You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
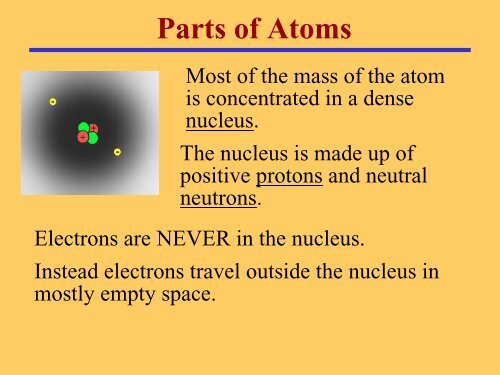
<strong>Parts</strong> <strong>of</strong> <strong>Atoms</strong>Most <strong>of</strong> the mass <strong>of</strong> the atomis concentrated in a densenucleus.The nucleus is made up <strong>of</strong>positive protons and neutralneutrons.Electrons are NEVER in the nucleus.Instead electrons travel outside the nucleus inmostly empty space.
Atomic Numbers10NeNeon20.18Atomic NumberThe atomic number <strong>of</strong> anelement is equal to thenumber <strong>of</strong> protons in itsnucleus.We know that a neon atomhas 10 protons because neonhas an atomic number <strong>of</strong> 10.
What about Mass?Protons and neutrons eachweight about 1000 times asmuch as electrons.So we calculate atomicmasses by adding up onlyneutrons and protons.We ignore the masses <strong>of</strong> electrons becausetheir mass is so small compared to the mass <strong>of</strong>protons and neutrons
Isotopesmass 1 mass 2 mass 3Isotopes are members <strong>of</strong> a family <strong>of</strong> elementswith the same number <strong>of</strong> protons (same atomicnumber) but a different number <strong>of</strong> neutrons(different atomic mass).Isotopes<strong>of</strong>hydrogen1 proton but no neutrons (mass 1)1 proton and 1 neutron (mass 2)1 proton and 2 neutrons (mass 3)
Weighted Average100 lbs 200 lbs 300 lbs98 skinnies 1 fat 1 superfat98 x 100 = 98001 x 200 = 2001 x 300 = 300100 10300The weightedaverage10300 divided by 100 = 103 lbs
10NeNeon20.18On the Periodic TableAtomic masses are <strong>of</strong>ten notwhole numbers.atomic mass (a weightedaverage <strong>of</strong> more thanone isotope)The atomic mass for neon is the weightedaverage <strong>of</strong> a common isotope with a mass <strong>of</strong> 20and a less common isotope with a mass <strong>of</strong> 21.The weighted average <strong>of</strong> 20.18 shows up on thePeriodic Table for neon.
Calculating Neutrons10NeNeon20.18Round <strong>of</strong>f the atomicmass to the nearestwhole number.So 20.18 is rounded <strong>of</strong>fto 20, a whole number.Subtract the number <strong>of</strong> protons(10) from the rounded atomicmass (20).So neon has 10 neutrons.
Calculating Electrons10NeNeon20.18Atomic NumberAn atom that has neither lostnor gained electrons isneutral.In a neutral atom, thenumber <strong>of</strong> electrons equalsthe number <strong>of</strong> protons (thesame as the atomic number)Neon (atomic number 10) has 10 electrons