Liquid Filling in Hard Gelatin Capsules - Preliminary Steps - Capsugel
Liquid Filling in Hard Gelatin Capsules - Preliminary Steps - Capsugel
Liquid Filling in Hard Gelatin Capsules - Preliminary Steps - Capsugel
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
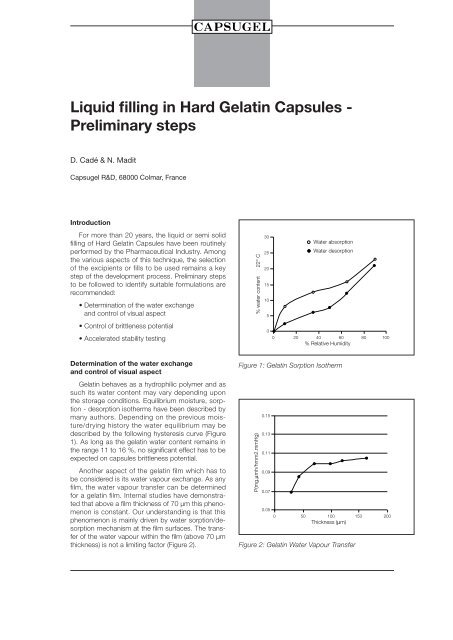
<strong>Liquid</strong> fill<strong>in</strong>g <strong>in</strong> <strong>Hard</strong> Gelat<strong>in</strong> <strong>Capsules</strong> -Prelim<strong>in</strong>ary stepsD. Cadé & N. Madit<strong>Capsugel</strong> R&D, 68000 Colmar, FranceIntroductionFor more than 20 years, the liquid or semi solidfill<strong>in</strong>g of <strong>Hard</strong> Gelat<strong>in</strong> <strong>Capsules</strong> have been rout<strong>in</strong>elyperformed by the Pharmaceutical Industry. Amongthe various aspects of this technique, the selectionof the excipients or fills to be used rema<strong>in</strong>s a keystep of the development process. Prelim<strong>in</strong>ary stepsto be followed to identify suitable formulations arerecommended:• Determ<strong>in</strong>ation of the water exchangeand control of visual aspect• Control of brittleness potential• Accelerated stability test<strong>in</strong>g% water content 22° C302520151050Water absorptionWater desorption0 20 40 60 80 100% Relative HumidityDeterm<strong>in</strong>ation of the water exchangeand control of visual aspectGelat<strong>in</strong> behaves as a hydrophilic polymer and assuch its water content may vary depend<strong>in</strong>g uponthe storage conditions. Equilibrium moisture, sorption- desorption isotherms have been described bymany authors. Depend<strong>in</strong>g on the previous moisture/dry<strong>in</strong>ghistory the water equilibrium may bedescribed by the follow<strong>in</strong>g hysteresis curve (Figure1). As long as the gelat<strong>in</strong> water content rema<strong>in</strong>s <strong>in</strong>the range 11 to 16 %, no significant effect has to beexpected on capsules brittleness potential.Another aspect of the gelat<strong>in</strong> film which has tobe considered is its water vapour exchange. As anyfilm, the water vapour transfer can be determ<strong>in</strong>edfor a gelat<strong>in</strong> film. Internal studies have demonstratedthat above a film thickness of 70 µm this phenomenonis constant. Our understand<strong>in</strong>g is that thisphenomenon is ma<strong>in</strong>ly driven by water sorption/desorptionmechanism at the film surfaces. The transferof the water vapour with<strong>in</strong> the film (above 70 µmthickness) is not a limit<strong>in</strong>g factor (Figure 2).Figure 1: Gelat<strong>in</strong> Sorption IsothermP(mg.µmh/hmm2.mmHg)0.150.130.110.090.070.050 50 100 150 200Thickness (µm)Figure 2: Gelat<strong>in</strong> Water Vapour Transfer
The importance of this water vapour transfer canbe illustrated by the determ<strong>in</strong>ation of the quantity ofwater absorbed by a hygroscopic dry powder (Carboxymethyl-CelluloseSodium salt from Sigma chemicalCo) filled <strong>in</strong>to a <strong>Hard</strong> Gelat<strong>in</strong> Capsule stored at50 % RH / room temperature (Figure 3).B- Lauroglycol FCC (Chemical denom<strong>in</strong>ation:Propylene glycol laurate. Supplier: Gattefossé)(Figure 4).3HYGROSCOPICITY AFTER 4 WEEKSLOD(%) of CMC20181614121086Water exchange %210-1-2Smedds 1014/bisLauroglycol FCC4200 50 100 150 200Time (hour)Figure 3: <strong>Hard</strong> Gelat<strong>in</strong> Capsule Water PermeabilityMore important for the liquid formulation <strong>in</strong>to<strong>Hard</strong> Gelat<strong>in</strong> <strong>Capsules</strong> is the determ<strong>in</strong>ation of thewater exchanges as a function of the Relative Humidityof the ambient air. Screen<strong>in</strong>g tests have beendef<strong>in</strong>ed to identify a potential hygroscopicity of thefilled excipients by storage <strong>in</strong>to dessicators atconstant RH.• 10 capsules are filled with the product to betested• These capsules are held <strong>in</strong> the upright positionand stored <strong>in</strong> dessicators at various relative humidities:2.5%, 10%, 30%, 50%, 65% RH• After 1, 2 & 4 weeks the water content changeis determ<strong>in</strong>ed by weigh<strong>in</strong>g the capsules.-32.5 10 30 50 65Storage conditions % RhFigure 4: Water exchanges of two Gattefossé productsfilled <strong>in</strong>to <strong>Hard</strong> Gelat<strong>in</strong> <strong>Capsules</strong>As per comparison other excipients have beentested for their hygroscopicity <strong>in</strong>to <strong>Hard</strong> Gelat<strong>in</strong><strong>Capsules</strong>.Water exchanges <strong>in</strong> % w/w of excipients filled<strong>in</strong>to <strong>Hard</strong> Gelat<strong>in</strong> capsules stored 4 weeks atconstant Relative Humidity:Excipient 35%RH/RT 60%RH/RTPEG 300 +4.6 +14.7PEG 600 +2.9 +11.8PEG 4000 0 +0.6Glycer<strong>in</strong> +10.5 +28.2Empty capsules -0.4 +1.9Examples of products tested:A- Pharmaceutical formulation DH 1014/BisSolid SMEDDS from Gattefossé.Example with Gelucire products from Gattefossé:Excipient 10%RH/RT 65%RH/RT% w/wGelucire 44/14 80Plurol Oleique 10Lauroglycol FCC 10Gelucire 39/01 -0.8 +0.5Gelucire 43/01 -0.7 +0.4Gelucire 44/14 -1.0 +0.8Gelucire 50/02 -0.8 +0.7
As a first <strong>in</strong>dication we would def<strong>in</strong>e suitability ofan excipient or a formulation for <strong>Hard</strong> Gelat<strong>in</strong> Capsulewhen water exchange is limited to -2% / +2%under above described test conditions.In parallel to this step, we recommend to controlthe visual aspects of the <strong>Hard</strong> Gelat<strong>in</strong> <strong>Capsules</strong>(soften<strong>in</strong>g or leakers).Control of brittleness potentialA second key aspect of the gelat<strong>in</strong> capsule stabilityto be determ<strong>in</strong>ed is the possible trend for brittleness.Few authors have published on this characteristic(1). <strong>Capsugel</strong> method is based on thedeterm<strong>in</strong>ation of the resistance to impact of theempty shells (measured with a modified Moutonpendulum - Figure 5). This technique gives an accurate<strong>in</strong>formation by test<strong>in</strong>g a limited number ofsamples (N=10).Figure 7: Schematic view of a modified tube testerExample of resistance to impact of capsules(stored filled with various Gattefossé products)tested on <strong>in</strong>dividual body or cap parts:Loss on Resistancedry<strong>in</strong>g to impact(%) (mJ/mm)SMEDDS 1014/Bis 12.2 27Lauroglycol FCC 12.4 25Acceptable range 12-15 >22Other methods have been described (2, 3) to determ<strong>in</strong>ethe potential brittleness of the capsulessuch as resistance to deformation of the filled capsules(Figure 6) or the tube test (Figure 7).Figure 5: Equipment for control of Capsule’sResistance to ImpactFigure 6: Test set up for capsule’s deformation testAccelerated stability test<strong>in</strong>gA further step is the determ<strong>in</strong>ation of the behaviourof capsules stored under accelerated stabilityconditions. From the various conditions recommendedby the ICH3 (4), we have selected as firstcondition to be tested the storage at 40°C/75% RH.We have selected the HDPE bottle as conta<strong>in</strong>erand checked the <strong>Hard</strong> Gelat<strong>in</strong> <strong>Capsules</strong> dissolutionstability after 1, 2, 3 and 6 months. Dissolutionmeasurements are performed on emptied capsulesrefilled with Acetam<strong>in</strong>ophen (USP method).We have studied the dissolution behaviour of gelat<strong>in</strong>ecapsules filled with lactose powder exposedto formaldehyde vapour (5). This technique did enableus and other authors to better understand theimportance of this mechanism (6). <strong>Capsules</strong> with
% Acetam<strong>in</strong>ophen dissolved1009080706050403020100Reference13 ppm22 ppm HCHO41 ppm0 15 30 45 60Time <strong>in</strong> m<strong>in</strong>utes% Acetam<strong>in</strong>ophen dissolved1101009080706050403020100ReferenceSmedds 1014/BisLauroglycol FCC0 15 30 45 60 75Time <strong>in</strong> m<strong>in</strong>utesFigure 8: Dissolution of Acetam<strong>in</strong>ophen from capsulesstressed with various levels of formaldehydeand stored at 50°C for 4 weeksdelayed dissolution <strong>in</strong> water could easily be prepared<strong>in</strong> our laboratory and tested with Acetam<strong>in</strong>ophen(Figure 8).Us<strong>in</strong>g the same approach, we have filled our gelat<strong>in</strong>ecapsules with various products selected fromthe range of excipients from Gattefossé and storedthe filled capsules at 40°C / 75 % RH. After the def<strong>in</strong>edperiod (1,2,3 & 6 months) the capsules areemptied from their fill and refilled with Acetam<strong>in</strong>ophento perform the dissolution test described. Resultsbelow (Figure 9), show that there is no <strong>in</strong>teractionbetween the SMEDDS 1014/Bis or theLauroglycol FCC and the gelat<strong>in</strong>e capsules.Figure 9: Dissolution of Acetam<strong>in</strong>ophen from capsulesstored at 40°C/75%RH with various fillsConclusionThe results obta<strong>in</strong>ed with the selected tests aresummarized below.With the three steps described above, prelim<strong>in</strong>aryselection of the appropriate excipient formulationfor liquid or semi-solid fill<strong>in</strong>g <strong>in</strong>to <strong>Hard</strong> Gelat<strong>in</strong><strong>Capsules</strong>, can be performed at an early developmentstage, <strong>in</strong> the laboratory, without <strong>in</strong>volv<strong>in</strong>g highcost or sophisticated equipment.Solid SMEDDSLauroglycol1014/BisFCCWater loss / ga<strong>in</strong> Less than 2% Less than 2%Visual aspect No change No changeBrittleness With<strong>in</strong> range With<strong>in</strong> rangeAccelerated stability 40°C/75% RH - Dissolution No change No changeReferences1. Mark J. Kontny and Carol A. Mulsky, Intern. Journal of Pharmaceutics, 54 (1989), 79-852. D. Cadé, APGI Colloque Nov 1994, Chatenay - Malabry, France3. T. Yamamoto and all, Pharm. Tech. Japan, Vol. 8, N°11, 19924. Eur. J. Pharm. Biopharm. 41 (3), 194-195, 19955. D. Cadé, N. Madit and E.T. Cole, AAPS Pharm. Research Vol. 11, No. 10, p. S 1476. G.A. Digenis; T.B. Gold; V.P. Shah; Journal of Pharmaceutical Sciences, 83 (7), 915-921, 1994®BAS 191 E 1998