Unit 2 Water and Weather - Spokane Public Schools
Unit 2 Water and Weather - Spokane Public Schools
Unit 2 Water and Weather - Spokane Public Schools
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
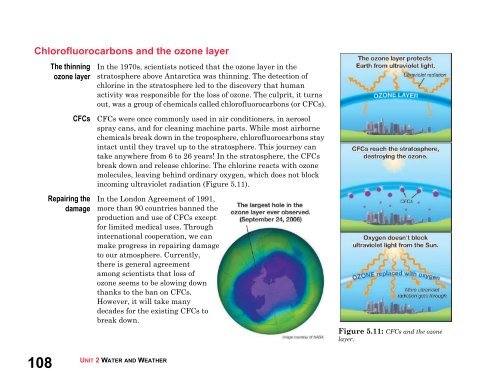
Chlorofluorocarbons <strong>and</strong> the ozone layerThe thinningozone layerCFCsRepairing thedamageIn the 1970s, scientists noticed that the ozone layer in thestratosphere above Antarctica was thinning. The detection ofchlorine in the stratosphere led to the discovery that humanactivity was responsible for the loss of ozone. The culprit, it turnsout, was a group of chemicals called chlorofluorocarbons (or CFCs).CFCs were once commonly used in air conditioners, in aerosolspray cans, <strong>and</strong> for cleaning machine parts. While most airbornechemicals break down in the troposphere, chlorofluorocarbons stayintact until they travel up to the stratosphere. This journey cantake anywhere from 6 to 26 years! In the stratosphere, the CFCsbreak down <strong>and</strong> release chlorine. The chlorine reacts with ozonemolecules, leaving behind ordinary oxygen, which does not blockincoming ultraviolet radiation (Figure 5.11).In the London Agreement of 1991,more than 90 countries banned theproduction <strong>and</strong> use of CFCs exceptfor limited medical uses. Throughinternational cooperation, we canmake progress in repairing damageto our atmosphere. Currently,there is general agreementamong scientists that loss ofozone seems to be slowing downthanks to the ban on CFCs.However, it will take manydecades for the existing CFCs tobreak down.Figure 5.11: CFCs <strong>and</strong> the ozonelayer.108UNIT 2 WATER AND WEATHER