SOP02V3 SOP for establishing and maintaining a TMF - Accord
SOP02V3 SOP for establishing and maintaining a TMF - Accord
SOP02V3 SOP for establishing and maintaining a TMF - Accord
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
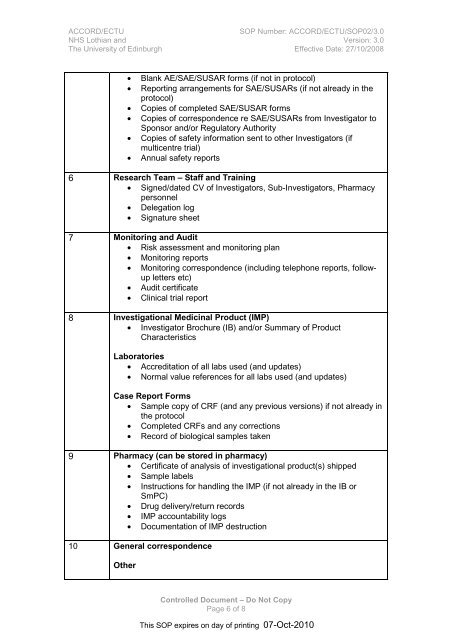
ACCORD/ECTU<strong>SOP</strong> Number: ACCORD/ECTU/<strong>SOP</strong>02/3.0NHS Lothian <strong>and</strong> Version: 3.0The University of Edinburgh Effective Date: 27/10/2008• Blank AE/SAE/SUSAR <strong>for</strong>ms (if not in protocol)• Reporting arrangements <strong>for</strong> SAE/SUSARs (if not already in theprotocol)• Copies of completed SAE/SUSAR <strong>for</strong>ms• Copies of correspondence re SAE/SUSARs from Investigator toSponsor <strong>and</strong>/or Regulatory Authority• Copies of safety in<strong>for</strong>mation sent to other Investigators (ifmulticentre trial)• Annual safety reports6 Research Team – Staff <strong>and</strong> Training• Signed/dated CV of Investigators, Sub-Investigators, Pharmacypersonnel• Delegation log• Signature sheet7 Monitoring <strong>and</strong> Audit• Risk assessment <strong>and</strong> monitoring plan• Monitoring reports• Monitoring correspondence (including telephone reports, followupletters etc)• Audit certificate• Clinical trial report8 Investigational Medicinal Product (IMP)• Investigator Brochure (IB) <strong>and</strong>/or Summary of ProductCharacteristicsLaboratories• Accreditation of all labs used (<strong>and</strong> updates)• Normal value references <strong>for</strong> all labs used (<strong>and</strong> updates)Case Report Forms• Sample copy of CRF (<strong>and</strong> any previous versions) if not already inthe protocol• Completed CRFs <strong>and</strong> any corrections• Record of biological samples taken9 Pharmacy (can be stored in pharmacy)• Certificate of analysis of investigational product(s) shipped• Sample labels• Instructions <strong>for</strong> h<strong>and</strong>ling the IMP (if not already in the IB orSmPC)• Drug delivery/return records• IMP accountability logs• Documentation of IMP destruction10 General correspondenceOtherControlled Document – Do Not CopyPage 6 of 8