Drug vs medical device clinical trial applications - TOPRA
Drug vs medical device clinical trial applications - TOPRA
Drug vs medical device clinical trial applications - TOPRA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
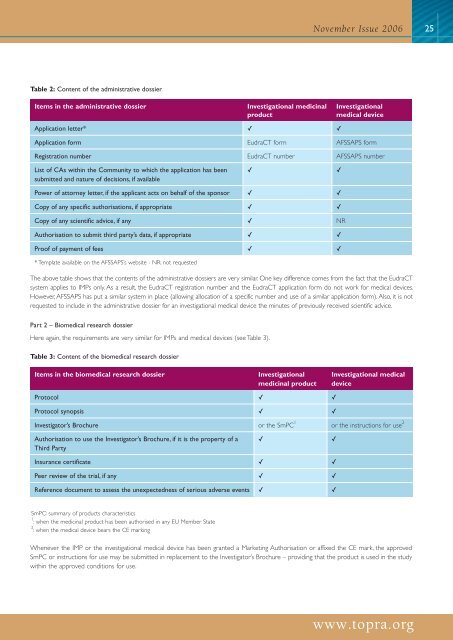
November Issue 200625Table 2: Content of the administrative dossierItems in the administrative dossierInvestigational medicinalproductInvestigational<strong>medical</strong> <strong>device</strong>Application letter* ✓ ✓Application form EudraCT form AFSSAPS formRegistration number EudraCT number AFSSAPS numberList of CAs within the Community to which the application has beensubmitted and nature of decisions, if available✓✓Power of attorney letter, if the applicant acts on behalf of the sponsor ✓ ✓Copy of any specific authorisations, if appropriate ✓ ✓Copy of any scientific advice, if any ✓ NRAuthorisation to submit third party’s data, if appropriate ✓ ✓Proof of payment of fees ✓ ✓* Template available on the AFSSAPS’s website - NR: not requestedThe above table shows that the contents of the administrative dossiers are very similar. One key difference comes from the fact that the EudraCTsystem applies to IMPs only. As a result, the EudraCT registration number and the EudraCT application form do not work for <strong>medical</strong> <strong>device</strong>s.However, AFSSAPS has put a similar system in place (allowing allocation of a specific number and use of a similar application form). Also, it is notrequested to include in the administrative dossier for an investigational <strong>medical</strong> <strong>device</strong> the minutes of previously received scientific advice.Part 2 – Bio<strong>medical</strong> research dossierHere again, the requirements are very similar for IMPs and <strong>medical</strong> <strong>device</strong>s (see Table 3).Table 3: Content of the bio<strong>medical</strong> research dossierItems in the bio<strong>medical</strong> research dossierInvestigationalmedicinal productInvestigational <strong>medical</strong><strong>device</strong>Protocol ✓ ✓Protocol synopsis ✓ ✓Investigator’s Brochure or the SmPC 1 or the instructions for use 2Authorisation to use the Investigator’s Brochure, if it is the property of aThird Party✓✓Insurance certificate ✓ ✓Peer review of the <strong>trial</strong>, if any ✓ ✓Reference document to assess the unexpectedness of serious adverse events ✓ ✓SmPC: summary of products characteristics1 : when the medicinal product has been authorised in any EU Member State2 : when the <strong>medical</strong> <strong>device</strong> bears the CE markingWhenever the IMP or the investigational <strong>medical</strong> <strong>device</strong> has been granted a Marketing Authorisation or affixed the CE mark, the approvedSmPC or instructions for use may be submitted in replacement to the Investigator’s Brochure – providing that the product is used in the studywithin the approved conditions for use.www.topra.org