Drug vs medical device clinical trial applications - TOPRA
Drug vs medical device clinical trial applications - TOPRA
Drug vs medical device clinical trial applications - TOPRA
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
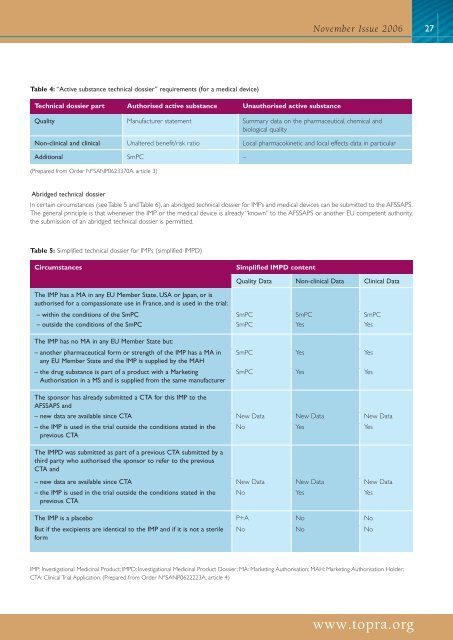
November Issue 200627Table 4: “Active substance technical dossier” requirements (for a <strong>medical</strong> <strong>device</strong>)Technical dossier part Authorised active substance Unauthorised active substanceQuality Manufacturer statement Summary data on the pharmaceutical, chemical andbiological qualityNon-<strong>clinical</strong> and <strong>clinical</strong> Unaltered benefit/risk ratio Local pharmacokinetic and local effects data in particularAdditional SmPC –(Prepared from Order N°SANP0623370A, article 3)Abridged technical dossierIn certain circumstances (see Table 5 and Table 6), an abridged technical dossier for IMPs and <strong>medical</strong> <strong>device</strong>s can be submitted to the AFSSAPS.The general principle is that whenever the IMP or the <strong>medical</strong> <strong>device</strong> is already “known” to the AFSSAPS or another EU competent authority,the submission of an abridged technical dossier is permitted.Table 5: Simplified technical dossier for IMPs (simplified IMPD)CircumstancesSimplified IMPD contentQuality Data Non-<strong>clinical</strong> Data Clinical DataThe IMP has a MA in any EU Member State, USA or Japan, or isauthorised for a compassionate use in France, and is used in the <strong>trial</strong>:– within the conditions of the SmPC SmPC SmPC SmPC– outside the conditions of the SmPC SmPC Yes YesThe IMP has no MA in any EU Member State but:– another pharmaceutical form or strength of the IMP has a MA inany EU Member State and the IMP is supplied by the MAH– the drug substance is part of a product with a MarketingAuthorisation in a MS and is supplied from the same manufacturerSmPC Yes YesSmPC Yes YesThe sponsor has already submitted a CTA for this IMP to theAFSSAPS and– new data are available since CTA New Data New Data New Data– the IMP is used in the <strong>trial</strong> outside the conditions stated in theprevious CTANo Yes YesThe IMPD was submitted as part of a previous CTA submitted by athird party who authorised the sponsor to refer to the previousCTA and– new data are available since CTA New Data New Data New Data– the IMP is used in the <strong>trial</strong> outside the conditions stated in theprevious CTANo Yes YesThe IMP is a placebo P+A No NoBut if the excipients are identical to the IMP and if it is not a sterile No No NoformIMP: Investigational Medicinal Product; IMPD: Investigational Medicinal Product Dossier; MA: Marketing Authorisation; MAH: Marketing Authorisation Holder;CTA: Clinical Trial Application. (Prepared from Order N°SANP0622223A, article 4)www.topra.org