View PDF - Doug Davis - Georgia Institute of Technology
View PDF - Doug Davis - Georgia Institute of Technology
View PDF - Doug Davis - Georgia Institute of Technology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
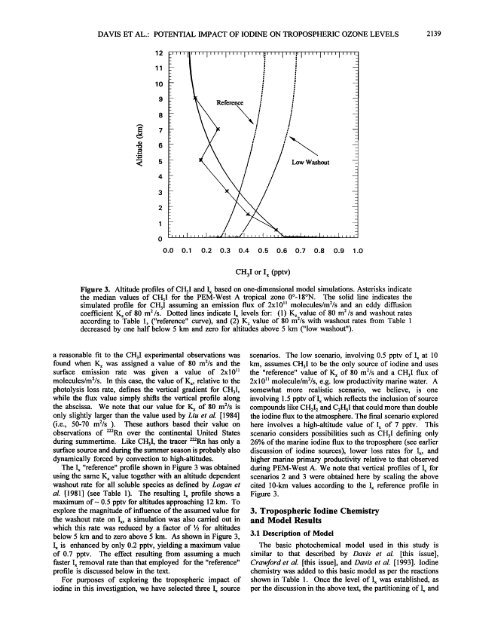
DAVIS ET AL.' POTENTIAL IMPACT OF IODINE ON TROPOSPHERIC OZONE LEVELS 21391211lOI;Reference:I;;Low Washoutoo.o0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 1.0CH3I or I x (pptv)Figure 3. Altitude pr<strong>of</strong>iles <strong>of</strong> CH3I and Ix based on one-dimensional model simulations. Asterisks indicatethe median values <strong>of</strong> CH3I for the PEM-West A tropical zone 0ø-18øN. The solid line indicates thesimulated pr<strong>of</strong>ile for CH3I assuming an emission flux <strong>of</strong> 2x10 • molecules/m2/s and an eddy diffusioncoefficient Kz <strong>of</strong> 80 m 2/s. Dotted lines indicate Ix levels for: (1) Kz value <strong>of</strong> 80 m 2/s and washout ratesaccording to Table 1, ("reference" curve), and (2) K z value <strong>of</strong> 80 m2/s with washout rates from Table 1decreased by one half below 5 km and zero for altitudes above 5 km ("low washout").a reasonable fit to the CH3I experimental observations wasfound when K z was assigned a value <strong>of</strong> 80 m2/s and thesurface emission rate was given a value <strong>of</strong> 2x10 •molecules/m2/s. In this case, the value <strong>of</strong> Kz, relative to thephotolysis loss rate, defines the vertical gradient for CH3I,while the flux value simply shifts the vertical pr<strong>of</strong>ile alongthe abscissa. We note that our value for K z <strong>of</strong> 80 m2/s isscenarios. The low scenario, involving 0.5 pptv <strong>of</strong> Ix at 10km, assumes CH3I to be the only source <strong>of</strong> iodine and usesthe "reference" value <strong>of</strong> K z <strong>of</strong> 80 m2/s and a CH3I flux <strong>of</strong>2x10 • molecule/m2/s, e.g. low productivity marine water. Asomewhat more realistic scenario, we believe, is oneinvolving 1.5 pptv <strong>of</strong> Ix which reflects the inclusion <strong>of</strong> sourcecompounds like CH2I 2 and C2H5I that could more than doubleonly slightly larger than the value used by Liu et al. [1984] the iodine flux to the atmosphere. The final scenario explored(i.e., 50-70 m2/s ). These authors based their value on here involves a high-altitude value <strong>of</strong> Ix <strong>of</strong> 7 pptv. Thisobservations <strong>of</strong> 222Rn over the continental United Statesduring summertime. Like CH3I, the tracer 222Rn has only asurface source and during the summer season is probably alsodynamically forced by convection to high-altitudes.The Ix "reference" pr<strong>of</strong>ile shown in Figure 3 was obtainedscenario considers possibilities such as CH3I defining only26% <strong>of</strong> the marine iodine flux to the troposphere (see earlierdiscussion <strong>of</strong> iodine sources), lower loss rates for Ix, andhigher marine primary productivity relative to that observedduring PEM-West A. We note that vertical pr<strong>of</strong>iles <strong>of</strong> Ix forusing the same K z value together with an altitude dependent scenarios 2 and 3 were obtained here by scaling the abovewashout rate for all soluble species as defined by Logan etal. [1981] (see Table 1). The resulting Ix pr<strong>of</strong>ile shows acited 10-km values according to the Ix reference pr<strong>of</strong>ile inFigure 3.maximum <strong>of</strong>- 0.5 pptv for altitudes approaching 12 km. Toexplore the magnitude <strong>of</strong> influence <strong>of</strong> the assumed value for 3. Tropospheric Iodine Chemistrythe washout rate on Ix, a simulation was also carried out in and Model Resultswhich this rate was reduced by a factor <strong>of</strong> « for altitudesbelow 5 km and to zero above 5 km. As shown in Figure 3,Ix is enhanced by only 0.2 pptv, yielding a maximum value<strong>of</strong> 0.7 pptv. The effect resulting from assuming a much3.1 Description <strong>of</strong> ModelThe basic photochemical model used in this study issimilar to that described by <strong>Davis</strong> et al. [this issue],faster Ix removal rate than that employed for the "reference" Crawford et al. [this issue], and <strong>Davis</strong> et al. [1993]. Iodinepr<strong>of</strong>ile is discussed below in the text.For purposes <strong>of</strong> exploring the tropospheric impact <strong>of</strong>iodine in this investigation, we have selected three Ix sourcechemistry was added to this basic model as per the reactionsshown in Table 1. Once the level <strong>of</strong> Ix was established, asper the discussion in the. above text, the partitioning <strong>of</strong> Ix and