CBA/CQA Comparison Chart - Ombu Enterprises LLC
CBA/CQA Comparison Chart - Ombu Enterprises LLC
CBA/CQA Comparison Chart - Ombu Enterprises LLC
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>CBA</strong>/<strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong><br />
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
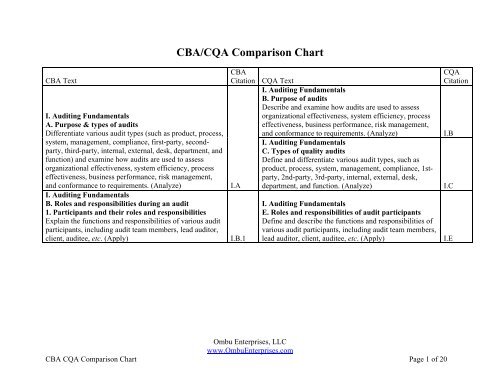
I. Auditing Fundamentals<br />
B. Purpose of audits<br />
Describe and examine how audits are used to assess<br />
Citation<br />
I. Auditing Fundamentals<br />
organizational effectiveness, system efficiency, process<br />
A. Purpose & types of audits<br />
effectiveness, business performance, risk management,<br />
Differentiate various audit types (such as product, process,<br />
and conformance to requirements. (Analyze) I.B<br />
system, management, compliance, first-party, second-<br />
I. Auditing Fundamentals<br />
party, third-party, internal, external, desk, department, and<br />
C. Types of quality audits<br />
function) and examine how audits are used to assess<br />
Define and differentiate various audit types, such as<br />
organizational effectiveness, system efficiency, process<br />
product, process, system, management, compliance, 1st-<br />
effectiveness, business performance, risk management,<br />
party, 2nd-party, 3rd-party, internal, external, desk,<br />
and conformance to requirements. (Analyze)<br />
I. Auditing Fundamentals<br />
I.A department, and function. (Analyze) I.C<br />
B. Roles and responsibilities during an audit<br />
I. Auditing Fundamentals<br />
1. Participants and their roles and responsibilities<br />
E. Roles and responsibilities of audit participants<br />
Explain the functions and responsibilities of various audit<br />
Define and describe the functions and responsibilities of<br />
participants, including audit team members, lead auditor,<br />
various audit participants, including audit team members,<br />
client, auditee, etc. (Apply) I.B.1 lead auditor, client, auditee, etc. (Apply) I.E<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 1 of 20
<strong>CBA</strong> Text<br />
I. Auditing Fundamentals<br />
B. Roles and responsibilities during an audit<br />
2. Ethical, legal, and professional issues<br />
Identify and apply factors of ethical and professional<br />
conduct, concepts of due diligence/due care with respect<br />
to confidentiality, conflict of interest, credibility,<br />
independence, objectivity, and qualifications. Identify<br />
legal and financial ramifications of improper auditor<br />
actions, such as carelessness, negligence, and discovery of<br />
illegal activities or unsafe conditions. Anticipate the effect<br />
that certain audit results can have on an auditee's<br />
regulatory and civil liability. (Apply) I.B.2<br />
ABOVE<br />
ABOVE<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
I. Auditing Fundamentals<br />
F. Ethical, legal, and professional Issues<br />
1. Audit Credibility<br />
Identify and apply ethical factors that influence audit<br />
credibility, such as auditor independence, objectivity, and<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 2 of 20<br />
<strong>CQA</strong><br />
Citation<br />
qualifications. (Apply) I.F.1<br />
I. Auditing Fundamentals<br />
F. Ethical, legal, and professional Issues<br />
2. Liability Issues<br />
Identify potential legal and financial ramifications of<br />
improper auditor actions, such as carelessness and<br />
negligence, in various situations, and anticipate the effect<br />
that certain audit results can have on an auditee's<br />
liability. (Apply) I.F.2<br />
I. Auditing Fundamentals<br />
F. Ethical, legal, and professional Issues<br />
3. Professional Conduct and Responsibilities<br />
Define and apply the concepts of due diligence and due<br />
care, with respect to confidentiality, conflict of interest,<br />
the discovery of illegal activities or unsafe conditions.<br />
(Apply) I.F.3
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
Citation<br />
II. Auditing & Inspection Processes<br />
II. Audit Process<br />
A. Audit preparation & planning<br />
A. Audit preparation and planning<br />
1. Elements of the audit planning process<br />
1. Elements of the audit planning process<br />
Determine and implement steps in audit preparation and<br />
Identify and implement steps in audit preparation and<br />
planning, such as verifying audit authority, establishing<br />
planning, such as verifying audit authority, determining<br />
the purpose, scope, and type of audit, the requirements to<br />
the purpose, scope, type, requirements to audit against,<br />
audit against, and the resources necessary, including the<br />
and identifying the resources necessary, including the<br />
size and number of audit teams. (Evaluate)<br />
II. Auditing & Inspection Processes<br />
A. Audit preparation & planning<br />
2. Auditor selection<br />
Identify and examine various auditor selection criteria,<br />
II.A.1 size and number of audit teams. (Evaluate) II.A.1<br />
such as education, experience, industry background, and<br />
II. Audit Process<br />
subject-matter expertise, and the characteristics that make<br />
A. Audit preparation and planning<br />
auditors effective, such as interpersonal skills, problem-<br />
2. Auditor selection<br />
solving skills, attention to detail, cultural sensitivity,<br />
Identify and examine various auditor selection criteria,<br />
ability to work independently and in a group or on a team.<br />
such as education, experience, industry background, and<br />
(Analyze) II.A.2 subject matter expertise. (Analyze)<br />
III. Auditor Competencies<br />
A. Auditor characteristics<br />
Identify characteristics that make auditors effective, such<br />
as interpersonal skills, problem-solving skills, close<br />
attention to detail, cultural sensitivity, ability to work<br />
II.A.2<br />
ABOVE<br />
independently and in a group or on a team. (Apply) III.A<br />
II. Auditing & Inspection Processes<br />
II. Audit Process<br />
A. Audit preparation & planning<br />
A. Audit preparation and planning<br />
3. Audit-related documentation<br />
3. Audit-related documentation<br />
Identify sources of pre-audit information and examine<br />
Identify the sources of pre-audit information and<br />
audit-related documentation, such as reference materials<br />
examine audit-related documentation, such as reference<br />
and prior audits. (Analyze) II.A.3 materials and prior audits. (Analyze) II.A.3<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 3 of 20
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
II. Audit Process<br />
A. Audit preparation and planning<br />
Citation<br />
II. Auditing & Inspection Processes<br />
5. Auditing tools<br />
A. Audit preparation & planning<br />
Select, prepare, and use checklists, log sheets, sampling<br />
4. Auditing tools<br />
plans, and procedural guidelines in various audit<br />
Select, prepare, and use checklists, log sheets, sampling<br />
situations. (Create)<br />
plans, and procedural guidelines in various audit<br />
[NOTE: Checklists as working papers are covered in II.<br />
situations. (Analyze) II.A.4 B. 3] II.A.5<br />
II. Auditing & Inspection Processes<br />
II. Audit Process<br />
A. Audit preparation & planning<br />
A. Audit preparation and planning<br />
5. Auditing strategies<br />
6. Auditing strategies<br />
Identify and use various tactical methods for conducting<br />
Identify and use various tactical methods for conducting<br />
an audit, such as forward and backward tracing and<br />
an audit, such as forward and backward tracing and<br />
discovery. (Apply) II.A.5 discovery. (Apply) II.A.6<br />
II. Auditing & Inspection Processes<br />
II. Audit Process<br />
A. Audit preparation & planning<br />
A. Audit preparation and planning<br />
6. Logistics<br />
4. Logistics<br />
Identify and organize various audit-related logistics, such<br />
Identify and organize various audit-related logistics, such<br />
as travel, security considerations, and escorts. (Apply)<br />
II. Auditing & Inspection Processes<br />
II.A.6 as travel, security considerations, and escorts. (Analyze) II.A.4<br />
B. Audit performance<br />
II. Audit Process<br />
1. Opening meeting<br />
B. Audit performance<br />
Describe the purpose and scope of an opening meeting,<br />
1. Opening meeting<br />
and the necessary elements for conducting such a meeting.<br />
Describe its purpose, scope, and elements and conduct an<br />
(Apply) II.B.1 opening meeting. (Apply) II.B.1<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 4 of 20
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
Citation<br />
II. Auditing & Inspection Processes<br />
II. Audit Process<br />
B. Audit performance<br />
B. Audit performance<br />
2. Data collection and analysis<br />
2. Data collection and analysis<br />
Select and apply various data collection methods, such as<br />
Select and apply various data collection methods, such as<br />
observing work activities, taking physical measurements,<br />
interviewing people, observing work activities, taking<br />
examining paper and electronic documents, etc., and<br />
physical measurements, and examining paper and<br />
analyze the results. (Evaluate)<br />
II. Auditing & Inspection Processes<br />
II.B.2 electronic documents; perform analysis. (Create) II.B.2<br />
B. Audit performance<br />
III. Auditor Competencies<br />
3. Communication techniques<br />
E. Interviewing techniques<br />
Define and apply appropriate interviewing techniques<br />
Define and apply appropriate interviewing techniques<br />
(e.g., when to use various question types, the significance<br />
(e.g., when to use open-ended and closed question types,<br />
of pauses and their length, when and how to prompt a<br />
determining the significance of pauses and their length,<br />
response), in various situations, such as when supervisors<br />
and when and how to prompt a response), based on<br />
are present, when conducting multiple interviews, and<br />
various factors, such as when supervisors are present,<br />
when using a translator. Identify typical conflict situations<br />
when interviewing a group of workers, and when using a<br />
and appropriate techniques for resolving them. (Apply) II.B.3 translator. (Apply)<br />
II. Auditing & Inspection Processes<br />
III.E<br />
II. Auditing & Inspection Processes<br />
B. Audit performance<br />
B. Audit performance<br />
3. Working papers<br />
4. Working papers<br />
Identify types of working papers, such as checklists,<br />
Identify types of working papers, such as completed<br />
auditor notes, and attendance rosters, and determine their<br />
checklists, auditor notes, and attendance rosters, and<br />
importance in providing evidence for an audit trail.<br />
determine their importance in providing evidence for an<br />
(Evaluate) [NOTE: Checklists as auditing tools are<br />
audit trail. (Create) II.B.4 covered in II. A. 5.] II.B.3<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 5 of 20
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
II. Auditing & Inspection Processes<br />
Citation<br />
II. Auditing & Inspection Processes<br />
B. Audit performance<br />
B. Audit performance<br />
4. Objective evidence<br />
5. Objective evidence<br />
Identify and differentiate various characteristics of<br />
Identify and differentiate various characteristics of<br />
objective evidence, such as observed, measured, verified,<br />
objective evidence, such as observed, measured, verified,<br />
and documented. (Analyze)<br />
and documented. (Evaluate)<br />
II. Auditing & Inspection Processes<br />
B. Audit performance<br />
II.B.5 [NOTE: The definition of evidence is covered in I. A.] II.B.4<br />
6. Observations<br />
II. Auditing & Inspection Processes<br />
Evaluate the significance of observations in terms of<br />
B. Audit performance<br />
positive, negative, chronic, isolated, and systemic.<br />
5. Observations<br />
(Evaluate)<br />
Evaluate the significance of observations in terms of<br />
[NOTE: This topic area includes general audit observation<br />
positive, negative, chronic, isolated, and systemic.<br />
classification only; FDA classification criteria are covered<br />
(Evaluate)<br />
in Body of Knowledge area II.E.3.] II.B.6 [NOTE: The definition of observation is covered in I. A.]<br />
II. Auditing & Inspection Processes<br />
B. Audit performance<br />
II.B.5<br />
II. Auditing & Inspection Processes<br />
6. Nonconformances<br />
B. Audit performance<br />
Classify nonconformances in terms of significance,<br />
7. Classifying nonconformances<br />
severity, frequency, and level of risk. (Evaluate)<br />
Classify nonconformances in terms of significance,<br />
[NOTE: The definition of nonconformance is covered in<br />
severity, frequency, and level of risk. (Analyze) II.B.7 I.A.] II.B.6<br />
II. Auditing & Inspection Processes<br />
II. Auditing & Inspection Processes<br />
B. Audit performance<br />
B. Audit performance<br />
8. Audit process management<br />
7. Audit process management<br />
Define and apply elements of managing an audit,<br />
Define and apply elements of managing an audit,<br />
including coordinating team activities, reallocating<br />
including coordinating team activities, re-allocating<br />
resources, adjusting the audit plan, and communicating<br />
resources, adjusting audit plan, and communicating with<br />
with the auditee during the audit. (Apply) II.B.8 the auditee. (Analyze) II.B.7<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 6 of 20
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
Citation<br />
II. Auditing & Inspection Processes<br />
II. Auditing & Inspection Processes<br />
B. Audit performance<br />
B. Audit performance<br />
9. Exit meeting<br />
8. Exit meeting<br />
Describe the purpose and scope of an exit meeting and the<br />
Describe its purpose, scope, and elements, and conduct<br />
necessary elements for conducting such a meeting,<br />
an exit meeting, including determining post-audit<br />
including determining post-audit activities and who is<br />
activities and who is responsible for performing them.<br />
responsible for performing them. (Apply) II.B.9 (Apply) II.B.8<br />
II. Auditing & Inspection Processes<br />
II. Auditing & Inspection Processes<br />
C. Audit reporting<br />
C. Audit reporting<br />
1. Basic elements<br />
1. Basic steps<br />
Define, plan, and apply the steps in generating an audit<br />
Define, plan, and implement the steps in generating an<br />
report, including reviewing and finalizing results,<br />
audit report, including reviewing and finalizing results,<br />
organizing details, obtaining necessary approvals, and<br />
organizing and summarizing details, obtaining necessary<br />
distributing the report. (Create)<br />
II. Auditing & Inspection Processes<br />
II.C.1 approvals, and distributing the report. (Create) II.C.1<br />
C. Audit reporting<br />
II. Auditing & Inspection Processes<br />
2. Effective audit reports<br />
C. Audit reporting<br />
Report observations and nonconformances accurately, cite<br />
2. Effective audit reports<br />
objective evidence, procedures, and requirements, and<br />
Identify what makes an audit report effective, and<br />
develop and evaluate various components, such as<br />
develop and evaluate various components, such as<br />
executive summaries, prioritized data, graphic<br />
executive summaries, prioritized data, graphic<br />
presentation, and the impact of conclusions. (Create) II.C.2 presentation, and the impact of conclusions. (Create) II.C.2<br />
II. Auditing & Inspection Processes<br />
II. Auditing & Inspection Processes<br />
C. Audit reporting<br />
C. Audit reporting<br />
3. Record retention<br />
3. Records retention<br />
Identify and apply record retention requirements,<br />
Identify and apply record retention requirements, such as<br />
including the type of documents and storage<br />
type of documents, length of time, and storage<br />
considerations, for various audits. (Apply) II.C.3 considerations, for various audits. (Apply) II.C.3<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 7 of 20
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
II. Auditing & Inspection Processes<br />
Citation<br />
II. Auditing & Inspection Processes<br />
D. Audit follow-up and closure<br />
D. Audit follow-up and closure<br />
1. Elements of the corrective and preventive action<br />
1. Elements of corrective and preventive action<br />
processes<br />
Identify and apply the elements of these processes,<br />
Identify and apply the elements of these processes,<br />
including problem identification, assignment of<br />
including problem identification, assignment of<br />
responsibility, root cause analysis, and recurrence<br />
responsibility, root cause analysis, and recurrence<br />
prevention. (Analyze) II.D.1 prevention. (Apply) II.D.1<br />
II. Auditing & Inspection Processes<br />
II. Auditing & Inspection Processes<br />
D. Audit follow-up and closure<br />
D. Audit follow-up and closure<br />
2. Review of corrective action plan<br />
2. Review of corrective action plan<br />
Use various criteria to evaluate the acceptability of<br />
Use various criteria to evaluate the acceptability of<br />
corrective action plans, and identify and apply strategies<br />
corrective action plans, and identify and apply strategies<br />
for negotiating changes to unacceptable plans. (Evaluate)<br />
II. Auditing & Inspection Processes<br />
D. Audit follow-up and closure<br />
3. Conducting audit follow-up<br />
Use various methods to verify and evaluate the adequacy<br />
II.D.2 for negotiating changes to unacceptable plans. (Evaluate) II.D.2<br />
of corrective actions taken, such as re-examining<br />
II. Auditing & Inspection Processes<br />
procedures, observing revised processes, and conducting<br />
D. Audit follow-up and closure<br />
follow-up audits or re-audits. Develop strategies when<br />
3. Verification of corrective action<br />
corrective actions are not implemented or are not<br />
Use various methods to verify and evaluate the adequacy<br />
effective, such as communicating to the next level of<br />
of corrective actions taken, such as re-examining<br />
management, re-issuing the corrective action request, etc.<br />
procedures, observing revised processes, and conducting<br />
(Evaluate) II.D.3 follow-up audits. (Evaluate) II.D.3<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 8 of 20
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
II. Auditing & Inspection Processes<br />
D. Audit follow-up and closure<br />
4. Follow up on ineffective corrective action<br />
Identify and develop strategies to use when corrective<br />
actions are not implemented or are not effective, such as<br />
communicating to the next level of management, re-<br />
Citation<br />
ABOVE<br />
issuing the corrective action, and re-auditing. (Create) II.D.4<br />
II. Auditing & Inspection Processes<br />
II. Auditing & Inspection Processes<br />
D. Audit follow-up and closure<br />
D. Audit follow-up and closure<br />
4. Audit closure<br />
5. Audit closure<br />
Identify and apply various elements of, and criteria for,<br />
Identify and apply various elements of, and criteria for,<br />
audit closure. (Evaluate)<br />
II. Auditing & Inspection Processes<br />
E. Audit procedural references<br />
1. Guidelines for auditing quality systems<br />
Describe general auditing principles and approaches as<br />
II.D.4 audit closure. (Evaluate) II.D.5<br />
described in the ISO 19011 standard. (Understand)<br />
II. Auditing & Inspection Processes<br />
E. Audit procedural references<br />
2. Quality System Inspection Technique (QSIT)<br />
Explain the purpose of QSIT and its related terms, and<br />
compare QSIT to other audit approaches. Differentiate the<br />
principal subsystems of QSIT, and use and interpret QSIT<br />
II.E.1 N/A --<br />
sampling tables. (Analyze) II.E.2 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 9 of 20
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
II. Auditing & Inspection Processes<br />
E. Audit procedural references<br />
3. US compliance programs for medical devices (FDA<br />
CPG 7382.845)<br />
Explain the purpose and scope of various FDA<br />
inspections, including the criteria for the FDA taking<br />
action as a result of quality system audits, and categorize<br />
observations according to FDA classification criteria.<br />
(Apply)<br />
[NOTE: This topic area includes FDA classification<br />
criteria only; general audit observation classification is<br />
covered in Body of Knowledge area II.B.6.] II.E.3 N/A --<br />
II. Auditing & Inspection Processes<br />
E. Audit procedural references<br />
4. International auditing guidelines for medical devices<br />
(GHTF SG4 (99)28, GHTF SG4 (99)14, GHTF SG4<br />
(00)3)<br />
Apply general auditing principles for medical device<br />
audits. Assess the adequacy of auditors' training, their<br />
qualifications to conduct audits of a medical device<br />
manufacturer's quality system, and ongoing training to<br />
maintain their qualifications. Assess an audit team's ability<br />
to read, speak, write, and understand the native language<br />
used by auditee personnel and the auditee's quality system<br />
documentation; assess the team's ability to arrange for an<br />
interpreter in advance of the audit. (Apply) II.E.4 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 10 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
III Biomedical Quality Management System<br />
Requirements<br />
A. Regulatory requirements & guidance<br />
1. European directive: Medical Device Directive<br />
93/42/EEC of 14 June 1993 (Article 1)<br />
Interpret the applications of the directive. (Understand) III.A.1 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
A. Regulatory requirements & guidance<br />
2. US requirements (FD&C Act, sections 301-304, 501-<br />
502, 704, 518, 513)<br />
Identify how the FD&C Act defines and is applied to<br />
medical devices and differentiate between device<br />
classifications and pre-market requirements. Define<br />
misbranding and adulteration and the implications of each.<br />
Describe the FDA's authority to perform establishment<br />
inspections and mandate product recalls. (Apply) III.A.2 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
A. Regulatory requirements & guidance<br />
3. Labeling: 21 CFR 801, subpart A<br />
Apply general labeling provisions for medical devices.<br />
(Apply) III.A.3 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
A. Regulatory requirements & guidance<br />
4. Establishment registration and device listing: 21<br />
CFR 807<br />
Define these requirements. (Understand) III.A.4 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 11 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
III Biomedical Quality Management System<br />
Requirements<br />
A. Regulatory requirements & guidance<br />
5. Electronic records and electronic signatures: 21<br />
CFR 11<br />
Define these requirements. (Understand) III.A.5 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
A. Regulatory requirements & guidance<br />
6. FDA guideline for the manufacture of in vitro<br />
diagnostic (IVD) products (Jan 10, 1994)<br />
Define IVD devices and other terms related to the use of<br />
good manufacturing practices (GMPs) for IVDs.<br />
(Understand) III.A.6 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
B. Quality systems regulations & standards<br />
1. 21 CFR 820<br />
Identify FDA explanations and justifications for the<br />
content of the QSReg from the preamble; apply the scope<br />
and defined terms to medical devices. (Understand) III.B.1 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
B. Quality systems regulations & standards<br />
2. ISO 9001, ISO 9002, ISO 13485, ISO 13488<br />
Evaluate the selection, interpretation, and implementation<br />
of these various quality system standards. (Evaluate) III.B.2 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 12 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
III Biomedical Quality Management System<br />
Requirements<br />
B. Quality systems regulations & standards<br />
3. GHTF.SG3.N99-8<br />
Evaluate the selection and use of this guidance for an<br />
auditee's quality system. (Evaluate) III.B.3 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
C. Management controls<br />
Examine management responsibility for the quality<br />
system, including organization, resources, management<br />
review, quality audits, and personnel requirements.<br />
(Analyze) III.C N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
D. Design controls<br />
Evaluate the scope and purpose of design control elements<br />
and implementation of a design control system. Assess the<br />
design control system for compliance to the Medical<br />
Device Directive (MDD), including Essential<br />
Requirements, harmonized standards, risk analysis, and<br />
clinical investigation. (Evaluate) III.D N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
E. Corrective and preventive actions (CAPA)<br />
1. Existing and potential problem resolution<br />
Distinguish correction, corrective action, and preventive<br />
action, and explain their importance in terms of<br />
management responsibility, methods of implementing<br />
these tools, etc. Describe how trending is used as it relates<br />
to corrective and preventive action data. (Evaluate) III.E.1 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 13 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
III Biomedical Quality Management System<br />
Requirements<br />
E. Corrective and preventive actions (CAPA)<br />
2. Identification and control of nonconforming product<br />
(820.90)<br />
Define and apply various methods for detecting and<br />
controlling nonconforming product. (Analyze) III.E.2 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
E. Corrective and preventive actions (CAPA)<br />
3. Post-market surveillance<br />
Review and analyze complaint handling and servicing<br />
processes. Distinguish between vigilance and medical<br />
device reporting (MDR) requirements and processes.<br />
Evaluate the requirements and processes for product<br />
recall, corrections, removals, and medical device tracking.<br />
(Evaluate) III.E.3 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
F. Production and process controls (P&PC)<br />
1. Document and change control (820.40, 820.180-<br />
820.186)<br />
Identify and distinguish between device master records<br />
(DMRs), design history files (DHFs), device history<br />
records (DHRs), and quality system records. Explain<br />
document and change control. (Analyze) III.F.1 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 14 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
III Biomedical Quality Management System<br />
Requirements<br />
F. Production and process controls (P&PC)<br />
2. Purchasing controls and acceptance activities<br />
(820.50, 820.80, [including product identification &<br />
traceability] 820.60, 820.65)<br />
Explain the importance of having adequate controls in<br />
purchasing products, components, and services, and the<br />
use of appropriate identification and acceptance activities.<br />
(Understand) III.F.2 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
F. Production and process controls (P&PC)<br />
3. Handling, storage, distribution and installation<br />
(820.140-820.170)<br />
Identify the requirements for these processes.<br />
(Understand) III.F.3 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
F. Production and process controls (P&PC)<br />
4. Validation and process controls (820.70, 820.75,<br />
820.72, GHTF.SG3.N99-10)<br />
Assess a validation process and production and process<br />
controls in relation to components, materials, technology,<br />
product use, industry standards, production standards, test<br />
methods, and calibration. (Evaluate) III.F.4 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
F. Production and process controls (P&PC)<br />
5. Packaging and labeling controls (820.120, 820.130)<br />
Identify these requirements. (Understand) III.F.5 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 15 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
III Biomedical Quality Management System<br />
Requirements<br />
F. Production and process controls (P&PC)<br />
6. Sampling techniques (820.250(b))<br />
Identify, interpret, and use various sampling methods,<br />
such as acceptance, random, and stratified, and define<br />
related concepts (e.g., consumer and producer risk, and<br />
confidence level), as used in the biomedical field. (Apply) III.F.6 N/A --<br />
III Biomedical Quality Management System<br />
Requirements<br />
F. Production and process controls (P&PC)<br />
7. Statistical techniques (820.250(a))<br />
Identify, interpret, and use various measures of central<br />
tendency (mean, median, and mode), and dispersion, such<br />
as standard deviation and frequency distribution. Identify<br />
appropriate rationales for statistical techniques used in the<br />
biomedical field. (Apply) III.F.7 N/A --<br />
IV. Technical Biomedical Knowledge<br />
A. Risk management<br />
Identify the steps necessary for risk analysis of medical<br />
devices. Identify known or foreseeable hazards for<br />
medical devices in both normal and fault conditions, and<br />
describe suitable methods for risk estimation. Evaluate<br />
risk analysis reports for completeness, and use FMEA,<br />
FTA, and other tools to assess risk in a variety of<br />
situations. (Evaluate) IV.A N/A --<br />
IV. Technical Biomedical Knowledge<br />
B. Sterilization<br />
1. Definitions<br />
Distinguish between aseptically processed products and<br />
terminally sterilized products. (Understand) IV.B.1 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 16 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
IV. Technical Biomedical Knowledge<br />
B. Sterilization<br />
2. Standards<br />
Identify the standards applicable to each sterilization<br />
process, including ISO 11134, ISO 11135, ISO 11137,<br />
ISO 11737, ISO 11138, ISO/TIR 13409, EN 550/552/554,<br />
and EN 556. (Understand) IV.B.2 N/A --<br />
IV. Technical Biomedical Knowledge<br />
B. Sterilization<br />
3. Methods<br />
Identify elements of sterilization validation, including<br />
commissioning of equipment installation and process<br />
qualification (physical and microbiological) and determine<br />
appropriateness. Identify appropriate process controls and<br />
monitors for each sterilization process and determine<br />
whether they are incorporated and documented properly.<br />
(Understand) IV.B.3 N/A --<br />
IV. Technical Biomedical Knowledge<br />
B. Sterilization<br />
4. Packaging of sterile products<br />
Interpret the appropriate standard for sterile product<br />
packaging, including ISO 11607 and EN 868-1.<br />
(Understand) IV.B.4 N/A --<br />
IV. Technical Biomedical Knowledge<br />
C. Biocompatibility<br />
Define various biocompatibility terms, associated tests,<br />
and test selection rationale in accordance with ISO 10993,<br />
FDA Blue Book #G95-1, and U.S. Pharmacopoeia (USP)<br />
Classes V & VI. (Understand) IV.C N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 17 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
IV. Technical Biomedical Knowledge<br />
D. Controlled environments and utility systems<br />
1. Controlled environments<br />
Apply appropriate controlled environment classes for<br />
various medical devices. Identify and interpret controlled<br />
environment specifications as required by ISO 14644 and<br />
Federal Standard 209 E. Interpret qualifications,<br />
validation, and monitoring, including cleaning,<br />
disinfection, and sanitization in terms of controlled<br />
environment specifications, classifications, and standards.<br />
Verify that appropriate training and personnel practices<br />
are in use for controlled environments. (Evaluate) IV.D.1 N/A --<br />
IV. Technical Biomedical Knowledge<br />
D. Controlled environments and utility systems<br />
2. Utility systems<br />
Recognize water, compressed gas, and HVAC utilities<br />
used in medical device manufacturing and determine<br />
whether they require qualification, validation, or<br />
maintenance according to U.S. Pharmacopoeia (USP),<br />
ISO 14644, Federal Standard 209E, or ISO 11134<br />
standards. (Understand) IV.D.2 N/A --<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 18 of 20<br />
<strong>CQA</strong><br />
Citation
<strong>CBA</strong> Text<br />
<strong>CBA</strong><br />
Citation <strong>CQA</strong> Text<br />
IV. Technical Biomedical Knowledge<br />
E. Software development for products, processes, and<br />
quality systems<br />
Define the major elements of software development, such<br />
as requirements specifications, unit testing, integration and<br />
systems testing, verification, validation, etc., in<br />
accordance with FDA software Guidance for FDA<br />
Reviewers and Industry, (May 29, 1998), General<br />
Principles of Software Validation, 21 CFR Part 11, and<br />
ISPE Good Automated Manufacturing Practice (GAMP4)<br />
requirements. (Understand) IV.E N/A --<br />
IV. Technical Biomedical Knowledge<br />
F. Laboratory testing<br />
Review validation procedures used for laboratory test<br />
methods and determine whether they are appropriate.<br />
(Evaluate) IV.F N/A --<br />
V. Quality Tools & Techniques<br />
A. Fundamental quality control tools<br />
Interpret and apply Pareto charts, cause and effect<br />
diagrams, flowcharts, control charts, check sheets, scatter<br />
diagrams, and histograms. (Apply) V.A<br />
V. Quality Tools & Techniques<br />
B. Quality improvement tools<br />
Interpret and apply problem-solving tools, such as root<br />
cause analysis, the six sigma model (DMAIC), lean tools,<br />
Plan-Do-Check-Act (PDCA), and corrective and<br />
preventive action (CAPA) methods. (Apply) V.B<br />
V. Quality Tools & Techniques<br />
C. Process capability<br />
Identify and interpret various process capability indices,<br />
such as Cp and Cpk. (Understand) V.C<br />
V. Quality Tools And Techniques<br />
A. Fundamental quality control tools<br />
Identify, interpret, and apply Pareto charts, cause and<br />
effect diagrams, flowcharts, control charts, check sheets,<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 19 of 20<br />
<strong>CQA</strong><br />
Citation<br />
scatter diagrams, and histograms. (Analyze) V.A<br />
V. Quality Tools And Techniques<br />
B. Quality improvement tools<br />
Identify, interpret, and apply problem-solving tools, such<br />
as root cause analysis, the six sigma model (DMAIC),<br />
lean tools, Plan-Do-Check-Act (PDCA), and corrective<br />
and preventive action (CAPA) methods. (Apply) V.B<br />
V. Quality Tools And Techniques<br />
E. Process capability<br />
Identify and interpret various process capability indices,<br />
such as Cp and Cpk. (Understand) V.E
<strong>CBA</strong><br />
<strong>CQA</strong><br />
<strong>CBA</strong> Text<br />
Citation <strong>CQA</strong> Text<br />
Citation<br />
V. Quality Tools & Techniques<br />
V. Quality Tools And Techniques<br />
D. Qualitative and quantitative analysis<br />
F. Qualitative and quantitative analysis<br />
Describe and distinguish between qualitative and<br />
Describe and distinguish between qualitative and<br />
quantitative analyses, and attributes and variables data.<br />
quantitative analyses, and attributes and variables data.<br />
(Analyze) V.D (Analyze)<br />
V. Quality Tools And Techniques<br />
V.F<br />
V. Quality Tools & Techniques<br />
G. Cost of quality<br />
E. Cost of quality<br />
Identify the basic cost of quality (COQ) principles, and<br />
Identify the basic cost of quality (COQ) principles, and<br />
describe the four COQ categories: prevention, appraisal,<br />
describe the four COQ categories. (Understand) V.E internal failure, and external failure. (Understand) V.G<br />
<strong>Ombu</strong> <strong>Enterprises</strong>, <strong>LLC</strong><br />
www.<strong>Ombu</strong><strong>Enterprises</strong>.com<br />
<strong>CBA</strong> <strong>CQA</strong> <strong>Comparison</strong> <strong>Chart</strong> Page 20 of 20