INSIDE: - Medical Device Daily
INSIDE: - Medical Device Daily
INSIDE: - Medical Device Daily
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
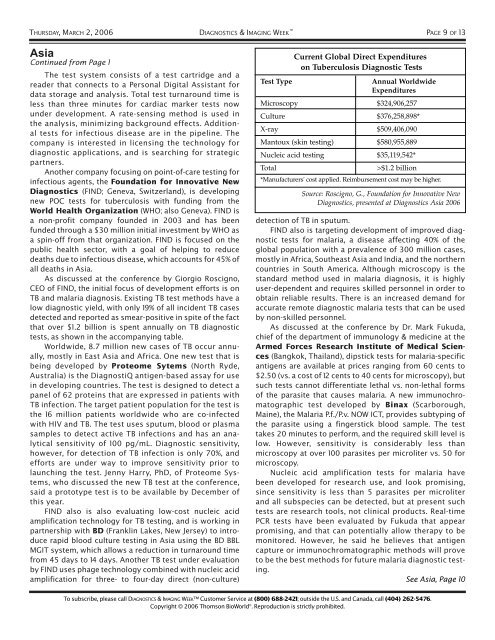
THURSDAY, MARCH 2, 2006 DIAGNOSTICS &IMAGING WEEK PAGE 9 OF 13AsiaContinued from Page 1The test system consists of a test cartridge and areader that connects to a Personal Digital Assistant fordata storage and analysis. Total test turnaround time isless than three minutes for cardiac marker tests nowunder development. A rate-sensing method is used inthe analysis, minimizing background effects. Additionaltests for infectious disease are in the pipeline. Thecompany is interested in licensing the technology fordiagnostic applications, and is searching for strategicpartners.Another company focusing on point-of-care testing forinfectious agents, the Foundation for Innovative NewDiagnostics (FIND; Geneva, Switzerland), is developingnew POC tests for tuberculosis with funding from theWorld Health Organization (WHO; also Geneva). FIND isa non-profit company founded in 2003 and has beenfunded through a $30 million initial investment by WHO asa spin-off from that organization. FIND is focused on thepublic health sector, with a goal of helping to reducedeaths due to infectious disease, which accounts for 45% ofall deaths in Asia.As discussed at the conference by Giorgio Roscigno,CEO of FIND, the initial focus of development efforts is onTB and malaria diagnosis. Existing TB test methods have alow diagnostic yield, with only 19% of all incident TB casesdetected and reported as smear-positive in spite of the factthat over $1.2 billion is spent annually on TB diagnostictests, as shown in the accompanying table.Worldwide, 8.7 million new cases of TB occur annually,mostly in East Asia and Africa. One new test that isbeing developed by Proteome Sytems (North Ryde,Australia) is the DiagnostiQ antigen-based assay for usein developing countries. The test is designed to detect apanel of 62 proteins that are expressed in patients withTB infection. The target patient population for the test isthe 16 million patients worldwide who are co-infectedwith HIV and TB. The test uses sputum, blood or plasmasamples to detect active TB infections and has an analyticalsensitivity of 100 pg/mL. Diagnostic sensitivity,however, for detection of TB infection is only 70%, andefforts are under way to improve sensitivity prior tolaunching the test. Jenny Harry, PhD, of Proteome Systems,who discussed the new TB test at the conference,said a prototype test is to be available by December ofthis year.FIND also is also evaluating low-cost nucleic acidamplification technology for TB testing, and is working inpartnership with BD (Franklin Lakes, New Jersey) to introducerapid blood culture testing in Asia using the BD BBLMGIT system, which allows a reduction in turnaround timefrom 45 days to 14 days. Another TB test under evaluationby FIND uses phage technology combined with nucleic acidamplification for three- to four-day direct (non-culture)Current Global Direct Expenditureson Tuberculosis Diagnostic TestsTest TypeAnnual WorldwideExpendituresMicroscopy $324,906,257Culture $376,258,898*X-ray $509,406,090Mantoux (skin testing) $580,955,889Nucleic acid testing $35,119,542*Total>$1.2 billion*Manufacturers' cost applied. Reimbursement cost may be higher.Source: Roscigno, G., Foundation for Innovative NewDiagnostics, presented at Diagnostics Asia 2006detection of TB in sputum.FIND also is targeting development of improved diagnostictests for malaria, a disease affecting 40% of theglobal population with a prevalence of 300 million cases,mostly in Africa, Southeast Asia and India, and the northerncountries in South America. Although microscopy is thestandard method used in malaria diagnosis, it is highlyuser-dependent and requires skilled personnel in order toobtain reliable results. There is an increased demand foraccurate remote diagnostic malaria tests that can be usedby non-skilled personnel.As discussed at the conference by Dr. Mark Fukuda,chief of the department of immunology & medicine at theArmed Forces Research Institute of <strong>Medical</strong> Sciences(Bangkok, Thailand), dipstick tests for malaria-specificantigens are available at prices ranging from 60 cents to$2.50 (vs. a cost of 12 cents to 40 cents for microscopy), butsuch tests cannot differentiate lethal vs. non-lethal formsof the parasite that causes malaria. A new immunochromatographictest developed by Binax (Scarborough,Maine), the Malaria P.f./P.v. NOW ICT, provides subtyping ofthe parasite using a fingerstick blood sample. The testtakes 20 minutes to perform, and the required skill level islow. However, sensitivity is considerably less thanmicroscopy at over 100 parasites per microliter vs. 50 formicroscopy.Nucleic acid amplification tests for malaria havebeen developed for research use, and look promising,since sensitivity is less than 5 parasites per microliterand all subspecies can be detected, but at present suchtests are research tools, not clinical products. Real-timePCR tests have been evaluated by Fukuda that appearpromising, and that can potentially allow therapy to bemonitored. However, he said he believes that antigencapture or immunochromatographic methods will proveto be the best methods for future malaria diagnostic testing.See Asia, Page 10To subscribe, please call DIAGNOSTICS &IMAGING WEEK Customer Service at (800) 688-2421; outside the U.S. and Canada, call (404) 262-5476.Copyright © 2006 Thomson BioWorld ® . Reproduction is strictly prohibited.