Guidelines for the Use of the MS16A Syringe Driver - Palliative Care ...
Guidelines for the Use of the MS16A Syringe Driver - Palliative Care ...
Guidelines for the Use of the MS16A Syringe Driver - Palliative Care ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>Guidelines</strong> <strong>for</strong> <strong>the</strong> <strong>Use</strong> <strong>of</strong> <strong>the</strong> <strong>MS16A</strong> <strong>Syringe</strong><strong>Driver</strong>These are <strong>Guidelines</strong> only – you have a pr<strong>of</strong>essionalresponsibility to be trained and competent to use <strong>the</strong><strong>MS16A</strong> <strong>Syringe</strong> <strong>Driver</strong>, and to appropriately assessand review <strong>the</strong> clinical needs <strong>of</strong> <strong>the</strong> patientAuthor:Nicky Ryan, <strong>Palliative</strong> <strong>Care</strong> Team; Marion Khan,Practice Development TeamDate <strong>of</strong> issue: March 2008Version: 1Owner:<strong>Palliative</strong> <strong>Care</strong> TeamPublisher:Ms Nichola Greenwood, Medical Governance ManagerReview date: March 2009
<strong>Guidelines</strong> <strong>for</strong> <strong>the</strong> <strong>Use</strong> <strong>of</strong> <strong>the</strong> <strong>MS16A</strong> <strong>Syringe</strong> <strong>Driver</strong>ContentsPage1 The <strong>MS16A</strong> <strong>Syringe</strong> <strong>Driver</strong> anddelivery <strong>of</strong> subcutaneous medication:Indications <strong>for</strong> <strong>Use</strong>Advantages <strong>of</strong> <strong>Use</strong>Contraindications to <strong>Use</strong> 32 <strong>Guidelines</strong> <strong>for</strong> Insertion <strong>of</strong>Yellow Side-Ported Saf-T-IntimaCannula 4Table 1: <strong>Guidelines</strong> <strong>for</strong> insertion <strong>of</strong> <strong>the</strong>Saf-t-Intima Cannula 43 The Graseby <strong>MS16A</strong> syringe driver 63.1 Measuring and setting <strong>the</strong> rate <strong>of</strong> <strong>the</strong><strong>MS16A</strong> syringe driver 6Figure 1: Measurement <strong>of</strong> millimetre(mm) fluid volume in luer lock syringe 63.2 Calculating rate <strong>of</strong> infusion <strong>of</strong> <strong>the</strong>syringe driver 7Figure 2: The MS 16A <strong>Syringe</strong> <strong>Driver</strong> 73.3 Setting up <strong>of</strong> <strong>the</strong> syringe driver 73.4 Risk assessment 103.5 Using two syringe drivers 103.6 Changing prescription and medication 113.7 Changing insertion sites, lines andcannula 113.8 <strong>Care</strong> and monitoring <strong>of</strong> patients 123.9 Checking <strong>the</strong> syringe driver 123.10 Problem solving <strong>the</strong> syringe driver 123.11 Discontinuing <strong>the</strong> syringe driver 134 Documentation and record keeping 135 Complications 146 O<strong>the</strong>r Related Issues 157 References 15Appendix 1: Risk Assessment 172
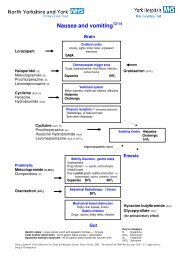
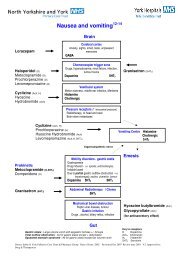
1. The <strong>MS16A</strong> <strong>Syringe</strong> <strong>Driver</strong> and delivery <strong>of</strong> subcutaneousmedication:Indications <strong>for</strong> useFor control <strong>of</strong> symptoms:- Pain- Nausea and vomiting- Restlessness- Increased secretions- Pain unrelieved by oralmedication orintermittent injectionPatients unable to swallow orabsorb oral medication:- Intractable nausea andvomiting- Gastro-intestinalobstruction/malabsorption- Mouth, throat, oesophageallesions- Pr<strong>of</strong>oundweakness/unconsciousnessAdvantages <strong>of</strong> use- Constant plasma drug levels resulting in increased com<strong>for</strong>tand confidence- Absorption <strong>of</strong> drugs more reliable- Change only every 24 hours- More effective assessment <strong>of</strong> symptom control.Contraindications to use- Infection and broken skin at infusion sites- Patients with clotting disorders because <strong>of</strong> risk <strong>of</strong> bleeding at<strong>the</strong> infusion site- Patients who have poor tissue perfusion- Peripheral vascular disease <strong>of</strong> lower extremities- Pre-existing oedema.(Ferry et al 1999, Sasson and Shvartzman 2001, Jackson 2004)3
2. <strong>Guidelines</strong> <strong>for</strong> Insertion <strong>of</strong> Yellow Side-PortedSaf-T-Intima CannulaIt is essential with any procedure, invasive or not, to discussfully with patients <strong>the</strong> intervention required and obtain <strong>the</strong>irin<strong>for</strong>med consent to continue. Be<strong>for</strong>e commencement <strong>of</strong>subcutaneous <strong>the</strong>rapy, <strong>the</strong> nurse must ensure that he or sheis confident that <strong>the</strong> prescription and prescribed <strong>the</strong>rapy aresuitable <strong>for</strong> this route. No nurse must undertake anyprocedure or practice <strong>for</strong> which he or she has not receivedadequate training and assessment <strong>of</strong> competence (Nursingand Midwifery Council (NMC) 2004). <strong>Guidelines</strong> <strong>for</strong> insertionare outlined in Table 1.Table 1. <strong>Guidelines</strong> <strong>for</strong> insertion <strong>of</strong> <strong>the</strong> Saf-t-Intima CannulaActionExplain procedure to patient and obtainin<strong>for</strong>med verbal consent (if possible).Consider site selection, involving patientin decision if possible.Wash hands and wear clean gloves.Clean selected insertion site with topicalcleaning agent and allow to dry.Remove white stopper from side armand prime line with infusion syringe.Attach bionector.Grasp “pebbled” side <strong>of</strong> <strong>the</strong> cannulawings, pinching wings firmly toge<strong>the</strong>r.This locks <strong>the</strong> needle and prevents isfrom retracting during insertionEnsure <strong>the</strong> needle is bevel-sideuppermost. If <strong>the</strong> bevel-side is notuppermost, open <strong>the</strong> wings and gentlytwist <strong>the</strong> white shield until <strong>the</strong> needle iscorrectly positionedGently pinch <strong>the</strong> skin into a fold. Inpatients with greater adipose tissue, itmay be necessary to keep <strong>the</strong> skin flatto ensure <strong>the</strong> needle is inserted into <strong>the</strong>RationaleTo ensure patient aware <strong>of</strong>, and consentsto, proposed procedure.To select <strong>the</strong> appropriate infusion siteand encourage patient concordance andtolerance.To minimise risk <strong>of</strong> infection.To ensure pre-insertion skin disinfection.To remove air from line to reduce <strong>the</strong> risk<strong>of</strong> air embolism. Ensure medications aredelivered as soon as <strong>the</strong> syringe driverstarts.To ensure effective insertion <strong>of</strong> needleinto subcutaneous tissueTo guide <strong>the</strong> needle through <strong>the</strong> tissueand reduce patient discom<strong>for</strong>t.To lift <strong>the</strong> subcutaneous layer away from<strong>the</strong> muscle layer4
subcutaneous layer.Insert <strong>the</strong> needle subcutaneously at anangle <strong>of</strong> approximately 45 degrees.Open <strong>the</strong> wings (pebbled side down) flatagainst <strong>the</strong> skinThere must be no evidence <strong>of</strong> bloodpresent in <strong>the</strong> giving set or cannula oninsertion or during treatment.To ensure <strong>the</strong> secure entry <strong>of</strong> <strong>the</strong> needleinto <strong>the</strong> subcutaneous tissue.To ensure correct positioning <strong>of</strong> <strong>the</strong>cannulaThis indicates a capillary has beenpunctured. The device should beremoved and resited.Apply firm finger-tip pressure over <strong>the</strong>wings <strong>of</strong> <strong>the</strong> cannula (avoiding <strong>the</strong>centre where <strong>the</strong> needle retracts) andsimultaneously grasp <strong>the</strong> pebbled end<strong>of</strong> <strong>the</strong> white shield and pull in a straightcontinuous motion until <strong>the</strong> needle hasfully withdrawn into <strong>the</strong> colouredcylinder and pops <strong>of</strong>fGently remove <strong>the</strong> coloured cylinderfrom <strong>the</strong> cannula port, if it has notreleased spontaneously, exposing <strong>the</strong>adapter with <strong>the</strong> rubber bungPlace any resultant sharps in sharpsbin.Remove rubber bung and connectVygon infusion set, open clampApply IV 3000 over insertion site.Adjust medication delivery rate asprescribed.Complete necessary nursingdocumentation and line labels detailingdate/time <strong>of</strong> insertion; insertion site anddevice used. Complete and sign DrugChart.To remove <strong>the</strong> insertion needle andactivate <strong>the</strong> safety mechanism.To ensure safety needle mechanism asworked effectively.To reduce <strong>the</strong> risk <strong>of</strong> needlestick injury.To maintain closed circuitTo ensure secure fixation, allow <strong>for</strong> siteobservation and moisture vapourpermeability.To ensure timely and effective delivery <strong>of</strong>prescription.Good record keeping helps protect <strong>the</strong>welfare <strong>of</strong> <strong>the</strong> patient, promotes bettercommunication and ensures <strong>the</strong>dissemination <strong>of</strong> in<strong>for</strong>mation betweenhealth pr<strong>of</strong>essionals.(Dawkins and Pugh 2003, RCN 2005)5
3. The Graseby <strong>MS16A</strong> syringe driver (Figure 2)3.1 Measuring and setting <strong>the</strong> rate <strong>of</strong> <strong>the</strong> <strong>MS16A</strong> syringe driver(Figure 1)The total fluid to be infused should be measured in millimetres (mm)and <strong>the</strong> rate set in millimetres per hour (mm/hr) <strong>for</strong> <strong>the</strong> <strong>MS16A</strong> (blue).The <strong>MS16A</strong> (blue) Graseby syringe driver will deliver 48mm <strong>of</strong> fluidover 24hrs at a rate <strong>of</strong> 2mm per hour. The length <strong>of</strong> 48mm is used tocalculate <strong>the</strong> rate per hour setting, i.e. 2mm/hr x 24hrs= 48mmThe length <strong>of</strong> liquid in <strong>the</strong> syringe is measured using <strong>the</strong> mm scale on<strong>the</strong> side <strong>of</strong> <strong>the</strong> machine. If <strong>the</strong> length <strong>of</strong> liquid in <strong>the</strong> syringe is 48mm,it will be delivered over 24hrs at a rate <strong>of</strong> 2mm per hour (Figure 4).Different brands <strong>of</strong> syringes give different total volumes <strong>of</strong> fluid whenmeasured at 48mm. This is why <strong>the</strong> volume in syringes is measuredin mm to ensure <strong>the</strong> prescribed medication delivery over 24hrs.Within <strong>the</strong> Trust, BD Plastipak luer lock syringes must be used <strong>for</strong>10ml and 20ml syringes, and Terumo luer lock syringes <strong>for</strong> 30mls.Figure 1: Measurement <strong>of</strong> millimetre (mm) fluid volume in luerlock syringeIf <strong>the</strong> total volume <strong>of</strong> required fluid becomes greater than 48mm inlength in <strong>the</strong> 10ml syringe <strong>the</strong>n change <strong>the</strong> fluid to a 20ml syringe,and measure <strong>the</strong> 20ml syringe length to 48mm adding in extradiluent as needed to bring <strong>the</strong> volume up to 48mm. 30ml syringescan also be used, but care must be taken to ensure that <strong>the</strong>syringe in this case is <strong>the</strong> Terumo due to <strong>the</strong> barrel width <strong>of</strong> <strong>the</strong>syringe. The rate remains set at 2mm/hr <strong>for</strong> <strong>MS16A</strong> (blue).6
3.2 Calculating rate <strong>of</strong> infusion <strong>of</strong> <strong>the</strong> syringe driverIn <strong>the</strong> <strong>MS16A</strong> (blue) syringe driver, <strong>the</strong> following rates <strong>of</strong> infusionapply:48mm set at 2mm runs <strong>for</strong> 24hrs48mm set at 4mm runs <strong>for</strong> 12hrsFigure 2: The MS 16A <strong>Syringe</strong> <strong>Driver</strong>3.3 Setting up <strong>of</strong> <strong>the</strong> syringe driverAction• Check service date <strong>of</strong> syringedriver• Check driver and plastictransparent cover is cleanedusing a detergent wipe be<strong>for</strong>euseRationale• To ensure syringe driver is inworking order and safe touse• To reduce risk <strong>of</strong> crosscontaminationand infection7
• Insert new 9v battery andpress ‘Start’ button• Check medications onsyringe driver prescriptionchart are correctly prescribedand signed• To activate syringe driver andensure it is working bysilencing <strong>the</strong> alarm andinitiating <strong>the</strong> light to flash• To confirm and ensureappropriate drugs areprescribed correctly and arecompatible• Explain procedure to patientand obtain in<strong>for</strong>med verbalconsent (if possible).• A Lock Box risk assessment<strong>for</strong>m (Appendix 1) should becompleted regarding <strong>the</strong> safeadministration <strong>of</strong> medicinesvia <strong>the</strong> syringe driver.Identified risk should berecorded and discussed with<strong>the</strong> appropriate pr<strong>of</strong>essionals(see Section 3.4).• Wash hands according toInfection Control Policy.Ensure all <strong>the</strong> equipment hasbeen cleaned prior to use.Check compatibility <strong>of</strong> drugsand diluent.If concerned please speakwith <strong>Palliative</strong> <strong>Care</strong> Team,Medicines In<strong>for</strong>mation, GP orprescriber. Out <strong>of</strong> hours,contact St Leonard’sHospice. There should be no morethan three different drugsin one syringe, due toincreased risk <strong>of</strong>incompatibility andconcentration <strong>of</strong> drugs. Mix drugs with diluents• To ensure patient aware <strong>of</strong>,and consents to, proposedprocedure.• <strong>Care</strong>ful explanation isnecessary to allay fears andanxieties regarding changing<strong>of</strong> medication route andmedications used. To ensuresafe administration <strong>of</strong>prescribed medication.• To reduce risk <strong>of</strong> infection• Correct drugs aredelivered according toprescription.• Reduce possibility <strong>of</strong>incompatibility.8
and dilute up to 48mm andmeasure against syringedriver gauge. (See Figure2) Dilute with water <strong>for</strong>injection or sodiumchloride 0.9% dependingon which diluent is mostcompatible with drugsprescribed. <strong>Use</strong> ampoule breaker ifavailable to avoid sharpsinjury• Complete additive labelwith in<strong>for</strong>mation regardingpatients name, date <strong>of</strong>birth/hospital unit number,drugs, dosage, diluent,date and time, andsignature. Attach label to<strong>the</strong> syringe trying toensure <strong>the</strong> syringemeasures can be seen.• Priming <strong>of</strong> line-Remember on initialpriming <strong>of</strong> line, <strong>the</strong> infusionwill finish 2 or 3 hoursearlier (use only 100cmsclamped tubing) This isbecause part <strong>of</strong> <strong>the</strong>syringe contents remainunused in <strong>the</strong> tubing untila new syringe is set up.• Select appropriate site• Applying syringe anddriver to patientCheck <strong>the</strong> identity <strong>of</strong> <strong>the</strong>patient as outlined in <strong>the</strong>‘Positive Patient• Ensures correctmedication given to correctpatient, and enablesvisible check <strong>of</strong> syringeflow• Good siting will allow evenabsorption <strong>of</strong> drugswithout discom<strong>for</strong>t topatient.• Ensure correct patientreceives treatment. Ensureprocedure <strong>for</strong> insertion iscorrectly followed9
Identification Policy’(2008)Using SAF-T-Intima givingset, insert and secure asdescribed in Section 2.Document on <strong>the</strong>monitoring section <strong>of</strong> <strong>the</strong>syringe driver drug chart<strong>the</strong> area used <strong>for</strong> siting,<strong>the</strong> date and <strong>the</strong> time.• Recheck drug chart andcheck against label.• Confirm correctprescription3.4 Risk assessmentA risk assessment must be carried out on all patients who requirea syringe driver to deliver medications. Appendix 1 is <strong>the</strong> approvedrisk assessment tool. The aim <strong>of</strong> <strong>the</strong> risk assessment is to identifywhich patients may be at risk <strong>of</strong> accidental or intentional drugoverdose from tampering <strong>of</strong> <strong>the</strong> syringe driver. If <strong>the</strong> riskassessment indicates that <strong>the</strong> patient requires a lock box <strong>the</strong>secan be obtained from <strong>the</strong> <strong>Palliative</strong> <strong>Care</strong> Team. Out <strong>of</strong> hours, <strong>the</strong>lock box can be obtained from <strong>the</strong> Emergency Drug Cupboard.During use, <strong>the</strong> lock box keys should be kept with <strong>the</strong> ControlledDrug keys.3.5 Using two syringe driversIf two syringe drivers are to be used on one patient, extra careshould be taken:• The same type <strong>of</strong> syringe driver must be used if two driversare needed <strong>for</strong> one patient to reduce <strong>the</strong> risk <strong>of</strong> error.• Ensure each syringe driver prescription is clearly identifiedon drug chart.• Ensure prescription chart drugs and labels are checkedcarefully to ensure correct drugs used <strong>for</strong> each syringe driver• <strong>Use</strong> separate documentation <strong>for</strong> each syringe driver10
3.6 Changing prescription and medicationIf prescription medication and/or dosages are changed, a newsyringe and line must be set up and primed. The prescription chartshould be re-written with new medication and dosages. Themonitoring section <strong>of</strong> <strong>the</strong> syringe driver drug chart and labelling <strong>of</strong><strong>the</strong> syringe should be completed accordingly.Rationale:This allows patient to get new drugs and dosage as soon aspossible. Documentation should be updated and completed toshow new delivery <strong>of</strong> drugs.3.7 Changing insertion sites, lines and cannulaWhen resiting or restarting an infusion, it is recommended that <strong>the</strong>site selected is rotated using a figure-<strong>of</strong>-eight principle to maximisesite absorption. Frequency <strong>of</strong> resiting <strong>of</strong> <strong>the</strong> selected device isdependent on <strong>the</strong> manufacturer’s instructions; volume and type <strong>of</strong>infusate and local policies. It is reported in <strong>the</strong> literature (Brownand Worobec 2000, RCN 2005) that <strong>the</strong> time in situ <strong>of</strong> <strong>the</strong> devicecan vary between one to seven days, and must <strong>the</strong>re<strong>for</strong>e beguided by regular site assessment and local policies.Record any site reaction on nursing documentation. If reactionoccurs again, may need to look at <strong>the</strong> compatibility <strong>of</strong> drugs andamount <strong>of</strong> diluent.The manufacturers instructions must be followed changingequipment:• Vygon 100cm line – every 24 hours and when <strong>the</strong> syringe ischanged• Saf-t-intima – Every 72 hours, after each infusion <strong>of</strong> 2 litres<strong>of</strong> fluid (see above) and if <strong>the</strong> area or site becomes inflamedor sore.• <strong>Syringe</strong> – each time a new syringe is made up11
3.8 <strong>Care</strong> and monitoring <strong>of</strong> patientsIt is <strong>the</strong> responsibility <strong>of</strong> healthcare pr<strong>of</strong>essionals to monitorpatients at regular intervals in accordance with local protocols.Regular monitoring <strong>of</strong> patients enables healthcare pr<strong>of</strong>essionals toevaluate <strong>the</strong> infusion site and assess patients’ tolerance andresponse to <strong>the</strong> intervention. This will enable prompt identificationand management <strong>of</strong> any complications.3.9 Checking <strong>the</strong> syringe driverThe following checks must be undertaken at least every 4 hoursAlso check• Check <strong>the</strong> syringe driver is still running• Light is flashing• Whirr sound can be heard• <strong>Syringe</strong> volume has decreased since last check andcontents clear• <strong>Syringe</strong> remains in place in driver• Correct rate• Record check on syringe driver documentation• The line <strong>for</strong> kinks and to ensure clamp is <strong>of</strong>f• The site <strong>for</strong> signs <strong>of</strong> inflammation• The patient <strong>for</strong> symptom control3.10 Problem solving <strong>the</strong> syringe driverProblem: Indication <strong>for</strong> <strong>the</strong>problem:Infusion running too Clamp may be on orslowtubing kinked, batterymay need changing. Sitemay be blocked orinflamedInfusion running to<strong>of</strong>astRate may be set wrongPatient may havetampered with syringeSolution:Unclamp line, unkinktubing, change battery,check site <strong>for</strong> blockage orinflammation and ifoccurred remove cannulaand resite.Check calculation andrate.Recheck reassessment12
driverThe light stays on The battery is low Change <strong>the</strong> battery assoon as possibleThe light stops The infusion may be Re-site syringe driver andflashingAlarm soundsblockedThis means <strong>the</strong> syringedriver has stopped. Thiscould be due to <strong>the</strong>following:a. <strong>Syringe</strong> emptyb. Tubing kinkedc. Tubing is clampedchange <strong>the</strong> lineRenew syringe.Unkink or replace tubing.Unclamp tubing.If none <strong>of</strong> <strong>the</strong> above- change syringe driver and send <strong>for</strong> servicing<strong>Syringe</strong> drivers must be serviced annually.3.11 Discontinuing <strong>the</strong> syringe driverWhen <strong>the</strong> syringe driver is discontinued: -• Remove Saf-T-Intima and place in clinical waste. Applysterile dry dressing (if appropriate)• Discard syringe, its contents and line into sharps box inaccordance with hospital policy. Ensure to record <strong>the</strong> amount<strong>of</strong> discontinued medication on <strong>the</strong> prescription chart orchecklist• Clean driver and discard battery be<strong>for</strong>e storing away.4. Documentation and record keepingDocumentation <strong>of</strong> site selection and assessment is fundamental to<strong>the</strong> care and monitoring <strong>of</strong> <strong>the</strong> patient receiving subcutaneousinfusions.Ensure <strong>the</strong> <strong>Syringe</strong> <strong>Driver</strong> Drug Chart [including MonitoringSections] are completed accurately in accordance with NMCrecord keeping guidelines. Documentation must be completed <strong>for</strong>each syringe driver set up, when <strong>the</strong> syringe driver is changed,and at every drug round in <strong>the</strong> hospital.13
Rationale:• Maintain safe practice.• Maintain safe accuracy <strong>of</strong> drug delivery5. ComplicationsWith appropriate and correct usage, <strong>the</strong> risks and complicationsassociated with subcutaneous infusions are generally minimal andeasily resolved. Any complication that does arise, however,requires potential resiting and review <strong>of</strong> patient needs andprescription.ComplicationsAt insertion site:OedemaInfectionIrritationPain andinflammationAbscessPrevention & Management <strong>of</strong>complicationsEnsure cannula inserted as per Section 2above, and cover and secure with steriledressing. Change equipment and rotatesites. If complications occur removecannula and resite. Treat patient symptomswith analgesia / antipyretics, may requireantibiotics / surgical intervention <strong>for</strong>abcess.Infusion induced:Drug incompatibility Ensure drugs are compatiblePatient drug allergies Ensure allergies identified prior toprescribing and allergy band in situ. Ifallergic reaction occurs, stop infusion treatsymptoms and document on alert sheetand in recordsCirculatory collapseStop infusion, support patient and summonmedical help.14
CrystallisationObserve <strong>for</strong> crystallization within <strong>the</strong> line orsyringe. Management:1. Increase <strong>the</strong> volume to allow <strong>for</strong> morediluent by:Using a 20ml/30ml syringe <strong>for</strong> <strong>the</strong>prescription instead <strong>of</strong> 10mlMeasure again up to 48mmContinue to run at 2mm= 02mm/hr in<strong>MS16A</strong>(blue) <strong>for</strong> 24hrs OR2. Change to a 12 hourly regime by:Measure <strong>the</strong> syringe contents up to 48mmRun at 4mm= 04mm/hr in <strong>MS16A</strong>(blue) <strong>for</strong>12 hour regimeIt must be considered that <strong>the</strong> more serious complications <strong>of</strong>abscess, allergies and circulatory collapse may be <strong>the</strong> result <strong>of</strong>inappropriate prescribing or administration.6. O<strong>the</strong>r Related IssuesADVICE and INFORMATION:<strong>Palliative</strong> <strong>Care</strong> Team Hospital – 01904 725835 are available <strong>for</strong>advice between: Monday – Friday, 8.30am – 4.30pmOut <strong>of</strong> hours – advice can be sought from:St Leonard’s Hospice – 01904 7085537. ReferencesBrown MK, Worobec F (2000) Hyperdermoclysis: ano<strong>the</strong>r way toreplace fluids. Nursing. 30, 5, 58-59Dawkins L, Pugh J (2003) Paper entitled “<strong>Guidelines</strong> <strong>for</strong> <strong>the</strong> use <strong>of</strong>Saf-T-Intima <strong>for</strong> <strong>the</strong> Administration <strong>of</strong> Subcutaneous Infusions andBolus Medication”. Earl Mountbatten Hospice, Barnsley15
Ferry M, Dardaine V, Constans T (1999) Subcutaneous infusion orhypodermoclysis: a practical approach. Journal <strong>of</strong> <strong>the</strong> AmericanGeriatrics Society. 47, 1, 93-95.Sasson M, Shvartzman P (2001) Hypodermoclysis: an alternativeinfusion technique. American Family Physician. 64, 9, 1575-1578.Jackson A (2004) Subcutaneous Fluid Administration(Hypodermoclysis). Ro<strong>the</strong>rham General Hospitals Trust,Ro<strong>the</strong>rham.Nursing and Midwifery Council (2004) The NMC Code <strong>of</strong>Pr<strong>of</strong>essional Conduct: Standards <strong>for</strong> Conduct, Per<strong>for</strong>mance andEthics. NMC, London.Royal College <strong>of</strong> Nursing (2005) Standards <strong>for</strong> Infusion Therapy.RCN, London.16
Appendix 1York Hospitals NHS Foundation TrustRisk Assessment <strong>for</strong> patients requiring syringe driverAim: The reduction <strong>of</strong> non-pr<strong>of</strong>essional incidents <strong>of</strong> drug overdoseto patients, by accident or intentionPatient name:Ward:Codes: (M) = medium risk (H) = InitialFur<strong>the</strong>rhigh riskassessment assessmentSignature <strong>of</strong> nurse carrying outassessment:Date assessment carried out:Identification <strong>of</strong> risk factors Yes No Yes No1. Is <strong>the</strong> patient confused? (M)2. Is <strong>the</strong> patient agitated? (M)3. Does <strong>the</strong> patient interfere wi<strong>the</strong>quipment in general? (H)4. Has <strong>the</strong> patient a history <strong>of</strong>attempted suicide, orexpressed a wish to self-harm?(H)5. Has <strong>the</strong> patient a history <strong>of</strong>drug abuse?6. If a syringe driver is in situ has<strong>the</strong> patient attempted to/dismantled it? (H)7. Has <strong>the</strong> patients delivered SDmedication to <strong>the</strong>mselves? (H)8. If independently mobile, does<strong>the</strong> patient continually <strong>for</strong>get aSD is in situ, allowing it to fall?(M)9. On checking SD medication, is<strong>the</strong>re an unaccountablediscrepancy in amountdelivered? (H)17
Assessment results: Risk: Action:No and/or N/A to all Low Reassess if changequestionsoccursYes to 1 or 2 (M) ModerateMonitor over 1 st 24 hoursquestions<strong>for</strong> increased score <strong>the</strong>nPRN– Consider Lock boxYes to all (M) questions High Lockbox neededYes to 1 or more (H) Very highLockbox neededquestionsOutcome <strong>of</strong> Initial assessment:Lock box required? Yes No Lock box available? Yes No If no state action taken:Lock box in place? Yes No Date put in place:If no state reason:Removal <strong>of</strong> lock box: Date:Reason <strong>for</strong> removal:18
Outcome <strong>of</strong> fur<strong>the</strong>r assessment:Lock box required? Yes No Lock box available? Yes No If no state action taken:Lock box in place? Yes No Date put in place:If no state reason:Removal <strong>of</strong> lock box: Date:Reason <strong>for</strong> removal:(Adapted with courtesy <strong>of</strong> St Leonards’ Hospice)19