moj seminar P ... - F9
moj seminar P ... - F9
moj seminar P ... - F9
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
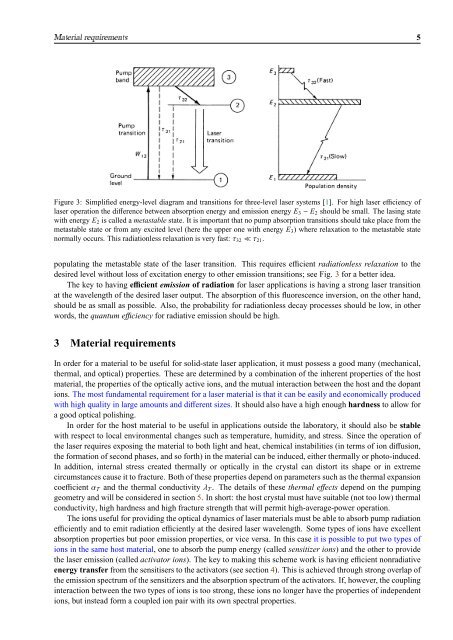
Material requirements 5Figure 3: Simplified energy-level diagram and transitions for three-level laser systems [1]. For high laser efficiency oflaser operation the difference between absorption energy and emission energy E 3 − E 2 should be small. The lasing statewith energy E 2 is called a metastable state. It is important that no pump absorption transitions should take place from themetastable state or from any excited level (here the upper one with energy E 3 ) where relaxation to the metastable statenormally occurs. This radiationless relaxation is very fast: τ 32 ≪ τ 21 .populating the metastable state of the laser transition. This requires efficient radiationless relaxation to thedesired level without loss of excitation energy to other emission transitions; see Fig. 3 for a better idea.The key to having efficient emission of radiation for laser applications is having a strong laser transitionat the wavelength of the desired laser output. The absorption of this fluorescence inversion, on the other hand,should be as small as possible. Also, the probability for radiationless decay processes should be low, in otherwords, the quantum efficiency for radiative emission should be high.3 Material requirementsIn order for a material to be useful for solid-state laser application, it must possess a good many (mechanical,thermal, and optical) properties. These are determined by a combination of the inherent properties of the hostmaterial, the properties of the optically active ions, and the mutual interaction between the host and the dopantions. The most fundamental requirement for a laser material is that it can be easily and economically producedwith high quality in large amounts and different sizes. It should also have a high enough hardness to allow fora good optical polishing.In order for the host material to be useful in applications outside the laboratory, it should also be stablewith respect to local environmental changes such as temperature, humidity, and stress. Since the operation ofthe laser requires exposing the material to both light and heat, chemical instabilities (in terms of ion diffusion,the formation of second phases, and so forth) in the material can be induced, either thermally or photo-induced.In addition, internal stress created thermally or optically in the crystal can distort its shape or in extremecircumstances cause it to fracture. Both of these properties depend on parameters such as the thermal expansioncoefficient α T and the thermal conductivity λ T . The details of these thermal effects depend on the pumpinggeometry and will be considered in section 5. In short: the host crystal must have suitable (not too low) thermalconductivity, high hardness and high fracture strength that will permit high-average-power operation.The ions useful for providing the optical dynamics of laser materials must be able to absorb pump radiationefficiently and to emit radiation efficiently at the desired laser wavelength. Some types of ions have excellentabsorption properties but poor emission properties, or vice versa. In this case it is possible to put two types ofions in the same host material, one to absorb the pump energy (called sensitizer ions) and the other to providethe laser emission (called activator ions). The key to making this scheme work is having efficient nonradiativeenergy transfer from the sensitisers to the activators (see section 4). This is achieved through strong overlap ofthe emission spectrum of the sensitizers and the absorption spectrum of the activators. If, however, the couplinginteraction between the two types of ions is too strong, these ions no longer have the properties of independentions, but instead form a coupled ion pair with its own spectral properties.