saykally, Leforestier, J. Chem. Phys. 110 (1999) 6306)24