Chapter 19: Optical Properties
Chapter 19: Optical Properties
Chapter 19: Optical Properties
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
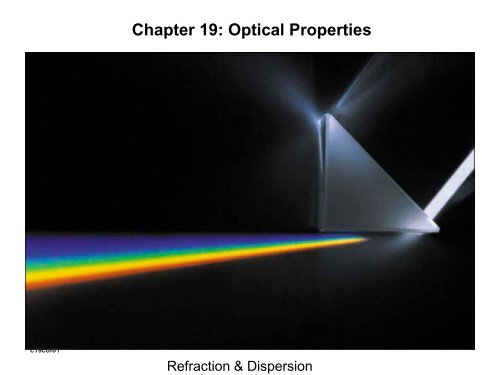
<strong>Chapter</strong> <strong>19</strong>: <strong>Optical</strong> <strong>Properties</strong>c<strong>19</strong>cof01Refraction & Dispersion
LIGHT INTERACTION WITH SOLIDS• Incident light is either reflected, absorbed, ortransmitted:I o = I T + I A + I R• <strong>Optical</strong> classification of materials:Adapted from Fig. 21.10,Callister 6e. (Fig. 21.10 is by J.Telford, with specimenpreparation by P.A. Lessing.)2
c<strong>19</strong>f03Example: Isolated atom
Figure <strong>19</strong>.4 (a) Schematic representation of the mechanism of photonabsorption for metallic materials in which an electron is excited into a higher-energyunoccupied state. The change in energy of the electron E is equal to the energy ofthe photon. (b) Reemission of a photon of light by the direct transition of anelectron from a high to a low energy state.
OPTICAL PROPERTIES OFMETALS: ABSORPTION• Absorption of photons by electron transition:J sAdapted from Fig. 21.4(a), Callister 6e.• Metals have a fine succession of energy states.• Near-surface electrons absorb visible light.3
OPTICAL PROPERTIES OFMETALS: REFLECTION• Electron transition emits a photon.IRre-emittedphoton frommaterialsurfaceEnergy of electronunfilled states“conducting” electron∆Efilled states• Reflectivity = IR/Io is between 0.90 and 0.95.• Reflected light is same frequency as incident.• Metals appear reflective (shiny)!Adapted from Fig. 21.4(b), Callister 6e.4
c<strong>19</strong>tf01
TRANSMITTED LIGHT: REFRACTION• Transmitted light distorts electron clouds.notransmittedlight+• Result 1: Light is slower in a material vs vacuum.Index of refraction (n) = speed of light in a vacuumspeed of light in a material--Adding large, heavy ions (e.g., leadcan decrease the speed of light.--Light can be"bent"MaterialLead glassSilica glassSoda-lime glassQuartzPlexiglasPolypropylene• Result 2: Intensity of transmitted light decreaseswith distance traveled (thick pieces less transparent!)n2.11.461.511.551.491.49Selected values from Table 21.1,Callister 6e.7
Figure <strong>19</strong>.5 Non Metallic Materials—Materials with a bandgapan electron is excited across the band gapleaving behind a hole in the valence band.absorbed photon energy: E = EgEmission of a photon of light by adirect electron transition across theband gap.c<strong>19</strong>f05
Figure <strong>19</strong>.6Photon absorption via avalence band-conductionband electron excitation for amaterial that has an impuritylevel that lies within the bandgapEmission of twophotons involvingelectron decay first intoan impurity state, andfinally into the groundstateGeneration of both a phononand a photon as an excitedelectron falls first into animpurity level and finally backto its ground statec<strong>19</strong>f06
SELECTED ABSORPTION: NONMETALS• Absorption by electron transition occurs if hν > Egapincident photonenergy hνAdapted from Fig. 21.5(a), Callister 6e.• If Egap < 1.8eV, full absorption; color is black (Si, GaAs)• If Egap > 3.1eV, no absorption; colorless (diamond)• If Egap in between, partial absorption; material hasa color.5
c<strong>19</strong>f07Figure <strong>19</strong>.7 The transmission of lightthrough a transparentmedium for which there is reflection at frontand back faces, aswell as absorption within the medium.
Figure <strong>19</strong>.8 The variation withwavelength of the fractions ofincident light transmitted, absorbed, andreflected through a green glass.
c<strong>19</strong>f09<strong>19</strong>.9 COLOR
COLOR OF NONMETALS• Color determined by sum of frequencies of--transmitted light,--re-emitted light from electron transitions.• Ex: Cadmium Sulfide (CdS)-- Egap = 2.4eV,-- absorbs higher energy visible light (blue, violet),-- Red/yellow/orange is transmitted and gives it color.• Ex: Ruby = Sapphire (Al2O3) + (0.5 to 2) at% Cr2O3-- Sapphire is colorless(i.e., Egap > 3.1eV)-- adding Cr2O3 :• alters the band gap• blue light is absorbed• yellow/green is absorbed• red is transmitted• Result: Ruby is deepred in color.Adapted from Fig. 21.9, Callister 6e. (Fig. 21.9adapted from "The <strong>Optical</strong> <strong>Properties</strong> ofMaterials" by A. Javan, Scientific American,<strong>19</strong>67.)6
<strong>19</strong>.10 OPACITY AND TRANSLUCENCY IN INSULATORSthe light transmittance of three aluminum oxide specimens. From left to right: singlecrystalmaterial (sapphire), which is transparent; a polycrystalline and fully dense(nonporous) material, which is translucent; and a polycrystalline material that containsapproximately 5% porosity, which is opaque.
<strong>19</strong>.11 APPLICATION: LUMINESCENCE• Process:Energy of electronunfilled statesincidentradiationEgapfilled statesAdapted from Fig. 21.5(a), Callister 6e. Adapted from Fig. 21.5(a), Callister 6e.• Ex: fluorescent lampselectrontransition occursemittedlightFluorescence & phosphorescence8
<strong>19</strong>.12 APPLICATION: PHOTOCONDUCTIVITY• Description:• Ex: Photodetector (Cadmium sulfide)9
APPLICATION: SOLAR CELL• p-n junction:• Operation:--incident photon produces hole-elec. pair.--typically 0.5V potential.--current increases w/light intensity.• Solar powered weather station:polycrystalline SiLos Alamos High School weatherstation (photo courtesyP.M. Anderson)10
Light-Emitting Diodesc<strong>19</strong>f11
c<strong>19</strong>f12OLED
the ruby laser and xenon flash lamp.
c<strong>19</strong>f14The ruby laser
(a) The chromium ions before excitation(b) Electrons in some chromium atoms areexcited into higher energy states by thexenon light flash.(c) Emission from metastable electron statesis initiated or stimulated by photons thatare spontaneously emitted.The stimulated emission and lightamplification for a ruby laser.(d) Upon reflection from the silvered ends,the photons continue to stimulateemissions as they traverse the rod length.(e) The coherent and intense beam is finallyemitted through the partially silvered end.
c<strong>19</strong>f16semiconductor laser
Figure <strong>19</strong>.17layered cross sectionof a GaAssemiconducting laser.Holes, excitedelectrons, and thelaser beam areconfined to the GaAslayer by theadjacent n- and p-type GaAlAs layers.c<strong>19</strong>f17
c<strong>19</strong>tf02
c<strong>19</strong>f18components of an optical fiber communications system
A high-power pulse of photonscorresponds to a “one” in thebinary formatA lowpower photon pulserepresents a “zero.”Digital encoding scheme for optical communications.
cross section of an optical fiberc<strong>19</strong>f20
Figure <strong>19</strong>.21 Step-index optical fiberdesign.(a) Fiber cross section.(b) Fiber radial index of refraction profile.(c) Input light pulse.(d) Internal reflection of light rays.(e) Output light pulse.
Figure <strong>19</strong>.22 Graded-index optical fiberdesign.(a) Fiber cross section.(b) Fiber radial index of refraction profile.(c) Input light pulse.(d) Internal reflection of a light ray.(e) Output light pulse.
APPLICATION: FIBER OPTICS• Design with stepped index of refraction (n):Adapted from Fig. 21.<strong>19</strong>, Callister 6e. (Fig. 21.<strong>19</strong> adapted from S.R. Nagel,IEEE Communications Magazine, Vol. 25, No. 4, p. 34, <strong>19</strong>87.)• Design with parabolic index of refractionAdapted from Fig. 21.20, Callister 6e. (Fig. 21.<strong>19</strong> adapted from S.R. Nagel, IEEECommunications Magazine, Vol. 25, No. 4, p. 34, <strong>19</strong>87.)• Parabolic = less broadening = improvement!11
SUMMARY• When light (radiation) shines on a material, it may be:--reflected, absorbed and/or transmitted.• <strong>Optical</strong> classification:--transparent, translucent, opaque• Metals:--fine succession of energy states causes absorptionand reflection.• Non-Metals:--may have full (Egap < 1.8eV) , no (Egap > 3.1eV), orpartial absorption (1.8eV < Egap = 3.1eV).--color is determined by light wavelengths that aretransmitted or re-emitted from electron transitions.--color may be changed by adding impurities whichchange the band gap magnitude (e.g., Ruby)• Refraction:--speed of transmitted light varies among materials.12