Electronic Transitions
Electronic Transitions
Electronic Transitions
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
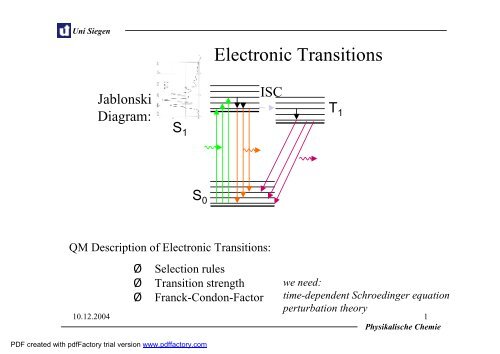
Uni SiegenJablonskiDiagram:<strong>Electronic</strong> <strong>Transitions</strong>ISCS 1S 0T 1QM Description of <strong>Electronic</strong> <strong>Transitions</strong>:‣ Selection rules‣ Transition strength‣ Franck-Condon-Factorwe need:time-dependentSchroedinger equationperturbation theory10.12.2004 1Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenQM Description of <strong>Electronic</strong> <strong>Transitions</strong>2. Ansatzfor thewavefunctions of the perturbed system:( )Ψ = ∑ Ψcn2nc t 0n nprobability that state n is populated2c 22c 1for t=0, system is in the state ground state n=0Ψ0( t = 0) = c0 ( t = 0) Ψ0( t = 0) = ϕ0c 0(t=0)=1 and c n(t=0)=0 for all other n(without perturbation the system would always stay in the ground state n=0)c 02at t=0, perturbation is switched on, with time c 0decreases and c nincrease⇒ system undergoes transitions between states10.12.2004 5Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenQM Description of <strong>Electronic</strong> <strong>Transitions</strong>integrationon both sides∫∑Vnc&Ψn0*mΨ0ndV= −Vih∫ ∑ncnΨ0*mH´ PΨ0ndVV: Volume of moleculewe use thateigenfunctionsare orthogonal∫V∫VΨΨ0*m0*mΨΨ0n0ndVdV= 1= 0for m=nfor m≠ni0*c&m= − ∑cn∫ ΨmHPΨhnV0ndVc&mi= − h∑ncnHPmn…..calculationstransition matrix elementP Pwith Hmn= Hmn( 0) exp( ωmnt)and ω 1 ( E − E )mn= hmnP 0*Hmn = ∫ ΨmHPΨV0ndVLight at frequency ω mnTransition energy hω mn10.12.2004 7Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenProbability of <strong>Electronic</strong> <strong>Transitions</strong>probability to find system in state mat time tif it was in state n at t=0transition probability n→mResult:wmn=cw( ω t)mmn2=( ωmnt/ 2)2( ω )24sinPH2mnhmnd cm=dt2sinmn mnH 02 Ph ωmn() 02transition probability depends on the transition matrix elementP 0*0Hmn = ∫ ΨmHPΨndV general formV-can be applied to any system( ) 22wavefunction of thefinal stateHamilton operatorof the perturbationwavefunctionof the initial state10.12.2004 8Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenQM Description of <strong>Electronic</strong> <strong>Transitions</strong>In our case: Perturbation by light⇒ light interacts with dipole momentH Pr r rrEp = eExr rp = ex=rrr r ri( )[ k ⋅x−ωt]Ex,t=dipole momentE0elight as harmonicemplane wavetransition matrixelementr r 0( x) dVrr0* i( ) e E e[ kx0 = Ψ]PHmn ∫ m 0ΨVnis extremely difficult to calculate…..Idea: Size of the molecule x (
Uni SiegenProbabiltyof <strong>Electronic</strong> <strong>Transitions</strong>Using the Electric dipole approximation we getrPrHmn ∫ mΨ0* 0( 0) = eE Ψ x dVVnPH mn( 0)is called “transitiondipole moment”because of exr⇒w mnr∝ E2∝I⇒transition probability (absorption) dependslinearly on the intensity of the lightPhoton picture:Excitation probability depends linearly on thenumber of photonsw mn∝ I =nhω10.12.2004 10Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni Siegen<strong>Electronic</strong> <strong>Transitions</strong> in H-AtomsApplication of the model:Hydrogen atom: lowest state 1Soptical transition between 1S and 2S?both states are symmetric:(angular momentum l=0)Ψ0 r 01SΨ1Sr( − x) = ( x)Ψy0 r 02SΨ2SSr( − x) = ( x)x(skippedEe)H P210H P210∞∞00* 00* 00* 0( ) = Ψ xΨdV = Ψ xΨdV + Ψ xΨdV∫−∞2r1∫02∞∞0* 00*0( ) = Ψ ( x) xΨ( x) dV + Ψ ( − x)( − x) Ψ ( − x)dV∫02rr1r∫0r12r∫−∞integral split in twor2r11rr r r= ∫∫∞∞0* 00* 0( 0) Ψ ( x) xΨ( x) dV − Ψ ( x) xΨ( x) dV 0H P21 2 12 1=00⇒10.12.2004 11Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.comrrrno electronic transition between 1S and 2S !!!
Uni Siegen<strong>Electronic</strong> <strong>Transitions</strong> in H-AtomsApplication of the model:Hydrogen atom: lowest state 1Soptical transition between 1S and 2P?2P is asymmetric:(angular momentum l=1)Ψ0 r 02PΨ2Pr( − x) = − ( x)-y+P xxH P210∞∞0* 00*0( ) = Ψ ( x) xΨ( x) dV + Ψ ( − x)( − x) Ψ ( − x)dV∫02rrr r r= ∫∫1r∞∞0* 00* 0( 0) Ψ ( x) xΨ( x) dV + Ψ ( x) xΨ( x) dV 0H P21 2 12 1≠00∫02rrrrr1r⇒transition 1S-2P is possible10.12.2004 12Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenA function withA function withSelection RulesrΨ −rΨ − xrr= −Ψ x( x) = Ψ( x)The operator of the electric field is ungerader r− x = −( ) ( )( ) x∞∞H P () 0 gugdV = udV 0Transition between two gerade(g) functions= ∫ ∫21=−∞−∞is called gerade(symmetric)is called ungerade(asymmetric)∆l=0“forbidden”Transition between gerade(g) and ungerade(u) function∞∞() 0 uugdV = gdV 0= ∫ ∫H P21≠−∞−∞“allowed”∆l=1Selection rule for electronic transitions: ∆l=1and ∆n≠010.12.2004 13because energy of the system is changed by photon energyPhysikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni Siegen<strong>Electronic</strong> <strong>Transitions</strong>: Particle in a boxground state: g-symmetryΨ0( − x) = ( x)00Ψ00x1 st exited state: u-symmetryΨ0( − x) = − ( x)01Ψ10x=> optical transition between ground and 1 st excited state is allowed2 nd exited state: g-symmetryΨ0( − x) = ( x)02Ψ20x=> optical transition between ground and 2 nd excited state is forbidden⇒selection rule ∆n = ± 110.12.2004 14Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni Siegen<strong>Electronic</strong> <strong>Transitions</strong>JablonskiDiagram:S 1ISCS 0T 1lightsourceAbsorption:dsampleI(d)detectorFluorescence / RamanlightsourcesampleI detectorHow do can we study these transitions?we need‣ 1. light source‣ 2. detector‣ 3. spectral resolution10.12.2004 15Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
1. Light sourcescharacterization of a light source:sourcelightarea An(λ)wavelength λ (energy per photon)E =hc/ λ = h ν = h / 2 πω = h / 2 π kintensity: energy / time / area (=power density)cεr2I = n E / (t A) or I = En number of photons02? source emits more than one wavelength (e.g. lamp)n dλ= n(λ) dλ(number of photons detected within wavelength interval dλ)Ttime averagedλλSpectral radiation density :I(λ) = n(λ) E(λ) / (t A)10.12.2004 16PDF created with pdfFactory trial version www.pdffactory.com
continous outputLamps10.12.2004 17PDF created with pdfFactory trial version www.pdffactory.com
continous outputLampsS 1OD(λ)=log(I0 λ)/I (λ))S 0used for absorption measurements10.12.2004 18PDF created with pdfFactory trial version www.pdffactory.com
Lampsline ouputS 1S 0spectral calibrationfluorescence10.12.2004 19PDF created with pdfFactory trial version www.pdffactory.com
LampsFIR (Far Infra Red): Nernst-Stift (rare earth oxides)Globar (SiC)NIR / Vis: Gas discharge lamps: (Hg, Xe, D 2..)Heated wires (CrNi, W, …)UV: Gas discharge lamps: (Hg, Xe, D 2..)10.12.2004 20PDF created with pdfFactory trial version www.pdffactory.com
LampsBlack body radiation (“heat radiation”)spectral radiant exitance:intensity per wavelengthinterval(number of photonswithin ∆λ)Planck’s law:W( λ,T )⎛⎜⎝ch−5= 8 ⎜ kλTωhcλe⎞−1⎟⎠−110.12.2004 21output spectrum depends on temperaturePDF created with pdfFactory trial version www.pdffactory.com
Laser: Stimulated emission(Light amplification by stimulated emission of radiation)Spontaneous emission:excited stateStimulated emission:excited stateexcited state relaxationone photon is emittedground stateground stateincident photon stimulates emissionof second photon => amplificationprocess is instantaneousemission is isotropic(“no preferred direction”)emission is directedtwo photons out of one: amplification!10.12.2004 22PDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenProbabiltyof <strong>Electronic</strong> <strong>Transitions</strong>P0* 0Transition dipole moment: Hmn( 0) = eE∫ ΨmxΨndVrVrgeneral rule for all states n and m of the systemstimulated emission:excited state nabsorption:excited state mground state mground state nPmn( ) 2 P0 H ( 0 ) 2H =nm⇒ probabilities for stimulated emission and absorption are the same10.12.2004 23Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenLaser: Inversioncompetition between absorption and stimulated emissionN 1,N 2number of atoms in ground state, excited stateexcited state (N 2)excited stateground state (N 1)ground stateN 1>>N 2N 1
Uni SiegenLaser: InversionNormal population:EN 2N 1N(E)N 2/ N 1= g 2/ g 1exp(-(E 2-E 1)/kT)(N 2 )(Boltzmann-Distribution)(e.g. E 2-E 1=2eV, T=300K, g 2=g 1N 2/N 1= 3.6*10 -34 )N 1>> N 2(N 1 )needed: N 1< N 2!Population inversion(N 2 )(N 1 )energy pump10.12.2004 25Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenLaser: 2, 3 and 4-level systems2-level 3-level 4-levelN 2pumpN 2N 1N 2pumppumpN 1N 1inversion notpossible*fast non-radiativerelaxationN 2should have long lifetimeN 1≈ 010.12.2004 26Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenLaser: Elementsmirror(high reflector)R HRdactivemediumenergy pumpR OCmirror(output coupler)essential elements:1. laser active gain medium (3-, 4-level system), amplifies incident radiation2. energy pump, produces population inversion3. optical resonator, stores energy in particular resonator modes10.12.2004 27Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni Siegenfeature:Gain medium:Pumping process:Resonator cavity:Ouput:Laser: Typessemiconductor materialdye moleculesgasdoped crystals, Ruby laser……lightelectric currentlinearringfolded……(1960)(1960) (1962) (1964)cw(continuous wave), pulsed (Q-switching, mode-locking..)fixed frequency, tunable10.12.2004 28Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenGain mediumexample: fluorescent dye moleculeRhodamin 6GN 24-levelabsorptionfluorescencelasingpumpN 1λvibrational relaxationpumplasingamplification (gain) only within fluorescence band10.12.2004 29Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenLaser: Longitudinal resonator modesλ= 2LLmetallic mirror,electric field in ametal is zeroλ=Lλ=L2/3standing wavesMode spectrum: λ= 2 L/m m=1, 2, 3……ν=m c/2Lmode spacing:∆ν=c/2Llasing not for arbitrary wavelength !only for resonator modes10.12.2004 30Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com
Uni SiegenLaser: Longitudinal resonator modesGainthreshold1∆ν=c/2Lν110.12.2004 31Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.comν
Uni SiegenLaser vs. LampsAdavantages of lasers:-high output powers-collimated output beams-narrow spectral linewidth-short output pulses (~4 fs)-coherence……Adavantages of lamps:-high output powers-broad spectral output-cheap……10.12.2004 32Physikalische ChemiePDF created with pdfFactory trial version www.pdffactory.com