Multiple Antigen Labeling - Vector Laboratories
Multiple Antigen Labeling - Vector Laboratories
Multiple Antigen Labeling - Vector Laboratories
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
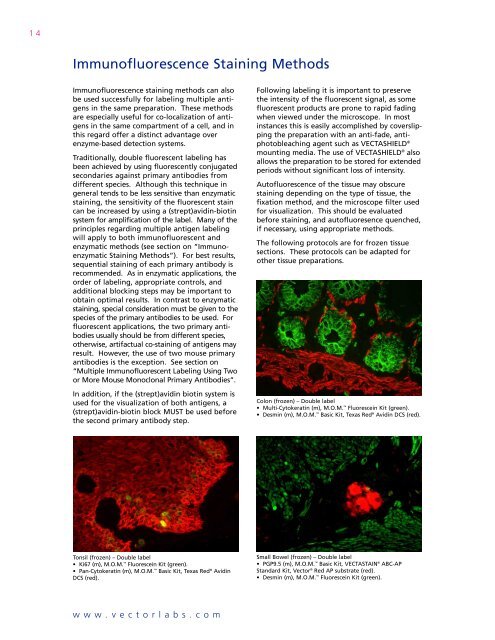
14Immunofluorescence Staining MethodsImmunofluorescence staining methods can alsobe used successfully for labeling multiple antigensin the same preparation. These methodsare especially useful for co-localization of antigensin the same compartment of a cell, and inthis regard offer a distinct advantage overenzyme-based detection systems.Traditionally, double fluorescent labeling hasbeen achieved by using fluorescently conjugatedsecondaries against primary antibodies fromdifferent species. Although this technique ingeneral tends to be less sensitive than enzymaticstaining, the sensitivity of the fluorescent staincan be increased by using a (strept)avidin-biotinsystem for amplification of the label. Many of theprinciples regarding multiple antigen labelingwill apply to both immunofluorescent andenzymatic methods (see section on “ImmunoenzymaticStaining Methods”). For best results,sequential staining of each primary antibody isrecommended. As in enzymatic applications, theorder of labeling, appropriate controls, andadditional blocking steps may be important toobtain optimal results. In contrast to enzymaticstaining, special consideration must be given to thespecies of the primary antibodies to be used. Forfluorescent applications, the two primary antibodiesusually should be from different species,otherwise, artifactual co-staining of antigens mayresult. However, the use of two mouse primaryantibodies is the exception. See section on“<strong>Multiple</strong> Immunofluorescent <strong>Labeling</strong> Using Twoor More Mouse Monoclonal Primary Antibodies”.In addition, if the (strept)avidin biotin system isused for the visualization of both antigens, a(strept)avidin-biotin block MUST be used beforethe second primary antibody step.Following labeling it is important to preservethe intensity of the fluorescent signal, as somefluorescent products are prone to rapid fadingwhen viewed under the microscope. In mostinstances this is easily accomplished by coverslippingthe preparation with an anti-fade, antiphotobleachingagent such as VECTASHIELD ®mounting media. The use of VECTASHIELD ® alsoallows the preparation to be stored for extendedperiods without significant loss of intensity.Autofluorescence of the tissue may obscurestaining depending on the type of tissue, thefixation method, and the microscope filter usedfor visualization. This should be evaluatedbefore staining, and autofluoresence quenched,if necessary, using appropriate methods.The following protocols are for frozen tissuesections. These protocols can be adapted forother tissue preparations.Colon (frozen) – Double label• Multi-Cytokeratin (m), M.O.M. Fluorescein Kit (green).• Desmin (m), M.O.M. Basic Kit, Texas Red ® Avidin DCS (red).Tonsil (frozen) – Double label• Ki67 (m), M.O.M. Fluorescein Kit (green).• Pan-Cytokeratin (m), M.O.M. Basic Kit, Texas Red ® AvidinDCS (red).Small Bowel (frozen) – Double label• PGP9.5 (m), M.O.M. Basic Kit, VECTASTAIN ® ABC-APStandard Kit, <strong>Vector</strong> ® Red AP substrate (red).• Desmin (m), M.O.M. Fluorescein Kit (green).www.vectorlabs.com