SEND Terminology 2012-12-21.pdf - EVS
SEND Terminology 2012-12-21.pdf - EVS
SEND Terminology 2012-12-21.pdf - EVS
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
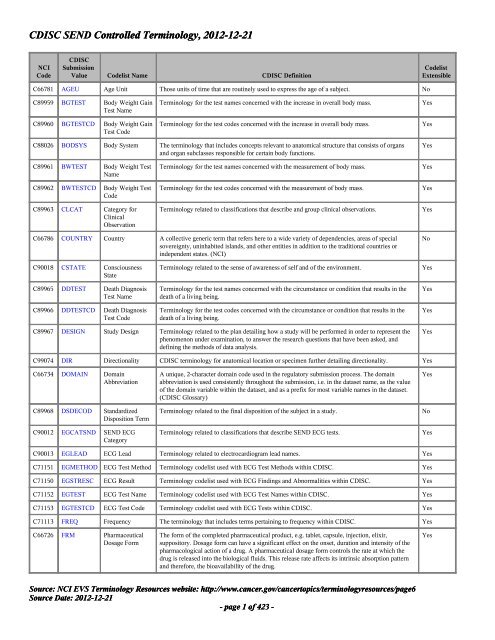
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21NCICodeCDISCSubmissionValue Codelist Name CDISC DefinitionCodelistExtensibleC66781 AGEU Age Unit Those units of time that are routinely used to express the age of a subject. NoC89959 BGTEST Body Weight GainTest NameC89960 BGTESTCD Body Weight GainTest Code<strong>Terminology</strong> for the test names concerned with the increase in overall body mass.<strong>Terminology</strong> for the test codes concerned with the increase in overall body mass.YesYesC88026 BODSYS Body System The terminology that includes concepts relevant to anatomical structure that consists of organsand organ subclasses responsible for certain body functions.YesC89961 BWTEST Body Weight TestNameC89962 BWTESTCD Body Weight TestCodeC89963 CLCAT Category forClinicalObservation<strong>Terminology</strong> for the test names concerned with the measurement of body mass.<strong>Terminology</strong> for the test codes concerned with the measurement of body mass.<strong>Terminology</strong> related to classifications that describe and group clinical observations.YesYesYesC66786 COUNTRY Country A collective generic term that refers here to a wide variety of dependencies, areas of specialsovereignty, uninhabited islands, and other entities in addition to the traditional countries orindependent states. (NCI)NoC90018 CSTATE ConsciousnessStateC89965 DDTEST Death DiagnosisTest NameC89966 DDTESTCD Death DiagnosisTest Code<strong>Terminology</strong> related to the sense of awareness of self and of the environment.<strong>Terminology</strong> for the test names concerned with the circumstance or condition that results in thedeath of a living being.<strong>Terminology</strong> for the test codes concerned with the circumstance or condition that results in thedeath of a living being.YesYesYesC89967 DESIGN Study Design <strong>Terminology</strong> related to the plan detailing how a study will be performed in order to represent thephenomenon under examination, to answer the research questions that have been asked, anddefining the methods of data analysis.YesC99074 DIR Directionality CDISC terminology for anatomical location or specimen further detailing directionality. YesC66734 DOMAIN DomainAbbreviationC89968 DSDECOD StandardizedDisposition TermC900<strong>12</strong> EGCATSND <strong>SEND</strong> ECGCategoryA unique, 2-character domain code used in the regulatory submission process. The domainabbreviation is used consistently throughout the submission, i.e. in the dataset name, as the valueof the domain variable within the dataset, and as a prefix for most variable names in the dataset.(CDISC Glossary)<strong>Terminology</strong> related to the final disposition of the subject in a study.<strong>Terminology</strong> related to classifications that describe <strong>SEND</strong> ECG tests.YesNoYesC90013 EGLEAD ECG Lead <strong>Terminology</strong> related to electrocardiogram lead names. YesC71151 EGMETHOD ECG Test Method <strong>Terminology</strong> codelist used with ECG Test Methods within CDISC. YesC71150 EGSTRESC ECG Result <strong>Terminology</strong> codelist used with ECG Findings and Abnormalities within CDISC. YesC71152 EGTEST ECG Test Name <strong>Terminology</strong> codelist used with ECG Test Names within CDISC. YesC71153 EGTESTCD ECG Test Code <strong>Terminology</strong> codelist used with ECG Tests within CDISC. YesC71113 FREQ Frequency The terminology that includes terms pertaining to frequency within CDISC. YesC66726 FRM PharmaceuticalDosage FormThe form of the completed pharmaceutical product, e.g. tablet, capsule, injection, elixir,suppository. Dosage form can have a significant effect on the onset, duration and intensity of thepharmacological action of a drug. A pharmaceutical dosage form controls the rate at which thedrug is released into the biological fluids. This release rate affects its intrinsic absorption patternand therefore, the bioavailability of the drug.YesSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 1 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21NCICodeCDISCSubmissionValue Codelist Name CDISC DefinitionCodelistExtensibleC89969 FWTEST Food and WaterConsumption TestNameC89970 FWTESTCD Food and WaterConsumption TestCode<strong>Terminology</strong> for the test names concerned with the subject's consumption of food and/or water.<strong>Terminology</strong> for the test codes concerned with the subject's consumption of food and/or water.YesYesC99073 LAT Laterality CDISC terminology for anatomical location or specimen further detailing the side(s) of interest. YesC67154 LBTEST Laboratory TestNameC65047 LBTESTCD Laboratory TestCodeC89971 MATEST MacroscopicFindings TestNameC89972 MATESTCD MacroscopicFindings TestCodeC90017 MIRESCAT MicroscopicHistopathologyResult CategoryC89973 MITEST MicroscopicFindings TestNameC89974 MITESTCD MicroscopicFindings TestCodeC89975 MTHTRM Method ofTermination<strong>Terminology</strong> used for Laboratory Tests of the CDISC Standard Data Tabulation Model.<strong>Terminology</strong> used for Laboratory Tests of the CDISC Standard Data Tabulation Model.<strong>Terminology</strong> for the test names concerned with the findings from a specimen that are visible tothe naked eye.<strong>Terminology</strong> for the test codes concerned with the findings from a specimen that are visible tothe naked eye.<strong>Terminology</strong> related to the classifications of the results from a microscopic histopathologicalanalysis.<strong>Terminology</strong> for the test names concerned with the findings from a specimen that are visible bymicroscopic analysis.<strong>Terminology</strong> for the test codes concerned with the findings from a specimen that are visible bymicroscopic analysis.<strong>Terminology</strong> related to the method by which an experimental organism is sacrificed.YesYesYesYesYesYesYesYesC66789 ND Not Done Indicates a task, process or examination that has either not been initiated or completed. (NCI) NoC88025 NEOPLASM Neoplasm Type The terminology that includes concepts relevant to benign or malignant tissue growth. YesC90004 NEOSTAT Neoplastic Status <strong>Terminology</strong> related to the classifications of the results from a histopathological analysis of atumor.C66742 NY No Yes Response A term that is used to indicate a question with permissible values of yes/no/unknown/notapplicable.NoNoC89976 OMTEST OrganMeasurement TestNameC89977 OMTESTCD OrganMeasurement TestCodeC95<strong>12</strong>0 PHSPRP PhysicalProperties TestNameC95<strong>12</strong>1 PHSPRPCD PhysicalProperties TestCode<strong>Terminology</strong> for the test names concerned with the measurement of organs.<strong>Terminology</strong> for the test codes concerned with the measurement of organs.<strong>Terminology</strong> relevant to the test names that describe the physical characteristics of an entity.<strong>Terminology</strong> relevant to the test codes that describe the physical characteristics of an entity.NoNoYesYesC85493 PKPARM PK Parameters Parameters used to describe the time-concentration curve. YesSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 2 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21NCICodeCDISCSubmissionValue Codelist Name CDISC DefinitionCodelistExtensibleC85839 PKPARMCD PK ParametersCodeC85494 PKUNIT PK ParameterUnits of MeasureParameter codes used to describe the time-concentration curve.Units of measure needed for pharmacokinetic parameters.YesYesC99075 PORTOT Portion/Totality Qualifier for anatomical location or specimen further detailing the portion or totality, whichmeans arrangement of, or apportioning of an entity.YesC71148 POSITION Position <strong>Terminology</strong> codelist used with Body Position within CDISC. YesC78737 RELTYPE Relationship Type The description of relationship types between a record or set of records. NoC66729 ROUTE Route ofAdministrationC89981 SBCCDSND <strong>SEND</strong> SubjectCharacteristicsTest CodeC89980 SBCSND <strong>SEND</strong> SubjectCharacteristicsTest NameThe course by which a substance was administered in order to reach the site of action in thebody.<strong>Terminology</strong> for the test codes concerned with the distinguishing qualities or prominent aspectof a person, object, action, process, or substance.<strong>Terminology</strong> for the test names concerned with the distinguishing qualities or prominent aspectof a person, object, action, process, or substance.YesYesYesC90000 SEV Severity <strong>SEND</strong> terminology related to the degree of an occurrence of something undesirable. NoC66731 SEX Sex The assemblage of physical properties or qualities by which male is distinguished from female;the physical difference between male and female; the distinguishing peculiarity of male orfemale. (NCI)NoC66732 SEXPOP Sex ofParticipantsC89982 SNDIGVER <strong>SEND</strong>ImplementationGuide VersionThe specific sex, either male, female, or mixed of the subject group being studied. (NCI)<strong>Terminology</strong> related to the version of the <strong>SEND</strong> implementation guide that is in use for thestudy.NoYesC77529 SPEC Specimen <strong>Terminology</strong> related to any material sample taken from a biological entity. YesC77808 SPECIES Species <strong>Terminology</strong> related to the common name for an animal used as the test system in a study (e.g.,dog, monkey, mouse, rabbit, rat).C90003 SSTYP Study Type The further classification of nonclinical studies performed e.g. absorption, distribution andmetabolism.C90002 STCAT Study Category The type of nonclinical study performed e.g. pharmacokinetics, safety pharmacology andtoxicology.C77530 STRAIN Strain/Substrain <strong>Terminology</strong> used to identify the vendor-supplied species, strain, substrain or breed designationfor the test system under study. It may combine the species, background strain, substrain, andassociated genetic modifications as supplied by the vendor (e.g., C57BL/6, A/J,B6.<strong>12</strong>9-Pparg/J, FISCHER 344, SPRAGUE-DAWLEY IGS, WISTAR Kyoto,BEAGLE, CYNOMOLGUS, RHESUS and CHIMPANZEE).YesYesYesYesC90007 STSPRM <strong>SEND</strong> TrialSummaryParameter TestNameC90009 STSPRMCD <strong>SEND</strong> TrialSummaryParameter TestCodeC90005 TFTEST Tumor FindingsTest Name<strong>Terminology</strong> related to the parameter names of the individual characteristics of a nonclinicalstudy.<strong>Terminology</strong> related to the parameter codes of the individual characteristics of a nonclinicalstudy.<strong>Terminology</strong> for the test names concerned with the assessment or evaluation of a neoplasticmass.YesYesYesSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 3 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21NCICodeCDISCSubmissionValue Codelist Name CDISC DefinitionCodelistExtensibleC90006 TFTESTCD Tumor FindingsTest Code<strong>Terminology</strong> for the test codes concerned with the assessment or evaluation of a neoplasticmass.YesC71620 UNIT Unit <strong>Terminology</strong> codelist used for units within CDISC. YesC66770 VSRESU Units for VitalSigns ResultsC67153 VSTEST Vital Signs TestNameC66741 VSTESTCD Vital Signs TestCodeThe unit used to record and describe the result of a test investigating a vital sign. (NCI)The name given to the test that analyzes a particular set of vital signs including temperature,respiratory rate, heart beat (pulse), and blood pressure. (NCI)The name given to the test that analyzes a particular set of vital signs including temperature,respiratory rate, heart beat (pulse), and blood pressure. (NCI)YesYesYesSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 4 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66781 - AGEU - Age UnitCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC25301 DAYS The time for Earth to make a complete rotation on itsaxis; ordinarily divided into twenty-four hours. Thisalso refers to a specific day. (NCI)C25529 HOURS Hour; hr A unit measure of time equal to 3,600 seconds or 60minutes. It is approximately 1/24 of a median day.(NCI)C29846 MONTHS One of the <strong>12</strong> divisions of a year as determined by acalendar. It corresponds to the unit of time ofapproximately to one cycle of the moon's phases,about 30 days or 4 weeks. (NCI)DayHourMonthC29844 WEEKS Any period of seven consecutive days. (NCI) WeekC29848 YEARS The period of time that it takes for Earth to make acomplete revolution around the sun, approximately365 days; a specific one year period. (NCI)YearSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 5 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89959 - BGTEST - Body Weight Gain Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90363 Average Body Weight Gain Average BodyWeight GainC62754 Body Weight Gain Body WeightGainC90434 Percentage Body Weight Gain Percentage BodyWeight GainThe mean value of the amount of weight change over aperiod of time, relative to the beginning of thecollection period.A change in overall body weight, relative to thebeginning of the collection period.The amount of weight change over a period of time inrelation to the total weight, relative to the beginning ofthe collection period.Average Body Weight GainWeight GainPercentage Body Weight GainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 6 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89960 - BGTESTCD - Body Weight Gain Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62754 BWGAIN Body WeightGainC90363 BWGAINA Average BodyWeight GainC90434 BWGAINP Percentage BodyWeight GainA change in overall body weight, relative to thebeginning of the collection period.The mean value of the amount of weight change over aperiod of time, relative to the beginning of thecollection period.The amount of weight change over a period of time inrelation to the total weight, relative to the beginning ofthe collection period.Weight GainAverage Body Weight GainPercentage Body Weight GainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 7 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88026 - BODSYS - Body SystemCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC35552CARDIOVASCULARSYSTEMSymptoms, physical examination results, and/orlaboratory test results related to the cardiovascularsystem. (NCI)Cardiovascular System FindingC36285 ENDOCRINE SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the endocrine system.(NCI)Endocrine System FindingC36279GASTROINTESTINALSYSTEMSymptoms, physical examination results, and/orlaboratory test results related to the gastrointestinalsystem. (NCI)Gastrointestinal System FindingC36289 HEMATOPOIETIC SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the hematopoieticsystem. (NCI)C39723 IMMUNE SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the immune system.(NCI)C36281 INTEGUMENTARY SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the integumentarysystem. (NCI)Hematopoietic System FindingImmune System FindingIntegumentary System FindingC36288MUSCULOSKELETALSYSTEMSymptoms, physical examination results, and/orlaboratory test results related to the musculoskeletalsystem, also including connective and soft tissue.Connective and Soft Tissue FindingC36280 NERVOUS SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the nervous system.(NCI)C36284 REPRODUCTIVE SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the reproductivesystem. (NCI)C45233 RESPIRATORY SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the respiratorysystem. (NCI)C36283 SPECIAL SENSES SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the organs of specialsense. (NCI)C36286 URINARY SYSTEM Symptoms, physical examination results, and/orlaboratory test results related to the urinary system.Nervous System FindingReproductive System FindingRespiratory System FindingEye and Ear FindingUrinary Tract FindingSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 8 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89961 - BWTEST - Body Weight Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC81328 Body Weight Body Weight The weight of a subject. (NCI) Body WeightC90464 Terminal Body Weight Terminal BodyWeightThe weight of a subject at a specified end point. (NCI)Terminal Body WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 9 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89962 - BWTESTCD - Body Weight Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC81328 BW Body Weight The weight of a subject. (NCI) Body WeightC90464 TERMBW Terminal BodyWeightThe weight of a subject at a specified end point. (NCI)Terminal Body WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 10 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89963 - CLCAT - Category for Clinical ObservationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC100104 CLINICAL SIGNS Clinical Signs Objective evidence of disease perceptible to theexaminer (sign) and subjective evidence of diseaseperceived by the subject (symptom).C25478 DERMAL Dermal Of or relating to or located in the dermis. When usedin the context of clinical observations, dermal mayalso include findings related to other components ofthe skin.C16939 OPHTHALMOLOGY Ophthalmology A medical specialty concerned with the structure andfunction of the eye and the medical and surgicaltreatment of its defects and diseases. (NCI)C20989 PHYSICAL EXAM Physical Exam A systemic evaluation of the body and its functionsusing visual inspection, palpation, percussion andauscultation. The purpose is to determine the presenceor absence of physical signs of disease or abnormalityfor an individual's health assessment.Sign or SymptomDermalOphthalmologyPhysical ExaminationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 11 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC17884 ABW ARUBA Island in the Caribbean Sea, north of Venezuela. (NCI) ArubaC16267 AFG AFGHANISTAN A country in Southern Asia, north and west of Pakistan,east of Iran. (NCI)C16292 AGO ANGOLA A country in Southern Africa, bordering the SouthAtlantic Ocean, between Namibia and DemocraticRepublic of the Congo. (NCI)C20133 AIA ANGUILLA An island in the Caribbean Sea, east of Puerto Rico.(NCI)AfghanistanAngolaAnguillaC44481 ALA ALANDISLANDSAn archipelago in the Baltic Sea at the entrance to theGulf of Bothnia between Sweden and Finland. (NCI)Aland IslandsC16271 ALB ALBANIA A country in Southeastern Europe, bordering theAdriatic Sea and Ionian Sea, between Greece andSerbia and Montenegro. (NCI)C16289 AND ANDORRA A country in Southwestern Europe, between France andSpain. (NCI)AlbaniaAndorraC17232 ARE UNITED ARABEMIRATESA country in the Middle East, bordering the Gulf ofOman and the Persian Gulf, between Oman and SaudiArabia. (NCI)United Arab EmiratesC16305 ARG ARGENTINA A country in Southern South America, bordering theSouth Atlantic Ocean, between Chile and Uruguay.(NCI)ArgentinaC16306 ARM ARMENIA A country in Southwestern Asia, east of Turkey. (NCI) ArmeniaC17739 ASM AMERICANSAMOAA group of islands in the South Pacific Ocean, abouthalf way between Hawaii and New Zealand. (NCI)American SamoaC18007 ATA ANTARCTICA The continent lying mostly south of the AntarcticCircle. (NCI)AntarcticaC20105 ATF FRENCHSOUTHERNTERRITORIESC16303 ATG ANTIGUA ANDBARBUDAIslands in the southern Indian Ocean, south of Africa,about equidistant between Africa, Antarctica, andAustralia. (NCI)Islands between the Caribbean Sea and the NorthAtlantic Ocean, east-southeast of Puerto Rico. (NCI)French Southern TerritoriesAntigua and BarbudaC16311 AUS AUSTRALIA The continent between the Indian Ocean and the SouthPacific Ocean. (NCI)C163<strong>12</strong> AUT AUSTRIA A country in Central Europe, north of Italy andSlovenia. (NCI)C16316 AZE AZERBAIJAN A country in Southwestern Asia, bordering the CaspianSea, between Iran and Russia. (NCI)C16371 BDI BURUNDI A country in Central Africa, east of DemocraticRepublic of the Congo. (NCI)C16329 BEL BELGIUM A country in Western Europe, bordering the North Sea,between France and the Netherlands. (NCI)AustraliaAustriaAzerbaijanBurundiBelgiumC16333 BEN BENINREPUBLICA country in Western Africa, bordering the NorthAtlantic Ocean, between Nigeria and Togo. (NCI)BeninSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong> of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC10<strong>12</strong>24 BES Bonaire, SintEustatius andSabaThree Caribbean islands that are part of the LesserAntilles; Bonaire is east of Aruba and Curacao off thecoast of Venezuela, Sint Eustatius and Saba are locatedsouth of Sint Maarten and northeast of Saint Kitts andNevis. (NCI)Bonaire, Sint Eustatius and SabaC16369 BFA BURKINA FASO A country in Western Africa, north of Ghana. (NCI) Burkina FasoC16323 BGD BANGLADESH A country in Southern Asia, bordering the Bay ofBengal, between Burma and India. (NCI)C16368 BGR BULGARIA A country in Southeastern Europe, bordering the BlackSea, between Romania and Turkey. (NCI)C16322 BHR BAHRAIN An archipelago in the Persian Gulf, east of SaudiArabia. (NCI)C16321 BHS BAHAMAS A chain of islands in the North Atlantic Ocean,southeast of Florida. (NCI)BangladeshBulgariaBahrainBahamasC16361 BIH BOSNIA-HERZEGOVINAC83609 BLM SAINTBARTHELEMYA country in Southeastern Europe, bordering theAdriatic Sea and Croatia. (NCI)An island in the Caribbean sea, between Saint Martinand Saint Kitts and Nevis. (NCI)Bosnia and HerzegovinaSaint BarthelemyC16372 BLR BELARUS A country in Eastern Europe, east of Poland. (NCI) BelarusC16331 BLZ BELIZE A country in Central America, bordering the CaribbeanSea, between Guatemala and Mexico. (NCI)C16334 BMU BERMUDA A group of islands in the North Atlantic Ocean, east ofSouth Carolina. (NCI)C16359 BOL BOLIVIA A country in Central South America, southwest ofBrazil. (NCI)C16364 BRA BRAZIL A country in Eastern South America, bordering theAtlantic Ocean. (NCI)C16324 BRB BARBADOS An island between the Caribbean Sea and the NorthAtlantic Ocean, northeast of Venezuela. (NCI)C16367 BRN BRUNEI A country in Southeastern Asia, bordering the SouthChina Sea and Malaysia. (NCI)C16336 BTN BHUTAN A country in Southern Asia, between China and India.(NCI)BelizeBermudaBolivia, Plurinational State ofBrazilBarbadosBrunei DarussalamBhutanC20104 BVT BOUVETISLANDAn island in the South Atlantic Ocean, south-southwestof the Cape of Good Hope (South Africa). (NCI)Bouvet IslandC16363 BWA BOTSWANA A country in Southern Africa, north of South Africa.(NCI)BotswanaC16409 CAF CENTRALAFRICANREPUBLICA country in Central Africa, north of DemocraticRepublic of the Congo. (NCI)Central African RepublicC16380 CAN CANADA A country in Northern North America, bordering theNorth Atlantic Ocean on the east, North Pacific Oceanon the west, and the Arctic Ocean on the north, northof the conterminous US. (NCI)CanadaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 13 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16445 CCK COCOS(KEELING)ISLANDSA group of islands in the Indian Ocean, south ofIndonesia, about halfway from Australia to Sri Lanka.(NCI)Cocos (Keeling) IslandsC17181 CHE SWITZERLAND A country in Central Europe, east of France, north ofItaly. (NCI)C16427 CHL CHILE A country in Southern South America, bordering theSouth Atlantic Ocean and South Pacific Ocean,between Argentina and Peru. (NCI)C16428 CHN CHINA A country in Eastern Asia, bordering the East ChinaSea, Korea Bay, Yellow Sea, and South China Sea,between North Korea and Vietnam. (NCI)C16762 CIV COTE D'IVOIRE A country in Western Africa, bordering the NorthAtlantic Ocean, between Ghana and Liberia. (NCI)C16379 CMR CAMEROON A country in Western Africa, bordering the Bight ofBiafra, between Equatorial Guinea and Nigeria. (NCI)SwitzerlandChileChinaCote d'IvoireCameroonC17266 COD DEMOCRATICREPUBLIC OFTHE CONGOA country in Central Africa, northeast of Angola.(NCI)Congo, the Democratic Republic oftheC16467 COG CONGO A country in Western Africa, bordering the SouthAtlantic Ocean, between Angola and Gabon. (NCI)C16469 COK COOK ISLANDS A group of islands in the South Pacific Ocean, aboutone-half of the way from Hawaii to New Zealand.(NCI)C16449 COL COLOMBIA A country in Northern South America, bordering theCaribbean Sea, between Panama and Venezuela, andbordering the North Pacific Ocean, between Ecuadorand Panama. (NCI)C16458 COM COMOROS A group of islands in the Mozambique Channel, abouttwo-thirds of the way between northern Madagascarand northern Mozambique. (NCI)C16382 CPV CAPE VERDE A group of islands in the North Atlantic Ocean, west ofSenegal. (NCI)C16470 CRI COSTA RICA A country in Central America, bordering both theCaribbean Sea and the North Pacific Ocean, betweenNicaragua and Panama. (NCI)C16477 CUB CUBA An island between the Caribbean Sea and the NorthAtlantic Ocean, 150 km south of Key West, Florida.(NCI)C10<strong>12</strong>25 CUW Curacao An island nation located in the Caribbean Sea off thecoast of Venezuela. (NCI)CongoCook IslandsColombiaComorosCape VerdeCosta RicaCubaCuracaoC44482 CXR CHRISTMASISLANDC16391 CYM CAYMANISLANDSAn Australian-administered island in the eastern IndianOcean south of Java, Indonesia. (NCI)An island group in the Caribbean Sea, nearly one-halfof the way from Cuba to Honduras. (NCI)Christmas IslandCayman IslandsC16480 CYP CYPRUS An island in the Mediterranean Sea, south of Turkey.(NCI)CyprusSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 14 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC17668 CZE CZECHREPUBLICA country in Central Europe, southeast of Germany.(NCI)Czech RepublicC16636 DEU GERMANY A country in Central Europe, bordering the Baltic Seaand the North Sea, between the Netherlands andPoland, south of Denmark. (NCI)C16506 DJI DJIBOUTI A country in Eastern Africa, bordering the Gulf ofAden and the Red Sea, between Eritrea and Somalia.(NCI)C16519 DMA DOMINICA An island between the Caribbean Sea and the NorthAtlantic Ocean, about one-half of the way from PuertoRico to Trinidad and Tobago. (NCI)C16496 DNK DENMARK A country in Northern Europe, bordering the BalticSea and the North Sea, on a peninsula north ofGermany (Jutland); also includes two major islands(Sjaelland and Fyn). (NCI)GermanyDjiboutiDominicaDenmarkC16520 DOM DOMINICANREPUBLICA country comprising the eastern two-thirds of theisland of Hispaniola, between the Caribbean Sea andthe North Atlantic Ocean, east of Haiti. (NCI)Dominican RepublicC16274 DZA ALGERIA A country in Northern Africa, bordering theMediterranean Sea, between Morocco and Tunisia.(NCI)C16528 ECU ECUADOR A country in Western South America, bordering thePacific Ocean at the Equator, between Colombia andPeru. (NCI)C16530 EGY EGYPT A country in Northern Africa, bordering theMediterranean Sea, between Libya and the Gaza Strip.(NCI)C16558 ERI ERITREA A country in Eastern Africa, bordering the Red Sea,between Djibouti and Sudan. (NCI)AlgeriaEcuadorEgyptEritreaC20113 ESH WESTERNSAHARAA country in Northern Africa, bordering the NorthAtlantic Ocean, between Mauritania and Morocco.(NCI)Western SaharaC17152 ESP SPAIN A country in Southwestern Europe, bordering the Bayof Biscay, Mediterranean Sea, North Atlantic Ocean,and Pyrenees Mountains, southwest of France. (NCI)C16562 EST ESTONIA A country in Eastern Europe, bordering the Baltic Seaand Gulf of Finland, between Latvia and Russia. (NCI)SpainEstoniaC16563 ETH ETHIOPIA A country in Eastern Africa, west of Somalia. (NCI) EthiopiaC16584 FIN FINLAND A country in Northern Europe, bordering the BalticSea, Gulf of Bothnia, and Gulf of Finland, betweenSweden and Russia. (NCI)C16582 FJI FIJI An island group in the South Pacific Ocean, abouttwo-thirds of the way from Hawaii to New Zealand.(NCI)FinlandFijiC17954 FLK FALKLANDISLANDSIslands in the South Atlantic Ocean, east of southernArgentina. (NCI)Falkland Islands (Malvinas)Source: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 15 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16654 GTM GUATEMALA A country in Central America, bordering the CaribbeanSea, between Honduras and Belize and bordering theNorth Pacific Ocean, between El Salvador and Mexico.(NCI)GuatemalaC16593 GUF FRENCHGUIANAA country in Northern South America, bordering theNorth Atlantic Ocean, between Brazil and Suriname.(NCI)French GuianaC16652 GUM GUAM Island in the North Pacific Ocean, about three-quartersof the way from Hawaii to the Philippines. (NCI)C16657 GUY GUYANA A country in Northern South America, bordering theNorth Atlantic Ocean, between Suriname andVenezuela. (NCI)C16695 HKG HONG KONG A special administrative region of China, bordering theSouth China Sea and China. (NCI)GuamGuyanaHong KongC20106 HMD HEARD ISLANDANDMCDONALDISLANDSIslands in the Indian Ocean, about two-thirds of the wayfrom Madagascar to Antarctica. (NCI)Heard Island and McDonald IslandsC16694 HND HONDURAS A country in Central America, bordering the CaribbeanSea, between Guatemala and Nicaragua and borderingthe North Pacific Ocean, between El Salvador andNicaragua. (NCI)C16474 HRV CROATIA A country in Southeastern Europe, bordering theAdriatic Sea, between Bosnia and Herzegovina andSlovenia. (NCI)C16660 HTI HAITI A country comprising the western one-third of theisland of Hispaniola, between the Caribbean Sea andthe North Atlantic Ocean, west of the DominicanRepublic. (NCI)C16699 HUN HUNGARY A country in Central Europe, northwest of Romania.(NCI)C16728 IDN INDONESIA A country in Southeastern Asia, comprising thearchipelago between the Indian Ocean and the PacificOcean. (NCI)C44480 IMN ISLE OF MAN An island in the Irish Sea, between Great Britain andIreland. (NCI)C16727 IND INDIA A country in Southern Asia, bordering the Arabian Seaand the Bay of Bengal, between Burma and Pakistan.(NCI)C16365 IOT BRITISHINDIAN OCEANTERRITORYAn archipelago in the Indian Ocean, about one-half theway from Africa to Indonesia. (NCI)C16757 IRL IRELAND A country in Western Europe, occupying five-sixths ofthe island of Ireland in the North Atlantic Ocean, westof Great Britain. (NCI)C16755 IRN IRAN A country in the Middle East, bordering the Gulf ofOman, the Persian Gulf, and the Caspian Sea, betweenIraq and Pakistan. (NCI)HondurasCroatiaHaitiHungaryIndonesiaIsle of ManIndiaBritish Indian Ocean TerritoryIrelandIran, Islamic Republic ofSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 17 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16756 IRQ IRAQ A country in the Middle East, bordering the PersianGulf, between Iran and Kuwait. (NCI)C16704 ISL ICELAND A country in Northern Europe, island between theGreenland Sea and the North Atlantic Ocean, northwestof the UK. (NCI)C16760 ISR ISRAEL A country in the Middle East, bordering theMediterranean Sea, between Egypt and Lebanon. (NCI)C16761 ITA ITALY A country in Southern Europe, occupying a peninsulaextending into the central Mediterranean Sea,northeast of Tunisia. (NCI)IraqIcelandIsraelItalyC16763 JAM JAMAICA An island in the Caribbean Sea, south of Cuba. (NCI) JamaicaC64374 JEY JERSEY Jersey and the other Channel Islands represent the lastremnants of the medieval Dukedom of Normandy thatheld sway in both France and England. Jersey is aBritish crown dependency, but is not part of the UK.(NCI)C16765 JOR JORDAN A country in the Middle East, northwest of SaudiArabia. (NCI)C16764 JPN JAPAN A country in Eastern Asia, occupying an island chainbetween the North Pacific Ocean and the Sea of Japan,east of the Korean Peninsula. (NCI)JerseyJordanJapanC20107 KAZ KAZAKHSTAN A country in Central Asia, northwest of China. (NCI) KazakhstanC16769 KEN KENYA A country in Eastern Africa, bordering the IndianOcean, between Somalia and Tanzania. (NCI)KenyaC16771 KGZ KYRGYZSTAN A country in Central Asia, west of China. (NCI) KyrgyzstanC16378 KHM CAMBODIA A country in Southeastern Asia, bordering the Gulf ofThailand, between Thailand, Vietnam, and Laos. (NCI)C16639 KIR KIRIBATI A group of 33 coral atolls in the Pacific Ocean,straddling the equator; the capital Tarawa is aboutone-half of the way from Hawaii to Australia. (NCI)CambodiaKiribatiC17885 KNA SAINT KITTSAND NEVISIslands in the Caribbean Sea, about one-third of theway from Puerto Rico to Trinidad and Tobago. (NCI)Saint Kitts and NevisC16774 KOR SOUTH KOREA A country in Eastern Asia, occupying the southern halfof the Korean Peninsula, bordering the Sea of Japanand the Yellow Sea. (NCI)C16775 KWT KUWAIT A country in the Middle East, bordering the PersianGulf, between Iraq and Saudi Arabia. (NCI)Korea, Republic ofKuwaitC16780 LAO LAO PEOPLE'SDEMOCRATICREPUBLICA country in Southeastern Asia, northeast of Thailand,west of Vietnam. (NCI)Lao People's Democratic RepublicC16784 LBN LEBANON A country in the Middle East, bordering theMediterranean Sea, between Israel and Syria. (NCI)C16791 LBR LIBERIA A country in Western Africa, bordering the NorthAtlantic Ocean, between Cote d'Ivoire and SierraLeone. (NCI)LebanonLiberiaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 18 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16793 LBY LIBYA A country in Northern Africa, bordering theMediterranean Sea, between Egypt and Tunisia. (NCI)C17113 LCA SAINT LUCIA A country in the Caribbean, occupying an islandbetween the Caribbean Sea and North Atlantic Ocean,north of Trinidad and Tobago. (NCI)LibyaSaint LuciaC16794 LIE LIECHTENSTEINA country in Central Europe, between Austria andSwitzerland. (NCI)LiechtensteinC17163 LKA SRI LANKA A country in Southern Asia, occupying an island in theIndian Ocean, south of India. (NCI)C16787 LSO LESOTHO A country in Southern Africa, an enclave of SouthAfrica. (NCI)C16799 LTU LITHUANIA A country in Eastern Europe, bordering the Baltic Sea,between Latvia and Russia. (NCI)C16803 LUX LUXEMBOURG A country in Western Europe, between France,Belgium, and Germany. (NCI)C16783 LVA LATVIA A country in Eastern Europe, bordering the Baltic Sea,between Estonia and Lithuania. (NCI)C16807 MAC MACAO A country in Eastern Asia, bordering the South ChinaSea and China. (NCI)Sri LankaLesothoLithuaniaLuxembourgLatviaMacaoC83610 MAF SAINT MARTIN,FRENCHAn island in the Caribbean sea, between Anguilla andSaint Barthelemy. (NCI)Saint Martin (French Part)C16878 MAR MOROCCO A country in Northern Africa, bordering the NorthAtlantic Ocean and the Mediterranean Sea, betweenAlgeria and Western Sahara. (NCI)C16874 MCO MONACO A country in Western Europe, bordering theMediterranean Sea on the southern coast of France,near the border with Italy. (NCI)MoroccoMonacoC16871 MDA MOLDOVA,REPUBLIC OFA country in Eastern Europe, northeast of Romania.(NCI)Moldova, Republic ofC16808 MDG MADAGASCAR A country in Southern Africa, occupying an island inthe Indian Ocean, east of Mozambique. (NCI)C16815 MDV MALDIVES A country in Southern Asia, occupying a group ofatolls in the Indian Ocean, south-southwest of India.(NCI)C16850 MEX MEXICO A country in Central America, bordering the CaribbeanSea and the Gulf of Mexico, between Belize and theUS and bordering the North Pacific Ocean, betweenGuatemala and the US. (NCI)MadagascarMaldivesMexicoC16822 MHL MARSHALLISLANDSC17654 MKD REPUBLIC OFMACEDONIAA group of atolls and reefs in the North Pacific Ocean,about one-half of the way from Hawaii to Australia.(NCI)A country in Southeastern Europe, north of Greece.(NCI)Marshall IslandsMacedonia, the Former YugoslavRepublic ofC16816 MLI MALI A country in Western Africa, southwest of Algeria.(NCI)MaliSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 19 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16817 MLT MALTA A country in Southern Europe, occupying islands in theMediterranean Sea, south of Sicily (Italy). (NCI)C16370 MMR MYANMAR A country in Southeastern Asia, bordering theAndaman Sea and the Bay of Bengal, betweenBangladesh and Thailand. (NCI)C64378 MNE MONTENEGRO A republic in Southeastern Europe, bordering theAdriatic Sea, between Albania and Bosnia andHerzegovina. Formerly part of Serbia and Montenegro(Federation Republic of Yugoslavia). (NCI)C16875 MNG MONGOLIA A country in Northern Asia, between China and Russia.(NCI)MaltaMyanmarMontenegroMongoliaC17882 MNP NORTHERNMARIANAISLANDSA country in the Pacific, comprising islands in theNorth Pacific Ocean, about three-quarters of the wayfrom Hawaii to the Philippines. (NCI)Northern Mariana IslandsC16882 MOZ MOZAMBIQUE A country in Southern Africa, bordering theMozambique Channel, between South Africa andTanzania. (NCI)C16826 MRT MAURITANIA A country in Northern Africa, bordering the NorthAtlantic Ocean, between Senegal and Western Sahara.(NCI)C16876 MSR MONTSERRAT A country in the Caribbean, occupying an island in theCaribbean Sea, southeast of Puerto Rico. (NCI)C16823 MTQ MARTINIQUE An island in the Caribbean Sea, north of Trinidad andTobago. (NCI)C16827 MUS MAURITIUS A country in Southern Africa, occupying an island inthe Indian Ocean, east of Madagascar. (NCI)MozambiqueMauritaniaMontserratMartiniqueMauritiusC16813 MWI MALAWI A country in Southern Africa, east of Zambia. (NCI) MalawiC16814 MYS MALAYSIA A country in Southeastern Asia, occupying a peninsulaand the northern one-third of the island of Borneo,bordering Indonesia and the South China Sea, south ofVietnam. (NCI)C16828 MYT MAYOTTE A country in Southern Africa, occupying an island inthe Mozambique Channel, about one-half of the wayfrom northern Madagascar to northern Mozambique.(NCI)C16891 NAM NAMIBIA A country in Southern Africa, bordering the SouthAtlantic Ocean, between Angola and South Africa.(NCI)MalaysiaMayotteNamibiaC16913 NCL NEWCALEDONIAA country in the Pacific, comprised of islands in theSouth Pacific Ocean, east of Australia. (NCI)New CaledoniaC16916 NER NIGER A country in Western Africa, southeast of Algeria.(NCI)NigerC16919 NFK NORFOLKISLANDA country in the Pacific, occupying an island in theSouth Pacific Ocean, east of Australia. (NCI)Norfolk IslandC16917 NGA NIGERIA A country in Western Africa, bordering the Gulf ofGuinea, between Benin and Cameroon. (NCI)NigeriaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 20 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16915 NIC NICARAGUA A country in Central America, bordering both theCaribbean Sea and the North Pacific Ocean, betweenCosta Rica and Honduras. (NCI)C16918 NIU NIUE A country in the Pacific, occupying an island in theSouth Pacific Ocean, east of Tonga. (NCI)C16903 NLD NETHERLANDS A country in Western Europe, bordering the North Sea,between Belgium and Germany. (NCI)C16920 NOR NORWAY A country in Northern Europe, bordering the North Seaand the North Atlantic Ocean, west of Sweden. (NCI)C16901 NPL NEPAL A country in Southern Asia, between China and India.(NCI)C16896 NRU NAURU A country in Oceania, occupying an island in the SouthPacific Ocean, south of the Marshall Islands. (NCI)C16914 NZL NEW ZEALAND A country in the Pacific, comprised of islands in theSouth Pacific Ocean, southeast of Australia. (NCI)C16933 OMN OMAN A country in the Middle East, bordering the ArabianSea, Gulf of Oman, and Persian Gulf, between Yemenand the United Arab Emirates. (NCI)C16949 PAK PAKISTAN A country in Southern Asia, bordering the Arabian Sea,between India on the east and Iran and Afghanistan onthe west and China in the north. (NCI)C16951 PAN PANAMA A country in Central America, bordering both theCaribbean Sea and the North Pacific Ocean, betweenColombia and Costa Rica. (NCI)C16993 PCN PITCAIRN A country in the Pacific, comprised of islands in theSouth Pacific Ocean, about midway between Peru andNew Zealand. (NCI)C16972 PER PERU A country in Western South America, bordering theSouth Pacific Ocean, between Chile and Ecuador.(NCI)C16978 PHL PHILIPPINES A country in Southeastern Asia, comprised of anarchipelago between the Philippine Sea and the SouthChina Sea, east of Vietnam. (NCI)C17733 PLW PALAU A country in the Pacific, comprising a group of islandsin the North Pacific Ocean, southeast of thePhilippines. (NCI)NicaraguaNiueNetherlandsNorwayNepalNauruNew ZealandOmanPakistanPanamaPitcairnPeruPhilippinesPalauC16952 PNG PAPUA NEWGUINEAA country in Southeastern Asia, comprising a group ofislands and including the eastern half of the island ofNew Guinea, between the Coral Sea and the SouthPacific Ocean, east of Indonesia. (NCI)Papua New GuineaC17002 POL POLAND A country in Central Europe, east of Germany. (NCI) PolandC17043 PRI PUERTO RICO An island between the Caribbean Sea and the NorthAtlantic Ocean, east of the Dominican Republic. (NCI)C16773 PRK NORTH KOREA A country in Eastern Asia, occupying the northern halfof the Korean Peninsula, bordering the Korea Bay andthe Sea of Japan, between China and South Korea.(NCI)Puerto RicoKorea, Democratic People'sRepublic ofSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 21 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC17006 PRT PORTUGAL A country in Southwestern Europe, bordering theNorth Atlantic Ocean, west of Spain. (NCI)C16953 PRY PARAGUAY A country in Central South America, northeast ofArgentina. (NCI)PortugalParaguayC20110 PSE PALESTINIANTERRITORY,OCCUPIEDC16594 PYF FRENCHPOLYNESIAA collective name for the West Bank and the GazaStrip, two territories in Palestine. (NCI)An archipelago in the South Pacific Ocean, aboutone-half of the way from South America to Australia.(NCI)Palestinian Territory, OccupiedFrench PolynesiaC17045 QAT QATAR A country in the Middle East, occupying a peninsulabordering the Persian Gulf and Saudi Arabia. (NCI)C17095 REU REUNION A country in Southern Africa, occupying an island inthe Indian Ocean, east of Madagascar. (NCI)C17108 ROU ROMANIA A country in Southeastern Europe, bordering the BlackSea, between Bulgaria and Ukraine. (NCI)QatarReunionRomaniaC17111 RUS RUSSIANFEDERATIONA country in Northern Asia (that part west of the Uralsis sometimes included with Europe), bordering theArctic Ocean, between Europe and the North PacificOcean. (NCI)Russian FederationC171<strong>12</strong> RWA RWANDA A country in Central Africa, east of DemocraticRepublic of the Congo. (NCI)C17117 SAU SAUDI ARABIA A country in the Middle East, bordering the PersianGulf and the Red Sea, north of Yemen. (NCI)C17170 SDN SUDAN A country in Northern Africa, bordering the Red Sea,between Egypt and Eritrea. (NCI)C17<strong>12</strong>1 SEN SENEGAL A country in Western Africa, bordering the NorthAtlantic Ocean, between Guinea-Bissau andMauritania. (NCI)C17134 SGP SINGAPORE A country in Southeastern Asia, comprised of islandsbetween Malaysia and Indonesia. (NCI)RwandaSaudi ArabiaSudanSenegalSingaporeC20111 SGS SOUTHGEORGIA ANDTHE SOUTHSANDWICHISLANDSA group of islands in the South Atlantic Ocean, east ofthe tip of South America. (NCI)South Georgia and the SouthSandwich IslandsC17164 SHN SAINT HELENA Islands in the South Atlantic Ocean, about midwaybetween South America and Africa. (NCI)Saint Helena, Ascension and Tristanda CunhaC17178 SJM SVALBARDAND JANMAYENC17148 SLB SOLOMONISLANDSIslands between the Arctic Ocean, Barents Sea,Greenland Sea, and Norwegian Sea, northeast ofIceland and north of Norway. (NCI)A group of islands in the South Pacific Ocean, east ofPapua New Guinea. (NCI)Svalbard and Jan MayenSolomon IslandsC17130 SLE SIERRA LEONE A country in Western Africa, bordering the NorthAtlantic Ocean, between Guinea and Liberia. (NCI)Sierra LeoneSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 22 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16532 SLV EL SALVADOR A country in Central America, bordering the NorthPacific Ocean, between Guatemala and Honduras.(NCI)C17115 SMR SAN MARINO A country in Southern Europe, an enclave in centralItaly. (NCI)C17149 SOM SOMALIA A country in Eastern Africa, bordering the Gulf ofAden and the Indian Ocean, east of Ethiopia. (NCI)El SalvadorSan MarinoSomaliaC17165 SPM SAINT PIERREANDMIQUELONA country in Northern North America, comprised ofislands in the North Atlantic Ocean, south ofNewfoundland (Canada). (NCI)Saint Pierre and MiquelonC64377 SRB SERBIA A republic in Southeastern Europe, bordering theAdriatic Sea, between Albania and Bosnia andHerzegovina. Formerly part of Serbia and Montenegro(Federation Republic of Yugoslavia). (NCI)C97351 SSD South Sudan A northeastern African country located in the Sahelregion and bordered by Sudan in the north, Uganda andKenya in the south and Ethiopia in the west. (NCI)SerbiaSouth SudanC17116 STP SAO TOMEAND PRINCIPEA country in Western Africa, comprised of islands inthe Gulf of Guinea, straddling the Equator, west ofGabon. (NCI)Sao Tome and PrincipeC17175 SUR SURINAME A country in Northern South America, bordering theNorth Atlantic Ocean, between French Guiana andGuyana. (NCI)SurinameC17669 SVK SLOVAKIA A country in Central Europe, south of Poland. (NCI) SlovakiaC17138 SVN SLOVENIA A country in Central Europe, bordering the AdriaticSea, between Austria and Croatia. (NCI)C17180 SWE SWEDEN A country in Northern Europe, bordering the BalticSea, Gulf of Bothnia, Kattegat, and Skagerrak, betweenFinland and Norway. (NCI)C17179 SWZ SWAZILAND A country in Southern Africa, between Mozambiqueand South Africa. (NCI)C10<strong>12</strong>26 SXM SINT MAARTEN(DUTCH)The southern portion of an island in the Caribbean sea,between Anguilla and Saint Barthelemy. (NCI)C17<strong>12</strong>9 SYC SEYCHELLES A country in Eastern Africa, comprised of a group ofislands in the Indian Ocean, northeast of Madagascar.(NCI)SloveniaSwedenSwazilandSint Maarten (Dutch Part)SeychellesC17182 SYR SYRIAN ARABREPUBLICC17224 TCA TURKS ANDCAICOSISLANDSA country in the Middle East, bordering theMediterranean Sea, between Lebanon and Turkey.(NCI)Two island groups in the North Atlantic Ocean,southeast of The Bahamas. (NCI)Syrian Arab RepublicTurks and Caicos IslandsC164<strong>12</strong> TCD CHAD A country in Central Africa, south of Libya. (NCI) ChadC17202 TGO TOGO A country in Western Africa, bordering the Bight ofBenin, between Benin and Ghana. (NCI)TogoSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 23 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC17192 THA THAILAND A country in Southeastern Asia, bordering theAndaman Sea and the Gulf of Thailand, southeast ofBurma. (NCI)ThailandC17183 TJK TAJIKISTAN A country in Central Asia, west of China. (NCI) TajikistanC17704 TKL TOKELAU A group of three atolls in the South Pacific Ocean,about one-half of the way from Hawaii to NewZealand. (NCI)TokelauC17223 TKM TURKMENISTANA country in Central Asia, bordering the Caspian Sea,between Iran and Kazakhstan. (NCI)TurkmenistanC17200 TLS TIMOR-LESTE A country in Southeastern Asia, northwest of Australiain the Lesser Sunda Islands at the eastern end of theIndonesian archipelago. East Timor includes theeastern half of the island of Timor, the Oecussi(Ambeno) region on the northwest portion of theisland of Timor, and the islands of Pulau Atauro andPulau Jaco. (NCI)C17205 TON TONGA An archipelago in the South Pacific Ocean, abouttwo-thirds of the way from Hawaii to New Zealand.(NCI)Timor-LesteTongaC17217 TTO TRINIDAD ANDTOBAGOIslands between the Caribbean Sea and the NorthAtlantic Ocean, northeast of Venezuela. (NCI)Trinidad and TobagoC17221 TUN TUNISIA A country in Northern Africa, bordering theMediterranean Sea, between Algeria and Libya. (NCI)C17222 TUR TURKEY A country in southeastern Europe and southwesternAsia (that portion of Turkey west of the Bosporus isgeographically part of Europe), bordering the BlackSea, between Bulgaria and Georgia, and bordering theAegean Sea and the Mediterranean Sea, betweenGreece and Syria. (NCI)C17225 TUV TUVALU An island group consisting of nine coral atolls in theSouth Pacific Ocean, about one-half of the way fromHawaii to Australia. (NCI)C17184 TWN TAIWAN A group of islands bordering the East China Sea,Philippine Sea, South China Sea, and Taiwan Strait,north of the Philippines, off the southeastern coast ofChina. (NCI)TunisiaTurkeyTuvaluTaiwan, Province of ChinaC17185 TZA TANZANIA,UNITEDREPUBLIC OFA country in Eastern Africa, bordering the IndianOcean, between Kenya and Mozambique. (NCI)Tanzania, United Republic ofC17228 UGA UGANDA A country in Eastern Africa, west of Kenya. (NCI) UgandaC17229 UKR UKRAINE A country in Eastern Europe, bordering the Black Sea,between Poland and Russia. (NCI)UkraineC201<strong>12</strong> UMI UNITEDSTATES MINOROUTLYINGISLANDSThe U.S. Minor Outlying Islands consist of BakerIsland, Howland Island, Jarvis Island, Johnston Atoll,Kingman Reef, Midway Island, Navassa Island, PalmyraAtoll, and Wake Island (Wake Atoll). (NCI)United States Minor Outlying IslandsC17244 URY URUGUAY A country in Southern South America, bordering theSouth Atlantic Ocean, between Argentina and Brazil.(NCI)UruguaySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 24 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66786 - COUNTRY - CountryCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC17234 USA UNITEDSTATESA country in North America, bordering both the NorthAtlantic Ocean and the North Pacific Ocean, betweenCanada and Mexico. (NCI)United StatesC17246 UZB UZBEKISTAN A country in Central Asia, north of Afghanistan. (NCI) UzbekistanC17249 VAT VATICAN CITYSTATEC17114 VCT SAINTVINCENT ANDTHEGRENADINESAn enclave of Rome (Italy). (NCI)A country in the Caribbean, comprised of islands in theCaribbean Sea, north of Trinidad and Tobago. (NCI)Holy See (Vatican City State)Saint Vincent and the GrenadinesC17250 VEN VENEZUELA A country in Northern South America, bordering theCaribbean Sea and the North Atlantic Ocean, betweenColombia and Guyana. (NCI)Venezuela, Bolivian Republic ofC17653 VGB VIRGINISLANDS,BRITISHC17255 VIR VIRGINISLANDS, U.S.Islands between the Caribbean Sea and the NorthAtlantic Ocean, east of Puerto Rico. (NCI)Islands between the Caribbean Sea and the NorthAtlantic Ocean, east of Puerto Rico. (NCI)Virgin Islands, BritishVirgin Islands, U.S.C17252 VNM VIETNAM A country in Southeastern Asia, bordering the Gulf ofThailand, Gulf of Tonkin, and South China Sea,alongside China, Laos, and Cambodia. (NCI)C17247 VUT VANUATU A group of islands in the South Pacific Ocean, aboutthree-quarters of the way from Hawaii to Australia.(NCI)Viet NamVanuatuC17259 WLF WALLIS ANDFUTUNAIslands in the South Pacific Ocean, about two-thirds ofthe way from Hawaii to New Zealand. (NCI)Wallis and FutunaC17740 WSM SAMOA A group of islands in the South Pacific Ocean, aboutone-half of the way from Hawaii to New Zealand.(NCI)C17264 YEM YEMEN A country in the Middle East, bordering the ArabianSea, Gulf of Aden, and Red Sea, between Oman andSaudi Arabia. (NCI)C17151 ZAF SOUTH AFRICA A country in Southern Africa, at the southern tip of thecontinent of Africa. (NCI)SamoaYemenSouth AfricaC17267 ZMB ZAMBIA A country in Southern Africa, east of Angola. (NCI) ZambiaC17268 ZWE ZIMBABWE A country in Southern Africa, between South Africaand Zambia. (NCI)ZimbabweSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 25 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90018 - CSTATE - Consciousness StateCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC88434 CONSCIOUS Conscious State A level of awareness that can be described as beingalert. (NCI)Conscious StateC78253 DEPRESSED Depressed LevelofConsciousnessC88440 SEMI-CONSCIOUS Semi-consciousStateC50635 UNCONSCIOUS UnconsciousState; Loss ofConsciousnessC90482 UNSPECIFIED UnspecifiedState ofConsciousnessA neurologic state characterized by decreased abilityto perceive and respond. (NCI)A level of awareness that can be described as variedand intermittent periods of consciousness andunconsciousness.The neurologic status characterized by the occurrenceof a loss of the ability to perceive and respond.The state of consciousness is not controlled. Thepossibility exists for having multiple conscious statesover a period of time. (NCI)Depressed Level Of ConsciousnessSemi-consciousLoss of ConsciousnessUnspecified State of ConsciousnessSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 26 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89965 - DDTEST - Death Diagnosis Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC8<strong>12</strong>39 Death Diagnosis Death Diagnosis The circumstance or condition that results in the deathof a living being. (NCI)Cause of DeathSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 27 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89966 - DDTESTCD - Death Diagnosis Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC8<strong>12</strong>39 DEATHD Death Diagnosis The circumstance or condition that results in the deathof a living being. (NCI)Cause of DeathSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 28 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89967 - DESIGN - Study DesignCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90368 BLOCKED A type of randomized study or trial in which studysubjects are arranged into groups by prespecifiedcriteria, such as race or sex, to reduce variability ofresults within a treatment group.C82637 CROSSOVER Participants receive one of two or more alternativeintervention(s) during the initial epoch of the study andreceive other intervention(s) during the subsequentepoch(s) of the study.C82638 FACTORIAL Two or more interventions, each alone and incombination, are evaluated in parallel against a controlgroup.C90402 LATIN SQUARE A type of continuous study or trial in which the studysubject receives each treatment during the entirecourse of the study.C82639 PARALLEL Participants are assigned to one of two or more armsin parallel for the duration of the study.Blocked StudyCrossover StudyFactorial StudyLatin Square StudyParallel StudyC90432PARTIALLY BLOCKEDBALANCEDA type of randomized study or trial in which studysubjects are arranged into groups with equal numbersby prespecified criteria, such as race or sex, to reducevariability of results within a treatment group.Partially Blocked Balanced StudyC90433PARTIALLY BLOCKEDUNBALANCEDA type of randomized study or trial in which studysubjects are arranged into groups with unequalnumbers by prespecified criteria, such as race or sex,to reduce variability of results within a treatmentgroup.Partially Blocked Unbalanced StudyC90475 TITRATION A study or trial in which the dosage of the interventionis increased by small increments until the desiredphysiological effect is seen.Titration StudySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 29 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C99074 - DIR - DirectionalityCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC25231 ANTERIOR Denoting the front portion of the body or a structure. AnteriorC25423 APICAL Relating to or located at the apex. ApicalC90067 BASAL Relating to or located at the lowest portion of astructure.BasalC73851 CAUDAL Toward the tail in a body. CaudalC25445 CENTRAL A point or area that is approximately central withinsome larger region or structure. (NCI)CenterC37936 CRANIAL Toward the head in a body. CranialC25240 DEEP Extending relatively far inward. (NCI) DeepC25237 DISTAL Situated farthest from a point of reference. DistalC45874 DORSAL Pertaining to the back or upper surface of the body. DorsalC90376 DORSOLATERAL Toward the back and side of a body. DorsolateralC90386 FORE Of or involving the front of a main body. (NCI) ForeC90393 HIND Of or involving the back of a main body. (NCI) HindC25353 INFERIOR Pertaining to a point below a given reference point. InferiorC37980 INNER Inside or closer to the inside of the body or object.(NCI)InnerC73705 INTERMEDIATE Located between two points or extremes. IntermediateC25309 LOWER The bottom one of two. (NCI) LowerC25232 MEDIAL Toward the middle or in a limb toward the medianplane.C81170 MIDLINE A medial line, especially the medial line or medialplane of the body (or some part of the body).C38166 OUTER Being on or toward the outside of the body or object.(NCI)C25233 PERIPHERAL On or near an edge or constituting an outer boundary;the outer area. (NCI)MedialMidlineOuterPeripheralC25622 POSTERIOR Denoting the back portion of the body or a structure. PosteriorC25236 PROXIMAL Situated nearest to a point of reference. ProximalC94393 ROSTRAL Toward the muzzle in the head. RostralC25239 SUPERFICIAL Of or pertaining to the exterior surface. (NCI) SuperficialC25235 SUPERIOR Pertaining to a point above a given reference point. SuperiorC25245 SURFACE The extended two-dimensional outer layer or area of athree-dimensional object. (NCI)SurfaceC90069 TIP The pointed end of a structure. TipC25355 UPPER The top one of two. UpperC45875 VENTRAL Pertaining to the front or lower surface of the body. VentralC98798 VENTROLATERAL Of or pertaining to the front and side of a main body.(NCI)VentrolateralSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 30 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66734 - DOMAIN - Domain AbbreviationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49563 AD Analysis Dataset Datasets used for statistical analysis and reporting bythe sponsor. These are submitted in addition to the datatabulation datasets.C49562 AE Adverse Events Adverse events may be captured either as free text or apre-specified list of terms.C49565 AU Autopsy Additional data about deaths, specific to findings fromautopsies.Analysis Dataset DomainAdverse Event DomainAutopsy DomainC95083 BG Body WeightGainBody weight gain is the actual difference between twobody weight measurements for any given interval for asubject. This is most commonly shown as thedifference between two consecutive body weightmeasurements.Body Weight Gain DomainC95084 BH Behavioral This domain captures behavioral testing measurementson individual subjects.Behavioral DomainC49566 BM BoneMeasurementsFindings resulting from evaluations of bone mineraldensity.Bone Measurements DomainC49567 BR Biopsy Findings resulting from biopsies. Biopsy DomainC95085 BW Body Weight This domain captures body weights collected forsubjects during the study and at the end of the study(terminal body weights).C85441 CE Clinical Events A dataset used to capture clinical events of interestthat would not be classified as adverse events.Body Weight DomainClinical Events DomainC95086 CL ClinicalObservationC49568 CM ConcomitantMedsThis domain captures clinical sign informationincluding ophthalmology, physical examination, anddermal examination collected in life while executingthe study.Case report form (CRF) data that captures theConcomitant and Prior Medications/Therapies used bythe subject. Examples are the ConcomitantMedications/Therapies given on an as needed basis andthe usual Background Medications/Therapies given fora condition.Clinical Observation DomainConcomitant Medication DomainC49569 CO Comments The Comments dataset accommodates two sources ofcomments: 1) those collected alongside other data ontopical case report form (CRF) pages such as AdverseEvents and 2) those collected on a separate pagespecifically dedicated to comments.Comment DomainC102605 CV CardiovascularSystem FindingsC49578 DA DrugAccountabilityC49570 DC DiseaseCharacteristicsA domain for physiological findings related to thecardiovascular system, including the heart, bloodvessels and lymphatic vessels.Data regarding the accountability of study drug, such asinformation on the receipt, dispensing, return, andpackaging.Data related to the characteristics of a specific diseasestate under study for a subject.Cardiovascular System FindingsDomainDrug Accountability DomainDisease Characteristics DomainC95087 DD Death Diagnosis This domain captures the diagnosis of the cause ofdeath for a subject.Death Diagnosis DomainC102616 DE Device Events A domain for device-related events. Device Events DomainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 31 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66734 - DOMAIN - Domain AbbreviationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102618 DI DeviceIdentifiersA domain for the submission of information thatidentifies a specific device unit.Device Identifiers DomainC49572 DM Demographics The Demographics domain includes a set of essentialstandard variables that describe each subject in aclinical study. It is the parent domain for all otherobservations for human clinical subjects.Demographics DomainC102619 DO DevicePropertiesC95088 DP DevelopmentalMilestoneC102620 DR Device toSubjectRelationshipA domain for the characteristics of the device that donot vary over the course of the study.This domain captures the outcome of developmentalmilestone testing during a scheduled period ofobservation.A domain for the linkage of each subject to thedevice(s) to which they may have been exposed.Device Properties DomainDevelopmental Milestone DomainDevice to Subject RelationshipDomainC49576 DS Disposition A subject domain utilized for the submission ofinformation encompassing and representing data,vocabulary or records related to disposition. (NCI)Disposition DomainC102621 DT Device Trackingand DispositionA domain for logistics and disposition informationspecific to a given device.Device Tracking and DispositionDomainC102622 DU Device-In-Use A domain for the findings for the values ofmeasurements and settings that are intentionally set ona device when it is in use. These are characteristics thatexist for the device, and have a specific setting for ause instance.Device-In-Use DomainC49585 DV ProtocolDeviationsThe intent of the domain is to capture protocolviolations and deviations during the course of the studyand will store only those criteria violation by ordeviated from by the subject and not a response to eachviolation or deviation.Protocol Deviations DomainC102617 DX Device Exposure A domain for a subject's exposure to a medical deviceunder study.Device Exposure DomainC102630 ED EndocrineSystem FindingsC49625 EE ElectroencephalogramC49626 EG ElectrocardiogramA domain for physiological findings related to theendocrine system, including the glands and cells thatmake hormones that are released directly into theblood and travel to tissues and organs all over the body.Findings resulting from electroencephalogram (EEG)tests.Findings related to the collection of ECG data,including position of the subject, method ofevaluation, all cycle measurements and all findingsfrom the ECG including an overall interpretation ifcollected or derived.Endocrine System Findings DomainElectroencephalogram DomainElectrocardiogram DomainC49587 EX Exposure The Exposure domain model records the details of asubject's exposure to protocol-specified studytreatment. Study treatment may be any intervention thatis prospectively defined as a test material within astudy, and is typically but not always supplied to thesubject.Exposure DomainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 32 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66734 - DOMAIN - Domain AbbreviationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85442 FA Findings AboutEvents orInterventionsA dataset used to capture the findings about an event orintervention that cannot be represented within an eventor intervention record or as a supplemental qualifier.Findings About Events orInterventions DomainC95089 FE Fertility This domain captures test results relative to male andfemale fertility.C102637 FF Fetal Findings Fetal examinations and measurements for nonclinicaldevelopmental and reproductive toxicology studies.C49588 FH Family History A subject domain utilized for the submission ofinformation encompassing and representing data,vocabulary or records related to family history. (NCI)C95090 FW Food And Water This domain captures food/water consumption ofanimals in the study. The data in this domain is deriveddata.Fertility DomainFetal Findings DomainFamily History DomainFood and Water ConsumptionDomainC95091 FX Fetal andNeonatalDevelopmentalMorphologyC102640 GI GastrointestinalSystem FindingsC102641 HM HematopoieticSystem FindingsC49589 HU HealthcareResourceUtilizationC95092 IC ImplantationClassificationC61536 IE Inclusion/ExclusionC102651 IG IntegumentarySystem FindingsC102649 IM Immune SystemFindingsThis domain captures observations for fetal and pupmorphological examinations.A domain for physiological findings related to thegastrointestinal system, including the esophagus,stomach, small and large intestine, anus, liver, biliarytract and pancreas.A domain for physiological findings related to thehematopoietic system, including the bone marrow andlymph nodes involved in the production of blood.Healthcare resource utilization data such as subjectvisits to physicians, hospitalizations, and nursing homestays.The Implantation Classification domain provides arecord for each implantation identified for thescheduled cesarean section component of a study.The intent of the domain model is to only collect thosecriteria that cause the subject to be in violation of theinclusion/exclusion criteria not a response to eachcriterion.A domain for physiological findings related to theintegumentary system, including the epidermis,dermis, all of the derivatives of the epidermis, hairs,nails, sudoriferous and sebaceous glands, andmammary glands.A domain for physiological findings related to theimmune system, including the thymus, spleen andtonsils.Fetal and Neonatal DevelopmentalMorphology DomainGastrointestinal System FindingsDomainHematopoietic System FindingsDomainHealthcare Resource UtilizationDomainImplantation Classification DomainInclusion Exclusion DomainIntegumentary System FindingsDomainImmune System Findings DomainC49592 LB Laboratory Data Laboratory test findings including, but is not limited tohematology, clinical chemistry and urinalysis data.This domain does not include microbiology orpharmacokinetic data, which are stored in separatedomains.Laboratory Data DomainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 33 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66734 - DOMAIN - Domain AbbreviationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC95093 LR Cesarean Sectionand DeliveryLitter ResultsC95094 MA MacroscopicFindingsThis domain captures litter based results in femaleanimals for cesarean section and/or deliverycomponents of a study, including litter survival duringpreweaning.The gross pathology findings recorded at necropsy.Cesarean Section and Delivery LitterResults DomainMacroscopic Findings DomainC49602 MB Microbiology Microbiology specimen findings, including gram stainresults, and organisms found.C49603 MH Medical History The medical history dataset includes the subject's priorhistory at the start of the trial. Examples of subjectmedical history information could include generalmedical history, gynecological history, and primarydiagnosis.Microbiology Specimen DomainMedical History DomainC95095 MI MicroscopicFindingsC102674 MK MusculoskeletalFindings,Connective andSoft TissueFindingsThe histopathology findings and microscopicevaluations recorded.A domain for physiological findings related to thesystem of muscles, tendons, ligaments, bones, jointsand associated tissues.Microscopic Findings DomainMusculoskeletal Findings,Connective and Soft Tissue FindingsDomainC49604 ML Meal Data Information regarding the subject's meal consumption,such as fluid intake, amounts, form (solid or liquidstate), frequency, etc., typically used forpharmacokinetic analysis.Meal Data DomainC102671 MO MorphologyFindingsC61531 MS MicrobiologySusceptibilityC102677 NV Nervous SystemFindingsC49605 OM OrganMeasurementsA domain relevant to the science of the form andstructure of an organism or of its parts.This includes microbiology susceptibility test results,plus results of any other organism-related tests.A domain for physiological findings related to thenervous system, including the brain, spinal cord, thecranial and spinal nerves, autonomic ganglia andplexuses.Findings from organ measurement evaluations.Morphology Findings DomainMicrobiology Susceptibility DomainNervous System Findings DomainOrgan Measurement DomainC102694 PA Pairing Events Nonclinical pairing records for the fertility componentof a study.Pairing Events DomainC49606 PC PharmacokineticConcentrationConcentrations of drugs/metabolites in fluids ortissues as a function of time.Pharmacokinetic ConcentrationDomainC49608 PE Physical Exam Findings collected during a physical examination ofthe subject. It has findings that are discovered that arerelated to body systems. Does not include vital signsmeasurements, which are stored in the vital signsdomain.Physical Exam DomainC61529 PG PharmacogenomicsPharmacogenomics findings that initially focus ongenotype and gene expression data.Pharmacogenomics DomainC95097 PM Palpable Masses This domain captures information of any palpablemasses examined during the experimental phase.Palpable Masses DomainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 34 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66734 - DOMAIN - Domain AbbreviationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49607 PP PharmacokineticParametersPharmacokinetic parameters derived frompharmacokinetic concentration-time (PC) data.Pharmacokinetic Parameters DomainC102700 PR Procedure A domain relevant to an interventional activity that isintended to have diagnostic, preventive, therapeutic, orpalliative effects.Procedure DomainC102678 PY NonclinicalPregnancyResultsPregnancy results of female nonclinical subjects.Nonclinical Pregnancy ResultsDomainC49609 QS Questionnaires Questionnaire data may include, but are not limited tothe following: subject reported outcomes, validated ornon-validated questionnaires, and validated ornon-validated diaries (i.e. data with a commontopicality).Questionnaire DomainC95098 RE RespiratorySystem FindingsC102707 RP ReproductiveSystem FindingsA domain for physiological findings related to therespiratory system, including the organs that areinvolved in breathing such as the nose, throat, larynx,trachea, bronchi and lungs.A domain for physiological findings related to themale and female reproductive systems.Respiratory DomainReproductive System FindingsDomainC102722 RS Tumor Response A domain for tumor response evaluations determinedfrom the data in the tumor results domain.Tumor Response DomainC49610 SC SubjectCharacteristicsThe subject characteristics domain is for data notcollected in other domains that is subject-related.Subject Characteristics DomainC49616 SE Subject Element The subject element table describes the actual order ofelements followed by the subject, together with thestart date/time and end date/time for each element.Subject Element DomainC49611 SG Surgery Surgical information. Surgery DomainC95099 SJ Subject Stages The Subject Stages domain captures subject levelnon-treatment related blocks of time known as stages,such as Gestation, Lactation and Premating.Subject Stages DomainC496<strong>12</strong> SK Skin Test Findings from diagnostic skin tests. Skin Test DomainC49613 SL SleepPolysomnography DataC49614 ST Stress (Exercise)Test DataFindings from diagnostic sleep tests(polysomnography).Findings from exercise stress tests.Sleep Polysomnography DomainStress Exercise Test Data DomainC49615 SU Substance Use The intent of the domain is to capture substance useinformation that may be used to assess the efficacyand/or safety of therapies that look to mitigate theeffects of chronic substance use.C49617 SV Subject Visits The subject visits table describes the actual start andend data/time for each visit of each individual subject.C49618 TA Trial Arms The trial arms table describes each planned arm in thetrial. An arm is described as an ordered sequence ofelements.Substance Use DomainSubject Visits DomainTrial Arms DomainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 35 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66734 - DOMAIN - Domain AbbreviationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49619 TE Trial Elements The trial elements table describes the element codethat is unique for each element, the elementdescription, and the rules for starting and ending anelement.C95100 TF Tumor Findings This domain captures the tumor findings of thenonclinical subject.Trial Elements DomainTumor Findings DomainC49620 TI TrialInclusion/ExclusionCriteriaThe trial summary information domain is not subjectoriented. It contains one record for each of theinclusion and exclusion criteria for the trial.Trial Inclusion and ExclusionCriteria DomainC95101 TP Trial Paths The trial paths domain is not subject oriented. Thisdomain provides the complete planned non-treatmentrelated sequences of stages for each path in a study.C53483 TS Trial Summary The trial summary information domain is not subjectoriented. It contains one record for each trial summarycharacteristic.C95102 TT Trial Stages The trial stages domain is not subject oriented. Itcaptures non-treatment related planned blocks of timeknown as stages, such as Gestation, Lactation andPremating.C49621 TV Trial Visits The trial visits table describes the planned order andnumber of visits in the study within each arm.C95103 TX Trial Sets The trial stages domain is not subject oriented. Itcontains one record for each trial set characteristicincluding experimental factors, treatment factors,inherent characteristics, or distinct sponsordesignations.Trial Paths DomainTrial Summary DomainTrial Stages DomainTrial Visits DomainTrial Sets DomainC102726 UR Urinary SystemFindingsA domain for physiological findings related to theurinary tract, including the organs involved in thecreation and excretion of urine such as the kidneys,ureters, bladder and urethra.Urinary System Findings DomainC49622 VS Vital Signs Measurements including but not limited to bloodpressure, temperature, respiration, body surface area,BMI, height and weight.Vital Signs DomainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 36 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89968 - DSDECOD - Standardized Disposition TermCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90351 ACCIDENTAL DEATH An indication that the subject's death or sacrifice wasdue to a mishap or technical/operational error.C90387 FOUND DEAD An indication that a subject was found in a deceasedstate. (NCI)C96372 MISSING An indication that the subject could not be found, inwhich case, its disposition was not known, and nopostmortem data was available.C90425 MORIBUND SACRIFICE An indication that a subject was euthanized due toethical reasons, such as being near death.Accidental DeathFound DeadMissing Study AnimalMoribund SacrificeC90436PLANNED INTERIMSACRIFICEAn indication that the study subject was sacrificed at apredetermined time before the end of the treatmentperiod.Planned Interim SacrificeC90445 RECOVERY SACRIFICE An indication that the subject did not receive treatmentfor a specific period of time following the end of thetreatment period before it was sacrificed.Recovery SacrificeC90447REMOVED FROM STUDYALIVEAn indication that the subject was alive when taken outof the study. (NCI)Removed From Study AliveC90465 TERMINAL SACRIFICE An indication that the subject was sacrificed at the endof the treatment period.Terminal SacrificeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 37 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C900<strong>12</strong> - EGCATSND - <strong>SEND</strong> ECG CategoryCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC15220 DIAGNOSIS The investigation, analysis and recognition of thepresence and nature of disease, condition, or injuryfrom expressed signs and symptoms; also, thescientific determination of any kind; the conciseresults of such an investigation. (NCI)C4<strong>12</strong>55 INTERPRETATION Interpretation An act or process of elucidation; explication, orexplanation of the meaning of the event or thing via theassignment of objects from the domain to theconstants of a formal language, truth-values to theproposition symbols, truth-functions to theconnectives, other functions to the function symbols,and extensions to the predicates, if any. Theassignments are result of human logic application andare not native to the symbols of the formal language.C25209 MEASUREMENT Annotation used to indicate the size or magnitude ofsomething that was determined by comparison to astandard. (NCI)DiagnosisInterpretationMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 38 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90013 - EGLEAD - ECG LeadCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90404 LEAD CM5 A bipolar EKG lead with the right thoracic limbelectrode placed on the manubrium and left thoraciclimb electrode placed at the surface marking of the V5position (just above the 5th interspace in the anterioraxillary line). The left pelvic limb lead acts as a neutraland may be placed anywhere. The C refers to 'clavicle'where it is often placed. (NCI)C90405 LEAD CV5RL A unipolar chest lead used mostly in large animals.Placed on the sixth ICS on the right side of the thoraxalong a line parallel to the level of the point of thehumeralradial joint.C90406 LEAD CV6LL V1 electrode (+) placed in the 6th intercostal space onthe left side of the thorax along a line parallel to thelevel of the point of the humeralradial joint.C90407 LEAD CV6LU V2 electrode (+) placed in the 6th intercostal space onthe left side of the thorax along a line parallel to thelevel of the point of the shoulder. (NCI)C90408 LEAD I A bipolar electrocardiogram limb lead which recordsthe voltage between the negative electrode on the rightthoracic limb and the positive electrode on the leftthoracic limb. (NCI)C90409 LEAD II A bipolar electrocardiogram limb lead which recordsthe voltage between the negative electrode on the rightthoracic limb and the positive electrode on the leftpelvic limb. (NCI)C90410 LEAD III A bipolar electrocardiogram limb lead which recordsthe voltage between the negative electrode on the leftthoracic limb and the positive electrode on the leftpelvic limb. (NCI)C904<strong>12</strong> LEAD V1 A unipolar electrocardiogram lead site; the electrodeis placed at the fourth intercostal space on the anteriorchest wall (between ribs 4 and 5) to the right of thesternal border. In small animals, it is placed at the rightfifth intercostal space near the sternum. (NCI)C90413 LEAD V10 A unipolar chest lead at which the electrode is placedover the dorsal spinous process of 7th thoracicvertebra. (NCI)C90414 LEAD V2 A unipolar electrocardiogram lead site; the electrodeis placed on the anterior chest wall at the fourthintercostal space (between ribs 4 and 5) to the left ofthe sternal border. In small animals it corresponds toV2-V3 where it is placed at the 6th left intercostalspace near the sternum. In large animals it is placedover the 6th rib at the level of the costochondraljunction on the left side of the thorax.C90415 LEAD V3 A unipolar electrocardiogram lead site; the electrodeis placed on the anterior chest wall midway betweenleads V2 and V4. In large and small animals, it isplaced over the dorsal spinous process of the 7ththoracic vertebra. (NCI)Lead Site CM5Lead Site CV5RLLead Site CV6LLLead Site CV6LULead Site ILead Site IILead Site IIILead Site V1Lead Site V10Lead Site V2Lead Site V3Source: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 39 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90013 - EGLEAD - ECG LeadCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90416 LEAD V4 A unipolar electrocardiogram lead site; the electrodeis placed at the fifth intercostal space on the anteriorchest wall (between ribs 5 and 6) at the leftmidclavicular line. In small animals it corresponds toV4-V6 where it is placed at the 6th left intercostalspace near the costochondral junction. In large animalsit is placed over the 6th rib at the level of a horizontalline drawn through the scapulohumeral articulation onthe left side of the thorax. (NCI)C90417 LEAD V5 A unipolar electrocardiogram lead site; the electrodeis placed on the anterior chest wall level with lead V4at the left anterior axillary line. In large animals it isplaced on the sixth ICS on the right side of the thoraxalong a line parallel to the level of the point of theshoulder corresponding to the electrical center of theheart (central terminal). (NCI)C90418 LEAD V6 A unipolar electrocardiogram lead site at which theelectrode is placed on the anterior chest wall levelwith lead V5 at the left midaxillary line .C90403 LEAD aV6 An augmented unipolar lead placed at the sixthintercostal space on the midaxillary line. (NCI)C90360 LEAD aVF An augmented unipolar electrocardiogram limb lead inwhich the positive (red) electrode is situated on theleft pelvic limb and the negative electrode is acombination of the right thoracic limb (white)electrode and the left thoracic limb (black) electrode.Measures the electrical activity of the electrode on theleft pelvic limb.C90361 LEAD aVL An augmented unipolar electrocardiogram limb lead inwhich the positive (black) electrode is situated on theleft thoracic limb and the negative electrode is acombination of the right thoracic limb (white)electrode and the left pelvic limb (red) electrode.Measures the electrical activity of the electrode on theleft thoracic limb. (NCI)C90362 LEAD aVR An augmented unipolar electrocardiogram limb lead inwhich the positive (white) electrode is situated on theright thoracic limb and the negative electrode is acombination of the left thoracic limb (black) electrodeand the left pelvic limb (red) electrode. Measures theelectrical activity of the electrode on the right thoraciclimb. (NCI)C90411 LEAD rV2 A unipolar precordial lead placed at the secondintercostal space to the left of the sternum. (NCI)Lead Site V4Lead Site V5Lead Site V6Lead Site aV6Augmented Vector FootAugmented Vector LeftAugmented Vector RightLead Site rV2Source: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 40 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71151 - EGMETHOD - ECG Test MethodCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90349 10 LEAD STANDARD 10 Lead Standard An electrocardiogram lead placement on the subjectusing a ten electrode lead set to synthesize standard <strong>12</strong>lead electrocardiograph data to elicit an electrical viewof the heart.10 Lead StandardC71<strong>12</strong>5 <strong>12</strong> LEAD 1 LEAD MISSING <strong>12</strong> Lead 1 LeadMissingAn electrocardiogram (ECG) lead placement whereby<strong>12</strong> leadpoints are recorded but one standard leadposition is missing therefore requiring a Mortarasource consistency filter. (NCI)<strong>12</strong> Lead Placement 1 Lead MissingC71116 <strong>12</strong> LEAD CABRERA <strong>12</strong> Lead Cabrera An electrocardiogram (ECG) lead placement wherebythe display of the <strong>12</strong> standard ECG leads is in anorderly sequence in a single horizontal display of:aVL, I, -aVR, II, aVF, III, V1 to V6. In the Cabreradisplay the limb lead aVR is inverted (-aVR) to obtainthe same positive leftward orientation as the other 5limbs. (NCI)<strong>12</strong> Lead Placement CabreraC71<strong>12</strong>3<strong>12</strong> LEAD EASI DOWERTRANSFORMATION<strong>12</strong> Lead EASIDowerTransformationAn electrocardiogram (ECG) lead placement whereby4 chest electrodes and 1 reference electrode are usedto allow for continuous monitoring at the clinicallevel. This placement creates a <strong>12</strong> lead ECG thatallows the acquisition of simultaneous events in thefrontal, horizontal and sagittal heart planes with thelinear transformation of vectors. This system providesa three-dimensional portrayal of the heart and usesmathematical and fixed coefficients for each lead.(NCI)Lead Placement EASI DowerTransformationC71103 <strong>12</strong> LEAD MASON LIKAR <strong>12</strong> Lead MasonLikarAn electrocardiogram (ECG) lead placement whereby<strong>12</strong> leadpoints are recorded but the standard leadpositions have been modified for ECG recordingduring exercise. Exercise stress testing requiresmoving the limb electrodes to more central positionson the thorax. The electrodes are placed in bonyprominences close to the bases of the respective limbsin order to avoid skeletal muscle artifact, providestability for recording electrodes and to recordwaveforms similar to the standard limb sites. (NCI)<strong>12</strong> Lead Placement Mason LikarC71110<strong>12</strong> LEAD MODIFIEDMASON LIKAR<strong>12</strong> LeadModified MasonLikarAn electrocardiogram (ECG) lead placement whereby<strong>12</strong> leadpoints are recorded but the Mason Likar leadpositions have been modified so that V1 to V6 on thechest are part of a single electrode pad. In addition,lead CM5 is substituted for lead aVR. (NCI)<strong>12</strong> Lead Placement Mason LikarModifiedC71114 <strong>12</strong> LEAD NON-STANDARD <strong>12</strong> LeadNon-StandardC711<strong>12</strong> <strong>12</strong> LEAD SINGLE PAD <strong>12</strong> Lead SinglePadAn electrocardiogram (ECG) lead placement wherebythe limb leads are placed on the torso for easier andfaster application in emergency situations. (NCI)An electrocardiogram (ECG) lead placement whereby<strong>12</strong> leadpoints are recorded but the standard leadpositions have been modified so that all leads on thechest are part of a single electrode pad. (NCI)<strong>12</strong> Lead Placement Non-Standard<strong>12</strong> Lead Placement ChestSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 41 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71151 - EGMETHOD - ECG Test MethodCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71102 <strong>12</strong> LEAD STANDARD <strong>12</strong> Lead Standard An electrocardiogram (ECG) lead placement whereby<strong>12</strong> leads are recorded, with each lead representing anelectrical view of the heart. The six leads recorded inthe frontal plane are derived from the placement of 3electrodes (RA or Right Arm, LA, or Left Arm, and LLor Left Leg). These bipolar frontal leads form the basisof Einthoven's triangle, and are represented by leads I,II, and III. Three other derived (or augmented) bipolarfrontal vectors are also recorded on a standard <strong>12</strong>-leadEKG, aVR, aVF, and aVL. 6 unipolar leads,corresponding to V1 - V6 measure the electricalactivity in the horizontal plane. The placement for theV leads is as follows: V1: right 4th intercostalspace,V2: left 4th intercostal space, V3: halfwaybetween V2 and V4, V4: left 5th intercostal space,mid-clavicular line, V5: horizontal to V4, anterioraxillary line, V6: horizontal to V5, mid-axillary line.(NCI)<strong>12</strong> Lead Placement StandardC71101 <strong>12</strong> LEAD UNSPECIFIED <strong>12</strong> LeadUnspecifiedAn electrocardiogram (ECG) lead placement whereby<strong>12</strong> leadpoints are recorded but the position of theleads is unspecified. (NCI)<strong>12</strong> Lead Placement UnspecifiedC90350 6 LEAD STANDARD 6 Lead Standard An electrocardiogram lead placement on the subjectusing a six electrode lead set with three standard leadsand three augmented derived leads to elicit anelectrical view of the heart.6 Lead StandardC71<strong>12</strong>1BIPOLAR UNCORRECTEDXYZ LEAD SYSTEMBipolaruncorrected XYZlead systemAn electrocardiogram (ECG) lead placement wherebythe X+ lead is placed at the right mid-axillary line atethe 4th intercostal space, X- at the left mid-axillaryline at he 4th intercostal space, Y+ at the proximal leftleg, Y- at the superior aspect of the manubrium, Z+ atthe direct posterior to Z- and Z- at the 4th intercostalspace at the left sternal margin. (NCI)Lead Placement Bipolar UncorrectedXYZC71<strong>12</strong>0 CUBE LEAD SYSTEM Cube lead system An electrocardiogram (ECG) lead placement that is atype of uncorrected vectorcardiograph. This leadsystem is based on a rectangular body axis. It uses anextra number of electrodes to make itthree-dimensional. (NCI)Lead Placement CubeC71118 FRANK LEAD SYSTEM Frank leadsystemAn electrocardiogram (ECG) lead placement fordetermining 3 orthogonal components X (right to leftdirection), Y (foot to head direction) and Z (back tofront direction) of the heart. For this method aminimum of 4 electrodes are needed that represent theright arm, left arm, left leg and back. However, usually7 electrodes are used to avoid dependence on thedipole location and facilitate interpretation. (NCI)Lead Placement FrankC71119MCFEE-PARUNGAO LEADSYSTEMMcFee-Parungaolead systemAn electrocardiogram (ECG) lead placement fordetermining 3 orthogonal components X (back tofront), Y (right to left) and Z (foot to head) of theheart. This system places the electrodes closer to theheart to achieve better orthogonality and ahomogeneous lead field. (NCI)Lead Placement McFee-ParungaoSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 42 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71151 - EGMETHOD - ECG Test MethodCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71<strong>12</strong>2PSEUDO-ORTHOGONALXYZ LEAD SYSTEMPseudo-orthogonal XYZ leadsystemAn electrocardiogram (ECG) lead placement thatallows monitoring and recording of cardiac electricalactivity. A V1-type lead is used whose positiveelectrode is localized in the 4th intercostal space,2.5cm from the sternum. Its negative electrode isplaced below the left clavicle. An addition of lead V5and aVF can be made to facilitate interpretation. (NCI)Lead Placement Pseudo OrthogonalXYZC71<strong>12</strong>8STANDARD <strong>12</strong>-LEAD ANDCC5-CM5-MLStandard <strong>12</strong>-leadandCC5-CM5-MLAn electrocardiogram (ECG) lead placement whereby<strong>12</strong> leadpoints are recorded but the standard leadpositions have been modified so that the negativereference is at CM5 and the active electrode is at theleft leg position. (NCI)<strong>12</strong> Lead Placement Standard AndCC5-CM5-MLC71<strong>12</strong>6STANDARD <strong>12</strong>-LEAD ANDCM5-CC5-CH5Standard <strong>12</strong>-leadandCM5-CC5-CH5An electrocardiogram (ECG) lead placement whereby<strong>12</strong> leadpoints are recorded but the standard leadpositions have been modified so that the bipolar leadgroups place the negative of the reference electrodeover the manubrium (CM5), the right scapula (CB5),V5R (CC5) or on the forehead (CH5) and the activeelectrode at V5. (NCI)<strong>12</strong> Lead Placement Standard AndCC5-CM5-CH5C71131STANDARD <strong>12</strong>-LEADEXTENDED LEFTStandard <strong>12</strong>-leadextended to theleft by V7, V8,V9An electrocardiogram (ECG) lead placement wherebythe standard lead placement is modified by havingleads V7, V8 and V9. (NCI)<strong>12</strong> Lead Placement StandardExtended LeftC71130STANDARD <strong>12</strong>-LEADEXTENDED RIGHTStandard <strong>12</strong>-leadextended to theright by V5R,V4R, V3RAn electrocardiogram (ECG) lead placement wherebythe rightward oriented V leads progress from V1R,placed instead of the standard V2, to V6R. The V3 leadin the standard placement is replaced by V4R. (NCI)<strong>12</strong> Lead Placement StandardExtended RightC71115STANDARD LEADS FORBICYCLE EXERCISEStandard leadsfor bicycleexercise. Limbleads on the back(shoulder and onthe hips)Limb leads on the back (shoulder and on the hips).(NCI)Lead Placement BicycleC71117STANDARD LEADS ONEINTERCOSTAL SPACEHIGHERStandard leadsone intercostalspace higherAn electrocardiographic lead placement schema inwhich the V leads are placed one intercostal spacecephalad to the position they would have in thestandard lead placement schema. (NCI)Lead Placement Standard IntercostalSpace HigherC71092VECTORCARDIOGRAPHCORRECTEDVectorcardiograph CorrectedA recording of the electrical activity of the heartdisplayed in the form of a vector loop, corrected foranatomic inconsistencies. (NCI)Vectorcardiograph CorrectedC71093VECTORCARDIOGRAPHUNCORRECTEDVectorcardiograph UncorrectedA recording of the electrical activity of the heartdisplayed in the form of a vector loop, uncorrected foranatomic inconsistencies. (NCI)Vectorcardiograph UncorrectedSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 43 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62015 1ST DEGREE AV BLOCK 1st degree AVblockA delay in the time required for the conduction of anelectrical impulse through the atrioventricular (AV)node beyond 0.2 seconds; prolongation of the PRinterval beyond 200 milliseconds. (NCI)AV Block First DegreeC71044 2:1 AV BLOCK 2:1 AV block A electrocardiographic finding of an electricalphenomenon in which the ratio of electrical impulsegenerated above the atrioventricular node to thenumber of impulses conducted through to theventricles is 2:1. (NCI)2:1 Atrioventricular BlockC62016 2ND DEGREE AV BLOCK 2nd degree AVblockC50501 3RD DEGREE AV BLOCK 3rd degree AVblockIntermittent failure of atrial electrical impulseconduction through the atrioventricular (AV) node tothe ventricles. (NCI)Complete failure of atrial electrical impulseconduction through the AV node to the ventricles.(NCI)AV Block Second DegreeAV Block Third DegreeC62266ACCELERATEDIDIOVENTRICULARRHYTHMAcceleratedidioventricularrhythmThe term accelerated idioventricular rhythm describesan ectopic ventricular rhythm with 3 or moreconsecutive ventricular premature beats with a ratefaster than the normal ventricular intrinsic escape rateof 30 to 40 beats per minute, but slower thanventricular tachycardia. Accelerated idioventricularrhythm differs from ventricular tachycardia byadditional features such as the onset with a longcoupling interval and the end by a gradual decrease ofthe ventricular rate or increase of the sinus rate. (NCI)Accelerated Idioventricular RhythmC71065ACUTE ANTERIOR WALLMIAcute AnteriorWall MyocardialInfarctionElectrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe anterior wall of the heart. (NCI)Acute Anterior Wall MyocardialInfarctionC102591ACUTE ANTEROLATERALWALL MYOCARDIALINFARCTIONAcuteAnterolateralWall MyocardialInfarctionAn electrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe anterolateral wall of the heart.Acute Anterolateral MyocardialInfarction by ECG FindingC102592ACUTE ANTEROSEPTALWALL MYOCARDIALINFARCTIONAcuteAnteroseptalWall MyocardialInfarctionAn electrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe anteroseptal wall of the heart.Acute Anteroseptal MyocardialInfarction by ECG FindingC102593ACUTE HIGH LATERALWALL MYOCARDIALINFARCTIONAcute HighLateral WallMyocardialInfarctionAn electrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe high lateral wall of the heart.Acute High Lateral MyocardialInfarction by ECG FindingC71066 ACUTE INFERIOR WALL MI Acute InferiorWall MyocardialInfarctionC71067 ACUTE LATERAL WALL MI Acute LateralWall MyocardialInfarctionElectrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe inferior wall of the heart. (NCI)Electrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe lateral wall of the heart. (NCI)Acute Inferior Wall MyocardialInfarctionAcute Lateral Wall MyocardialInfarctionC101596ACUTE MYOCARDIALINFARCTIONAcuteMyocardialInfarctionAn electrocardiographic finding showing a current ofinjury without specifying the anatomic region of theheart.Acute Myocardial Infarction by EKGFindingSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 44 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71068ACUTE POSTERIOR WALLMIAcute PosteriorWall MyocardialInfarctionElectrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe posterior wall of the heart. (NCI)Acute Posterior Wall MyocardialInfarctionC102594ACUTE RIGHTVENTRICULAR WALLMYOCARDIALINFARCTIONAcute RightVentricular WallMyocardialInfarctionAn electrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe right ventricular wall of the heart.Acute Right Ventricular MyocardialInfarction by ECG FindingC102595ACUTE SEPTAL WALLMYOCARDIALINFARCTIONAcute SeptalWall MyocardialInfarctionAn electrocardiographic finding showing a current ofinjury in leads corresponding to the anatomic region ofthe septal wall of the heart.Acute Septal Myocardial Infarctionby ECG FindingC71057ADENOSINE-SENSITIVEVENTRICULARTACHYCARDIAAdenosine-sensitive ventriculartachycardiaA condition that occurs as a result of cAMP-mediatedtriggered activity and typically originates from theright ventricular outflow tract (RVOT). This type oftachycardia occurs in patients with apparently normalhearts. (NCI)Adenosine-Sensitive VentricularTachycardiaC102642ADVANCED/HIGH GRADEAV BLOCKAdvanced/HighGrade AV BlockAn electrocardiographic finding of intermittent failureof atrial electrical impulse conduction through theatrioventricular (AV) node to the ventricles,characterized by two or more consecutivenon-conducted P waves.High Grade Atrioventricular BlockC71069ANTERIOR WALLMYOCARDIALINFARCTIONAnterior WallMyocardialInfarctionElectrocardiographic finding showing an injury inleads corresponding to the anatomic region of theanterior wall of the heart. (NCI)Anterior Wall Myocardial InfarctionC35303ANTEROLATERAL WALLMYOCARDIALINFARCTIONAnterolateralWall MyocardialInfarctionAn electrocardiographic finding showing an injury inleads corresponding to the anatomic region of theanterolateral wall of the heart.Anterolateral Myocardial InfarctionC35304ANTEROSEPTAL WALLMYOCARDIALINFARCTIONAnteroseptalWall MyocardialInfarctionAn electrocardiographic finding showing an injury inleads corresponding to the anatomic region of theanteroseptal wall of the heart.Anteroseptal Myocardial InfarctionC102596 ATRIAL BIGEMINY Atrial Bigeminy An electrocardiographic finding of a sinus beatfollowed by a premature atrial contraction for three ormore consecutive cycles; a regularly irregular rhythmof normal and abnormal P waves in a 1-1 ratio.C102597 ATRIAL COUPLETS Atrial Couplets An electrocardiographic finding in which twopremature atrial contractions occur in a row.Atrial BigeminyAtrial CoupletC71039 ATRIAL ENLARGEMENT AtrialEnlargementAn electrocardiographic finding which comprises left,right or bilateral atrial enlargement. (NCI)EKG Finding Atrial EnlargementC50466 ATRIAL FIBRILLATION Atrial fibrillation A supraventricular arrhythmia characterized byuncoordinated atrial myocardium activation due tomultiple reentry circuits with consequent deteriorationof atrial mechanical function. Instead of intermittentlycontracting, the atria quiver continuously in a chaoticpattern, causing a totally irregular, often tachycardiaventricular rate. On the ECG it is described by thereplacement of consistent P waves by rapidoscillations or fibrillatory waves that vary in size,shape, and timing, associated with an irregular,frequently rapid ventricular response whenatrioventricular conduction is intact. (NCI)Atrial FibrillationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 45 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC5<strong>12</strong>24 ATRIAL FLUTTER Atrial flutter An electrocardiographic finding of an organizedrhythmic contraction of the atria which is generally ata rate of 200-300 beats per minute. (NCI)Atrial FlutterC35481 ATRIAL TACHYCARDIA AtrialtachycardiaAny rapid heart rhythm originating in the atria. Atrialfibrillation and atrial flutter are varieties of atrialtachycardia. Atrial tachycardia is an electrical circuitoriginating in the upper or atrial chambers. Thiselectrical circuit or focus takes over and generatesrapid impulses across the atrial chambers. Theseimpulses are transmitted to the ventricular chambersresulting in a rapid rate. These arrhythmias mayoriginate from a variety of places across the atria. Theymay exist as an isolated problem or be related to anongoing structural problem within the heart. (NCI)Atrial TachycardiaC102598 ATRIAL TRIGEMINY Atrial Trigeminy An electrocardiographic finding of two sinus beatsfollowed by a premature atrial contraction for 3 ormore consecutive cycles; a regularly irregular rhythmof normal and abnormal P waves in a 2-1 ratio.Atrial TrigeminyC71045ATRIOVENTRICULARDISSOCIATIONAtrioventriculardissociationAn electrocardiographic finding in which the electricalactivity of the atria and ventricles are causallyindependent of one another. (NCI)Atrioventricular DissociationC62017 AV MOBITZ I AV Mobitz I Intermittent failure of atrial electrical impulseconduction through the atrioventricular (AV) node tothe ventricles, characterized by a progressivelylengthening PR interval prior to the block of an atrialimpulse.C62018 AV MOBITZ II AV Mobitz II Intermittent failure of atrial electrical impulseconduction through the atrioventricular (AV) node tothe ventricles, characterized by a relatively constantPR interval prior to the block of an atrial impulse.C35058 AV NODE RE-ENTRY AV node re-entry A supraventricular tachycardia due to reentry along acircuit contained within the AV node. AVNRT is themost common form of supraventricular tachycardiaand usually presents as a narrow QRS complextachycardia at a rate between 150 and 250 beats perminute. (NCI)AV Mobitz IAV Mobitz IIAtrioventricular Nodal ReentryTachycardiaC62261AV RE-ENTRANTTACHYCARDIAAV re-entranttachycardiaA supraventricular tachycardia due to reentry along acircuit including a concealed AV nodal bypass tract.This tachycardia circuit proceeds in an antegradefashion through the AV node and then is conductedretrograde through the bypass tract into the atria. (NCI)Atrioventricular ReentrantTachycardiaC71046 BIFASCICULAR BLOCK BifascicularblockAn electrocardiographic finding comprising rightbundle branch block and left anterior fascicular block,or right bundle branch block and left posteriorfascicular block. Defects occurring in two of the threedivisions of the conduction system of the heart areconsidered bifascicular blocks. Technically left bundlebranch block may be considered a bifascicular block.(NCI)Bifascicular BlockC71054 BIGEMINY Bigeminy An electrocardiographic finding of a normal QRSfollowed by a premature ventricular contraction; arhythmic pairing of normal and abnormal beatsoriginating in the ventricles in a 1-1 ratio. (NCI)BigeminySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 46 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92228 BORDERLINE QTcB Borderline QTcB An electrocardiographic finding in which the QTcB isapproximately 430-470 msec. There is variationamong subpopulations.C92229 BORDERLINE QTcF Borderline QTcF An electrocardiographic finding in which the QTcF isapproximately 430-470 msec. There is variationamong subpopulations.Borderline QTcBBorderline QTcFC37920 BRADYCARDIA Bradycardia Non-sinus origin slow heart rate. BradycardiaC71062BUNDLE BRANCHREENTRANTTACHYCARDIABundle branchreentranttachycardiaAn uncommon form of ventricular tachycardia (VT)incorporating both bundle branches into the reentrycircuit. Surface electrocardiogram (ECG) of sinusrhythm (SR) characteristically shows intraventricularconduction defects. Patients typically present withpresyncope, syncope or sudden death because of VTwith fast rates frequently above 200 beats per minute.The QRS morphology during VT is a typical bundlebranch block pattern, usually left bundle branch block,and may be identical to that in SR. (NCI)Bundle Branch Re-EntrantVentricular TachycardiaC62258 DELTA WAVE Delta wave An initial slurring (delta wave) of the QRS complexdue to the presence of an accessory pathway. Thischaracteristic EKG pattern is typically seen inWolff-Parkinson-White syndrome. (NCI)C102623 DEXTROCARDIA Dextrocardia An electrocardiographic finding suggestive ofdextrocardia with situs inversus, characterized byreversal of normal anterior R wave progression and theappearance of reversal of the right and left armelectrodes.EKG Delta WaveDextrocardia by ECG FindingC92230EARLY R WAVEPROGRESSIONEarly R WaveProgressionAn electrocardiographic finding in which the amplitudeof the R wave is abnormally increased in the anteriorprecordial leads.Early R Wave ProgressionC102628EARLY R WAVETRANSITIONEarly R WaveTransitionAn electrocardiographic finding where the amplitudeof the R wave becomes greater than the amplitude ofthe S wave in the QRS complex at an unusually earlypoint in the precordial leads, usually in leads V1 or V2.Early R Wave TransitionC102629 EARLY REPOLARIZATION EarlyRepolarizationC62245 ECTOPIC ATRIAL RHYTHM Ectopic atrialrhythmAn electrocardiographic finding of J point and STsegment elevation in the absence of other signs ofacute ischemia that is often a normal variant.A cardiac rhythm resulting from either an abnormalelectrical focus in the atria or intra-atrial reentry.Atrial ectopic beats (AEB) refer to a contraction of theupper heart chamber which occurs before it would beexpected. Atrial ectopic beats are also known aspremature atrial beats, premature atrial complex(PAC), or atrial extrasystole. (NCI)Early RepolarizationEctopic Atrial RhythmC71043ECTOPICSUPRAVENTRICULARRHYTHMEctopicsupraventricularrhythmAn electrocardiographic finding of a rhythmoriginating at a site other than the sinoatrial node,which is also above the atrioventricular node. (NCI)Ectopic Supraventricular RhythmSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 47 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71042ECTOPIC VENTRICULARRHYTHMEctopicventricularrhythmA cardiac rhythm resulting from either an abnormalelectrical focus in the ventricle. Ventricular ectopicbeats (VEB) refer to a contraction of the lower heartchamber which occurs before it would be expected.Ventricular ectopic beats are also known as prematureventricular beats, premature ventricular complex(PVC), or ventricular extrasystole. (NCI)Ectopic Ventricular RhythmC71035 ELECTRICAL ALTERNANS ElectricalalternansAn electrocardiographic finding in which there is analternating pattern of any of the waveformcomponents. (NCI)Electrical AlternansC71061FASCICULARTACHYCARDIAFasciculartachycardiaA distinct subgroup of idiopathic ventriculartachycardia (VT) that may be confused with eithertypical VT or supraventricular tachycardia (SVT). It ischaracterized by the absence of structural heart diseaseand classic electrocardiographic andelectrophysiological features. It originates from theregion of the posterior fascicle (or occasionally theanterior fascicle) of the left bundle branch and is partlypropagated by the His-Purkinje network. It thereforeproduces QRS complexes of relatively short duration(0.11-0.14)s. (NCI)Fascicular TachycardiaC102639 FUSION COMPLEX Fusion Complex An electrocardiographic finding that occurs whenelectrical activation of the atria or ventricles occursfrom two separate sites. This results in a P wave orQRS complex that displays merged characteristics ofbeats originating from the two different sites.Fusion ComplexC102643HIGH LATERAL WALLMYOCARDIALINFARCTIONHigh LateralWall MyocardialInfarctionAn electrocardiographic finding showing an injury inleads corresponding to the anatomic region of the highlateral wall of the heart.High Lateral Myocardial Infarctionby ECG FindingC71063IDIOPATHIC RIGHTBUNDLE BRANCH BLOCKVENTRICULARTACHYCARDIAIdiopathic rightbundle branchblock ventriculartachycardiaIdiopathic ventricular tachycardia (VT) is a type ofventricular tachycardia occurring unrelated to anyorganic cardiac disease. The abnormality is probablycongenital, be it a focus of abnormal automaticity ormyocardial tissue that can sustain re-entry. The clinicalpresentation for idiopathic VT can be very much like aparoxysmal supraventricular tachycardia. That is, mostpatients are generally younger without overt cardiacdisease and present with paroxysmal palpitations.Idiopathic ventricular tachycardia is not generallylife-threatening. (NCI)Idiopathic Right Bundle BranchBlockC50599IDIOVENTRICULARRHYTHMIdioventricularRhythmAn electrocardiographic finding of an ectopicventricular rhythm with three or more consecutiveventricular premature beats with a rate less than 100beats per minute.Idioventricular RhythmC62253INAPPROPRIATE SINUSTACHYCARDIAInappropriatesinus tachycardiaInappropriate sinus tachycardia (IST) is a rare form ofsupraventricular tachycardia involving the sinus node,in which a patient has an abnormally high heart rate,occurring both at rest and in response to stressors(such as physical activity). (NCI)Inappropriate Sinus TachycardiaC71049INCOMPLETE BUNDLEBRANCH BLOCKIncompletebundle branchblockAn electrocardiographic finding of bundle branchblock which does not meet the criteria for a morespecific bundle branch block. (NCI)Incomplete Bundle Branch BlockSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 48 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71047INCOMPLETE LEFTBUNDLE BRANCH BLOCKIncomplete leftbundle branchblockAn partial impairment of transmission of the cardiacelectrical impulse along the fibers of the left bundlebranch. (NCI)Incomplete Left Bundle BranchBlockC71048INCOMPLETE RIGHTBUNDLE BRANCH BLOCKIncomplete rightbundle branchblockVarious mechanisms may result in a pattern showing anincomplete right bundle branch block (RBBB). Theseinclude different degrees of conduction delay throughthe main trunk of the right bundle branch; increasedconduction time through an elongated right bundlebranch; diffuse Purkinje-myocardial delay due to rightventricular stretch or dilatation; surgical trauma ordisease-related interruption of the major ramificationsof the right branch (distal RBBB or right fascicularblocks); congenital variations of the distribution of themajor ramifications resulting in a slight delay in theactivation of the crista supraventricularis. (NCI)Incomplete Right Bundle BranchBlockC102701INDETERMINATE QRS AXIS QRS AxisIndeterminate;IndeterminateAxis;IndeterminateQRS AxisAn electrocardiographic finding in which the frontalplane QRS axis cannot be calculated.QRS Axis IndeterminateC35398INFERIOR WALLMYOCARDIALINFARCTIONInferior WallMyocardialInfarctionElectrocardiographic finding showing an injury inleads corresponding to the anatomic region of theinferior wall of the heart. (NCI)Inferior Wall Myocardial InfarctionC71073INTRAATRIALCONDUCTION DELAYIntraatrialConductionDelayA delay in impulse propagation through the atria. (NCI)Intra-Atrial Conduction DelayC62271INTRAVENTRICULARCONDUCTION DELAY,NONSPECIFICIntraventricularconductiondelay,nonspecificA delay in transmission of cardiac electrical impulsesin the His-Purkinje system, diagnosed when the QRS ismodestly prolonged (< <strong>12</strong>0 msec) and the QRS patternand axis are not typical of a hemiblock. The conductiondelay is considered to occur beyond the Purkinje'smyocardial gates and arises from slow cell-to-cellconduction. The phenomenon is common in patientswith acute MI. (NCI)Nonspecific IntraventricularConduction DelayC62248ISORHYTHMICDISSOCIATIONIsorhythmicdissociationA type of atrioventricular dissociation characterized byatria and ventricles beating at similar rates, althoughindependently. (NCI)Isorhythmic AtrioventricularDissociationC71030 J POINT ELEVATION J point elevation Elevation of the J point above the baseline; the J pointrepresents the end of ventricular depolarization andlocated at the terminus of the QRS complex. (NCI)J Point ElevationC71074JUNCTIONALBRADYCARDIAJunctionalbradycardiaAn electrocardiographic finding in which the pacer ofthe heart is located in the AV junction, normallypresenting with a narrow QRS complex at a rate lessthan 60 beats per minute. (NCI)Junctional BradycardiaC102652JUNCTIONAL PREMATURECOMPLEXJunctionalPrematureComplexAn ectopic impulse originating in the AV junctionpresenting as a QRS complex of supraventricularorigin which is not preceded by a P wave.Junctional Premature ComplexC71051 JUNCTIONAL RHYTHM JunctionalrhythmAn electrocardiographic finding in which the pacer ofthe heart is located in the AV junction, normallypresenting with a narrow QRS complex and a rate of60-100 beats per minute. (NCI)Junctional RhythmSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 49 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC35059JUNCTIONALTACHYCARDIAJunctionaltachycardiaAn automatic tachycardia originating in theatrioventricular junction, typically arising in theconduction tissues surrounding the atrioventricularnode. A junctional tachycardia tends to be a regular,narrow complex tachycardia and usually occurs duringmyocardial infarction, after heart surgery, or indigitalis intoxication. The rate may range from 140 to250 beats per minute. (NCI)Junctional TachycardiaC102653LATE R WAVE TRANSITION Late R WaveTransitionAn electrocardiographic finding where the amplitudeof the R wave does not become greater than theamplitude of the S wave until an unusually late point inthe precordial leads, usually in leads V4 or V5.Late R Wave TransitionC35586LATERAL WALLMYOCARDIALINFARCTIONLateral WallMyocardialInfarctionElectrocardiographic finding showing an injury inleads corresponding to the anatomic region of thelateral wall of the heart. (NCI)Lateral Wall Myocardial InfarctionC62267LEFT ANTERIORFASCICULAR BLOCKLeft anteriorfascicular blockAn impairment of transmission of the cardiacelectrical impulse along the fibers of the left anteriorfascicle. In left anterior fascicular block (LAFB) theposteroinferior regions of the left ventricularendocardium are activated abnormally before theanterosuperior left ventricular area. After emergingfrom the posteroinferior division of the left bundlebranch, the impulse first propagates in an inferior,rightward, and usually anterior direction for a shortperiod of time. This orientation is responsible for thesmall Q waves in leads 1 and aVL and for the R wavesin leads II, III, and aVF. (NCI)Left Anterior Fascicular BlockC71040LEFT ATRIALENLARGEMENTP-mitraleAn electrocardiographic finding suggesting underlyinghypertrophy or dilatation of the left atrium.Electrocardiographic criteria used for the diagnosis ofleft atrial abnormality may include a bifid p wave, abiphasic p wave and/or a p wave duration of greaterthan 0.<strong>12</strong> seconds. (NCI)P-mitraleC62269LEFT BUNDLE BRANCHBLOCKLeft bundlebranch blockAn impairment of transmission of the cardiacelectrical impulse along the fibers of the left mainbundle branch, or both the left anterior fascicle andleft posterior fascicle. This conduction disturbance ischaracterized by wide (greater than 0.11s) QRScomplexes. The diagnostic criteria consist ofprolongation of the QRS complexes (over 0.11s) withneither a Q wave nor an S wave in lead V1 and in theproperly placed V6. A wide R wave with a notch on itstop (plateau) is seen in these leads. In hearts with anelectrical (and anatomic) vertical position a small Qwave may be seen in AVL in the absence of MI. Rightchest lead V1 may or may not show an initial R wave,but the latter should be present in lead V2. (NCI)Left Bundle Branch BlockSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 50 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62268LEFT POSTERIORFASCICULAR BLOCKLeft posteriorfascicular blockAn impairment of transmission of the cardiacelectrical impulse along the fibers of the left posteriorfascicle. In pure left posterior fascicular block(LPFB), the impulse emerges from the unblockedanterosuperior division, thus producing small q wavesin leads II, III, and aVF. Thereafter, the impulse movesthrough the electrically predominant left ventricle inan inferior and rightward direction, thus explaining theS waves in leads I and aVL as well as the R waves inleads II, III, and aVF. (NCI)Left Posterior Fascicular BlockC92231LEFT VENTRICULARCONDUCTION DELAYLeft VentricularConductionDelayAn electrocardiographic finding in which there isevidence that electrical transmission through the leftventricle is impaired.Left Ventricular Conduction DelayC71076LEFT VENTRICULARHYPERTROPHYLeft VentricularHypertrophyElectrocardiographic findings suggestive of ahypertrophic left ventricle. (NCI)EKG Finding Left VentricularHypertrophyC102655LEFT VENTRICULARHYPERTROPHY WITHSTRAINLeft VentricularHypertrophyWith StrainAn electrocardiographic finding suggestive of ahypertrophied left ventricle, characterized by largeQRS amplitudes, ST depression and T wave inversion.Left Ventricular Hypertrophy withStrain by ECG FindingC71078 LOW QRS VOLTAGE Low QRS voltage An electrocardiographic finding of a QRS amplitudeless than or equal to 5mm in the limb leads or QRSamplitude less than or equal to 10mm in the precordialleads. (NCI)Low QRS VoltageC71050MULTIFOCAL ATRIALTACHYCARDIAMultifocal atrialtachycardiaAn electrocardiographic finding in which the electricalaxis of the p wave is variable and the rate is greaterthan 100 beats per minute. To meet criteria formultifocal atrial tachycardia, the p wave must exhibitthree distinct morphologies on the rhythm strip. (NCI)Multifocal Atrial TachycardiaC101589MYOCARDIALINFARCTIONMyocardialInfarctionAn electrocardiographic finding showing an injurywithout specifying the anatomic region of the heart.Myocardial Infarction byElectrocardiographic FindingC102675 MYOCARDIAL ISCHEMIA MyocardialIschemiaAn electrocardiographic finding of ST and T wavechanges consistent with impaired myocardialperfusion.Myocardial Ischemia by ECGFindingC102732NEW ANTERIOR WALLMYOCARDIALINFARCTIONNew AnteriorWall MyocardialInfarctionA new electrocardiographic finding showing an injuryin leads corresponding to the anatomic region of theanterior wall of the heart.New Anterior Myocardial Infarctionby ECG FindingC102733NEW ANTEROLATERALWALL MYOCARDIALINFARCTIONNewAnterolateralWall MyocardialInfarctionA new electrocardiographic finding showing an injuryin leads corresponding to the anatomic region of theanterolateral wall of the heart.New Anterolateral MyocardialInfarction by ECG FindingC102734NEW ANTEROSEPTALWALL MYOCARDIALINFARCTIONNewAnteroseptalWall MyocardialInfarctionA new electrocardiographic finding showing an injuryin leads corresponding to the anatomic region of theanteroseptal wall of the heart.New Anteroseptal MyocardialInfarction by ECG FindingC102735NEW EXTENSIVEANTERIOR WALLMYOCARDIALINFARCTIONNew ExtensiveAnterior WallMyocardialInfarctionA new electrocardiographic finding showing extensiveinjury in leads corresponding to the anatomic region ofthe anterior wall of the heart.New Extensive Anterior MyocardialInfarction by ECG FindingC102736NEW HIGH LATERALWALL MYOCARDIALINFARCTIONNew HighLateral WallMyocardialInfarctionA new electrocardiographic finding showing an injuryin leads corresponding to the anatomic region of thehigh lateral wall of the heart.New High Lateral MyocardialInfarction by ECG FindingSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 51 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102737NEW INFERIOR WALLMYOCARDIALINFARCTIONNew InferiorWall MyocardialInfarctionA new electrocardiographic finding showing an injuryin leads corresponding to the anatomic region of theinferior wall of the heart.New Inferior Myocardial Infarctionby ECG FindingC102738NEW LATERAL WALLMYOCARDIALINFARCTIONNew LateralWall MyocardialInfarctionA new electrocardiographic finding showing an injuryin leads corresponding to the anatomic region of thelateral wall of the heart.New Lateral Myocardial Infarctionby ECG FindingC102731NEW MYOCARDIALINFARCTIONNew MyocardialInfarctionA new electrocardiographic finding showing an injuryin leads without specifying the anatomic region of theheart.New Myocardial Infarction by ECGFindingC102739NEW SEPTAL WALLMYOCARDIALINFARCTIONNew Septal WallMyocardialInfarctionA new electrocardiographic finding showing an injuryin leads corresponding to the anatomic region of theseptal wall of the heart.New Septal Myocardial Infarction byECG FindingC71080NON Q WAVEMYOCARDIALINFARCTIONNon Q WaveMyocardialInfarctionA myocardial infarction in the absence of observable Qwave abnormalities in the ECG. (NCI)Non Q Wave Myocardial InfarctionC71031NON-SPECIFIC ST-TCHANGESNon-specificST-T changesAn electrocardiographic finding of changes in the STsegment and T wave that do not meet criteria forischemia or infarction. (NCI)Non-Specific ST-T ChangesC102680NON-SUSTAINED ATRIALTACHYCARDIANon-SustainedAtrialTachycardiaAn electrocardiographic finding of an atrial rategreater than 100 beats per minute, P wave morphologywhich is not consistent with sinus rhythm, and whichterminates in less than 30 seconds.Non-sustained Atrial TachycardiaC71053NON-SUSTAINEDVENTRICULARTACHYCARDIANon-sustainedventriculartachycardiaAn electrocardiographic finding of ventriculartachycardia less than 30 seconds in duration. (NCI)Non-Sustained VentricularTachycardiaC102681 NORMAL SINUS RHYTHM Normal SinusRhythmAn electrocardiographic finding of an atrial rhythmwhich originates from the sinoatrial node that isconsidered normal for the population.Normal Sinus RhythmC102634 NORTHWEST AXIS Northwest Axis Q axis from -90 to +180 degrees. Extreme Right Axis DeviationC71032 NOTCHED T-WAVES Notched T-waves Any depression occurring in a positive t wave. (NCI) Notched T-WavesC102684OLD OR AGEINDETERMINATEANTERIOR WALLMYOCARDIALINFARCTIONOld Or AgeIndeterminateAnterior WallMyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the anterior wall of the heart.Old or Age Indeterminate AnteriorMyocardial Infarction by ECGFindingC102685OLD OR AGEINDETERMINATEANTEROLATERAL WALLMYOCARDIALINFARCTIONOld Or AgeIndeterminateAnterolateralWall MyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the anterolateral wall of the heart.Old or Age IndeterminateAnterolateral Myocardial Infarctionby ECG FindingC102686OLD OR AGEINDETERMINATEANTEROSEPTAL WALLMYOCARDIALINFARCTIONOld Or AgeIndeterminateAnteroseptalWall MyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the anteroseptal wall of the heart.Old or Age IndeterminateAnteroseptal Myocardial Infarctionby ECG FindingSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 52 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102687OLD OR AGEINDETERMINATEEXTENSIVE ANTERIORWALL MYOCARDIALINFARCTIONOld Or AgeIndeterminateExtensiveAnterior WallMyocardialInfarctionAn electrocardiographic finding showing extensiveinjury of indeterminate age in leads corresponding tothe anatomic region of the anterior wall of the heart.Old or Age Indeterminate ExtensiveAnterior Myocardial Infarction byECG FindingC102688OLD OR AGEINDETERMINATE HIGHLATERAL WALLMYOCARDIALINFARCTIONOld Or AgeIndeterminateHigh LateralWall MyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the high lateral wall of the heart.Old or Age Indeterminate HighLateral Myocardial Infarction byECG FindingC102689OLD OR AGEINDETERMINATEINFERIOR WALLMYOCARDIALINFARCTIONOld Or AgeIndeterminateInferior WallMyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the inferior wall of the heart.Old or Age Indeterminate InferiorMyocardial Infarction by ECGFindingC102690OLD OR AGEINDETERMINATE LATERALWALL MYOCARDIALINFARCTIONOld Or AgeIndeterminateLateral WallMyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the lateral wall of the heart.Old or Age Indeterminate LateralMyocardial Infarction by ECGFindingC102691OLD OR AGEINDETERMINATEPOSTERIOR WALLMYOCARDIALINFARCTIONOld Or AgeIndeterminatePosterior WallMyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the posterior wall of the heart.Old or Age Indeterminate PosteriorMyocardial Infarction by ECGFindingC102692OLD OR AGEINDETERMINATE RIGHTVENTRICULAR WALLMYOCARDIALINFARCTIONOld Or AgeIndeterminateRight VentricularWall MyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the right ventricular wall of theheart.Old or Age Indeterminate RightVentricular Myocardial Infarction byECG FindingC102693OLD OR AGEINDETERMINATE SEPTALWALL MYOCARDIALINFARCTIONOld Or AgeIndeterminateSeptal WallMyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the septal wall of the heart.Old or Age Indeterminate SeptalMyocardial Infarction by ECGFindingC101597OLD OR AGEINDETERMINATE WALLMYOCARDIALINFARCTIONOld Or AgeIndeterminateWall MyocardialInfarctionAn electrocardiographic finding showing an injury ofindeterminate age in leads corresponding to theanatomic region of the wall of the heart.Old Myocardial Infarction by EKGFindingC71064OUTFLOW TRACTVENTRICULARTACHYCARDIAOutflow tractventriculartachycardiaRight ventricular outflow tract tachycardia (RVOT) isthe most common with a female predominance. It isoften exercise induced or enhanced. The site of originis the right ventricular outflow tract and hence theelectrocardiographic appearance is that of left bundlebranch block and inferior axis. The mechanism isthought to be abnormal automaticity that is sensitive toadenosine. (NCI)Right Ventricular Outflow TractTachycardiaC90430 P WAVE ABNORMALITY P WaveAbnormalityAn electrocardiographic finding for the P wave that isatypical either for the shape, duration, amplitude, axisor polarity. Abnormality of the P wave signifiesaberrant propagation of the electrical impulse throughthe atria. (NCI)P Wave AbnormalitySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 53 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90431 P WAVE NOTCHED P Wave Notched P waves with two peaks longer in duration than normaland amplitude greater than normal. (NCI)P Wave NotchedC92232PACED ATRIAL ANDVENTRICULAR RHYTHMPaced Atrial AndVentricularRhythmAn electrocardiographic finding in which both theatrial and ventricular rhythm is controlled by anelectrical impulse from a mechanical cardiacpacemaker.Paced Atrial And Ventricular RhythmC92233 PACED ATRIAL RHYTHM Paced AtrialRhythmC88140 PACED RHYTHM Paced Rhythm;Atrial and/orVentricularPaced RhythmAn electrocardiographic finding in which the atrialrhythm is controlled by an electrical impulse from amechanical cardiac pacemaker.An electrocardiographic finding in which the cardiacrhythm is controlled by an electrical impulse from amechanical cardiac pacemaker.Paced Atrial RhythmPaced RhythmC92234PACED VENTRICULARRHYTHMPacedVentricularRhythmAn electrocardiographic finding in which theventricular rhythm is controlled by an electricalimpulse from a mechanical cardiac pacemaker.Paced Ventricular RhythmC62250 PAROXYSMAL AV BLOCK Paroxysmal AVblockSudden onset AV block, often associated withpreexisting conduction disorders. (NCI)Paroxysmal Atrioventricular BlockC34902PAROXYSMALVENTRICULARTACHYCARDIAParoxysmalVentricularTachycardiaAn episodic form of ventricular tachycardia, withabrupt onset and termination. (NCI)Paroxysmal Ventricular TachycardiaC71033POOR R WAVEPROGRESSIONPoor R WaveProgressionLack of progression of R wave height acrossprecordial leads. (NCI)Poor R Wave ProgressionC71081POSTERIOR WALLMYOCARDIALINFARCTIONPosterior WallMyocardialInfarctionElectrocardiographic finding showing an injury inleads corresponding to the anatomic region of theposterior wall of the heart. (NCI)Posterior Wall MyocardialInfarctionC62247 PR PROLONGATION PR prolongation A PR interval of more than 0.20 seconds in adults.(NCI)C34940 PRE-EXCITATION Pre-excitation A condition characterized by the activation of thewhole or some part of the ventricle by thetransmission of an atrial impulse along an accessorypathway rather than by way of the normal specificconduction system which includes a delay at theatrioventricular node. (NCI)Prolonged PR IntervalPre-Excitation SyndromeC62257PREMATURE ATRIALCOMPLEXESPremature atrialcomplexesEctopic impulses originating in the atria. (NCI)Atrial Premature ComplexC102603PREMATURE ATRIALCOMPLEXES BLOCKEDPremature AtrialComplexesBlockedAn electrocardiographic finding of premature atrialcomplexes which are not conducted to the ventricle,and which are not followed by a QRS complex.Blocked Atrial Premature ComplexC102672PREMATURE ATRIALCOMPLEXES MULTIFOCALPremature AtrialComplexesMultifocalAn electrocardiographic finding of premature atrialcomplexes which have 2 or more distinctmorphologies, suggesting origin at more than oneatrial site.Multifocal Atrial PrematureComplexC102724PREMATURE ATRIALCOMPLEXES UNIFOCALPremature AtrialComplexesUnifocalAn electrocardiographic finding of premature atrialcomplexes which have a single distinct morphology,suggesting origin at one atrial site.Unifocal Atrial Premature ComplexC62256PREMATUREVENTRICULAR COMPLEXPVC Ectopic impulses originating in the ventricles. (NCI) Ventricular Premature ComplexSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 54 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102604PREMATUREVENTRICULARCOMPLEXESINTERPOLATEDPrematureVentricularComplexesInterpolatedAn electrocardiographic finding of a prematureventricular complex which occurs between two normalQRS complexes which have normal timing.Blocked Ventricular PrematureComplexC102673PREMATUREVENTRICULARCOMPLEXES MULTIFOCALPrematureVentricularComplexesMultifocalAn electrocardiographic finding of prematureventricular complexes which have two or more distinctmorphologies, suggesting origin at more than oneventricular site.Multifocal Ventricular PrematureComplexC102725PREMATUREVENTRICULARCOMPLEXES UNIFOCALPrematureVentricularComplexesUnifocalAn electrocardiographic finding of prematureventricular complexes which have a single distinctmorphology, suggesting origin at one ventricular site.Unifocal Ventricular PrematureComplexC71034 PROLONGED QT Prolonged QT A QT interval that is prolonged. The length of the QTinterval varies with the heart rate. (NCI)Prolonged QT IntervalC71094Q AXIS, LEFT AXISDEVIATIONQ Axis, Left axisdeviationQ axis from -30 to -90 degrees. (NCI)Q Axis Left Axis DeviationC71095Q AXIS, RIGHT AXISDEVIATIONQ Axis, Rightaxis deviationQ axis from +90 to +180 degrees. (NCI)Q Axis Right Axis DeviationC90440QRS COMPLEXABNORMALITYQRS ComplexAbnormalityAn electrocardiographic finding for the QRS complexthat is atypical either for the shape, duration,amplitude, axis or polarity. (NCI)QRS Complex AbnormalityC90441 QRS COMPLEX ABSENT QRS ComplexAbsentC83817 QTC PROLONGATION QTcProlongationA disturbance in the depolarization of the ventricularmyocardium which leads to an absence of the QRScomplex in the electrocardiogram tracing. (NCI)An electrocardiographic finding of a QT intervalcorrected for heart rate that is prolonged.QRS Complex AbsentCorrected Prolonged QT IntervalC71096QTCB PROLONGATION>500 MSECQTcBprolongation>500 msecCorrected QT interval longer than 500 msec, inaccordance with Bazett's correction formula. (NCI)QTcB Prolongation Greater Than500 msecC71097QTCF PROLONGATION>500 MSECQTcFprolongation>500 msecCorrected QT interval greater than 500 msec, inaccordance with Fridericia's correction formula. (NCI)QTcF Prolongation Greater Than 500msecC61395 R ON T PHENOMENON R on TphenomenonC90443 R WAVE ABNORMALITY R WaveAbnormalityAn electrocardiographic finding phenomena in whichthe R wave occurs on top of the T wave; this can triggerventricular fibrillation or ventricular tachycardia.(NCI)An abnormal R wave that is part of the QRS complex inan electrocardiographic recording, which occurs dueto a disturbance in the depolarization mechanism ofthe ventricular myocardium. (NCI)R On T PhenomenonR Wave AbnormalityC90444 R WAVE NOTCHED R Wave Notched An R wave variant in which there is a small deflectionof the R wave, with changing polarity, within the QRScomplex. (NCI)R Wave NotchedC102574REPOLARIZATIONABNORMALITYRepolarizationAbnormalityAn electrocardiographic finding of an abnormality of Twave duration or morphology or of earlyrepolarization.Ventricular RepolarizationAbnormalitySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 55 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102706REPOLARIZATIONABNORMALITYSECONDARY TOVENTRICULARHYPERTROPHYRepolarizationAbnormalitySecondary ToVentricularHypertrophyAn electrocardiographic finding of ST depression andT wave inversion in the presence of increased QRSamplitude which are thought to be due to leftventricular hypertrophy.Repolarization AbnormalitySecondary To VentricularHypertrophyC90450RESPIRATORY SINUSARRHYTHMIARespiratorySinus ArrhythmiaThe inherent change of a subject's heart rate associatedwith each excursion of breath, which can be traced inan electrocardiographic recording. (NCI)Respiratory Sinus ArrhythmiaC71041RIGHT ATRIALABNORMALITYP-pulmonaleAn electrocardiographic finding suggesting underlyinghypertrophy or dilatation of the right atrium.Electrocardiographic criteria used for the diagnosis ofright atrial abnormality may include a peaked p wavegreater than 2.5 millimeters in amplitude in theinferior leads. (NCI)P-pulmonaleC62270RIGHT BUNDLE BRANCHBLOCKRight BundleBranch BlockAn impairment of transmission of the cardiacelectrical impulse along the fibers of the right bundlebranch. A "complete RBBB pattern" (with QRSduration > 0.11s) does not necessarily reflect theexistence of a total conduction block in the rightbranch. This pattern only indicates that the entire ormajor parts of both ventricles are activated by theimpulse emerging from the left branch. Thus, asignificant degree of conduction delay ("high-grade" or"incomplete RBBB") can produce a similar pattern.(NCI)Right Bundle Branch BlockC92235RIGHT VENTRICULARCONDUCTION DELAYRight VentricularConductionDelay; RightVentricularDelayAn electrocardiographic finding in which there isevidence that electrical transmission through the rightventricle is impaired.Right Ventricular Conduction DelayC71077RIGHT VENTRICULARHYPERTROPHYRight ventricularHypertrophyElectrocardiographic findings suggestive of anenlarged right ventricle. (NCI)EKG Finding Right VentricularHypertrophyC92227 RSR PRIME RSR' An electrocardiographic finding in which there are twoR waves, which are two deflections above the baselineresulting from a single ventricular depolarization. Thefirst upward deflection in the complex is the R wave.The S is the first downward deflection. A secondupward deflection is called the R-prime wave.RSR'C90451 S WAVE ABNORMALITY S WaveAbnormalityAn abnormal S wave that is part of the QRS complex inan electrocardiographic recording, which occurs dueto a disturbance in the depolarization of the ventricularmyocardium. (NCI)S Wave AbnormalityC71082SEPTAL MYOCARDIALINFARCTIONSeptalmyocardialinfarctionElectrocardiographic findings suggesting an infarctionin the anatomic location of the cardiac septum. (NCI)Septal Myocardial InfarctionC62246 SHORT PR INTERVAL Short PR interval A PR interval of less than 0.<strong>12</strong> seconds. (NCI) Short PR IntervalC102709 SHORT QTC INTERVAL Short QTcIntervalAn electrocardiographic finding of a QT intervalcorrected for heart rate that is shorter than the lowerlimit of normal.Short QTc IntervalSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 56 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62244 SICK SINUS SYNDROME Sick sinussyndromeC50553 SINOATRIAL EXIT BLOCK Sinoatrial exitblockC62242 SINUS ARREST/PAUSE Sinusarrest/pauseA constellation of signs and symptoms which mayinclude syncope, fatigue, dizziness, and alternatingperiods of bradycardia and atrial tachycardia, which iscaused by sinoatrial node dysfunction. (NCI)A blockage of electrical conduction within thesinoatrial node resulting in the failure of impulsetransmission from the sinoatrial node. (NCI)A failure of impulse formation in the sinus node. Sinusarrest is indistinguishable from third degree sinoatrialexit block on the standard ECG. (NCI)Sick Sinus SyndromeSinoatrial Node Exit BlockSinus ArrestC62239 SINUS ARRHYTHMIA Sinus arrhythmia Irregularity of the heartbeat due to a variation in thesinus rhythm resulting in cyclic changes in the heartrate during breathing. This rhythm is most commonlyseen with breathing due to fluctuations inparasympathetic vagal tone. During inspiration stretchreceptors in the lungs stimulate the cardioinhibitorycenters in the medulla via fibers in the vagus nerve.Irregularity of the heartbeat due to a variation in thesinus rhythm resulting in cyclic changes in the heartrate during breathing. This rhythm is most commonlyseen with breathing due to fluctuations inparasympathetic vagal tone. During inspiration stretchreceptors in the lungs stimulate the cardioinhibitorycenters in the medulla via fibers in the vagus nerve.(NCI)C26923 SINUS BRADYCARDIA Sinus bradycardia A heart rate of less than 60 beats per minute, with itsorigin in the sinus node. (NCI)Sinus ArrhythmiaSinus BradycardiaC62243SINUS NODEDYSFUNCTION(BRADYCARDIA)Sinus nodedysfunction(bradycardia)A derangement in the normal functioning of thesinoatrial node. Typically, SA node dysfunction ismanifest as sinoatrial exit block or sinus arrest, butmay present as an absolute or relative bradycardia inthe presence of a stressor. It may be associated withbradycardia-tachycardia syndrome. (NCI)Sinus Node DysfunctionC100076 SINUS RHYTHM Sinus Rhythm An electrocardiographic finding of an atrial rhythmwhich originates from the sinoatrial node.C26889 SINUS TACHYCARDIA Sinus tachycardia A heart rate of more than 100 beats per minute, withits origin in the sinus node. (NCI)C71027 ST DEPRESSION ST depression An electrocardiographic finding of ST segmentdepression below the baseline, often described as upsloping, down sloping or horizontal. (NCI)C50540 ST ELEVATION ST elevation An electrocardiographic finding of ST segmentelevation above the baseline. (NCI)Sinus RhythmSinus TachycardiaST DepressionST ElevationC71029ST ELEVATIONPERICARDITISST elevationpericarditisAn electrocardiographic finding of ST changesconsistent with pericarditis. (NCI)ST Elevation PericarditisC71026ST SEGMENTABNORMALITYST segmentabnormalityAn electrocardiographic finding of the ST segmentelevation or depression which is not indicative ofischemia or infarction. (NCI)ST Segment AbnormalityC35061SUPRAVENTRICULARTACHYCARDIASupraventriculartachycardiaA generic expression for any tachycardia thatoriginates in the atria or the atrioventricular node.(NCI)Supraventricular TachycardiaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 57 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71052SUSTAINED VENTRICULARTACHYCARDIASustainedventriculartachycardiaAn electrocardiographic finding of ventriculartachycardia greater than 30 seconds in duration. (NCI)Sustained Ventricular TachycardiaC71083 T WAVE ABNORMALITY T waveabnormalityC102718 T WAVE ALTERNANS T WaveAlternansAn electrocardiographic finding of a T wave whichappears peaked, inverted, flattened or biphasic. (NCI)An electrocardiographic finding in which there arevariations in the shape, amplitude, or direction of the Twave from one beat to the next.T Wave AbnormalityT Wave AlternansC71085 T WAVE INVERSION T Wave Inversion An electrocardiographic finding of an inversion of theT wave from the expected axis. (NCI)C71086 T WAVE PEAKED T wave peaked An electrocardiographic finding in which the T waveappears increased in amplitude and cresting at a point.(NCI)C71087 T WAVES BIPHASIC T waves biphasic An electrocardiographic finding in which the T wave isasymmetric. (NCI)C71088 T WAVES FLAT T waves flat An electrocardiographic finding in which the T waveappears decreased in amplitude. (NCI)C38029 TACHYCARDIA Tachycardia An abnormally rapid heartbeat, usually applied to aheart rate above 100 per minute.T Wave InversionT Wave PeakedT Waves BiphasicT Waves FlatTachycardiaC50779 TORSADES DE POINTES Torsades depointesAn atypical rapid polymorphic ventricular tachycardiawith a characteristic rotation of the QRS complexaround the isoelectric baseline. In addition the QRScomplex displays a periodic waxing and waning ofamplitude on the electrogram. (NCI)Torsades De PointesC71055 TRIGEMINY Trigeminy An electrocardiographic finding of normal QRSfollowed by a premature ventricular contraction; arhythmic pairing of normal and abnormal beatsoriginating from the ventricles in a 1-2 ratio. (NCI)C71089 U WAVES U waves An electrocardiographic finding of a small humpfollowing the T wave occurring during the final phaseof Purkinje repolarization. (NCI)TrigeminyU WavesC71059VENTRICULARARRHYTHMIAASSOCIATED WITHBRUGADA SYNDROMEVentriculararrhythmiaassociated withBrugadasyndromePolymorphic ventricular tachycardia in the absence ofstructural heart disease, associated with a baselineECG pattern during sinus rhythm showing right bundlebranch block with ST segment elevation in leads V1through V3. It can also be characterized bydocumentation of ECG patterns associated withBrugada Syndrome, some of which may be unmaskedwhen provoked with drugs. The most common geneticmutations identified for Brugada syndrome are in thesodium channel gene SCN5A.Brugada SyndromeC34786VENTRICULARARRHYTHMIAASSOCIATED WITH LONGQT SYNDROMEVentriculararrhythmiaassociated withlong QTsyndromeLong QT Syndrome includes prolongation of thecorrected QT interval beyond 440 ms for adult males,460 ms for adult females and 50 ms in the presence ofventricular depolarization abnormalities (i.e., bundlebranch blocks or IVCB more than <strong>12</strong>0 ms. A normalQT interval in a resting ECG with a failure to shortenwith an increase in heart rate qualifies as Long QTSyndrome.Long QT SyndromeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 58 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71060VENTRICULARARRHYTHMIAASSOCIATED WITH SHORTQT SYNDROMEVentriculararrhythmiaassociated withshort QTsyndromeShort QT Syndrome is characterized by a QT intervalof less than or equal to 300 ms.Short QT SyndromeC62259 VENTRICULAR COUPLET VentricularcoupletTwo sequential ventricular premature complexes. Anotherwise normal finding, but possibly indicative ofelectrical heart disease particularly in comparison witha single ventricular premature complexes. (NCI)Ventricular CoupletC90483VENTRICULAR ESCAPEBEATVentricularEscape BeatA compensatory ectopic beat that occurs in theventricle after a pause in the ventricular rhythm, whichis due to an absence in electrical activity to theventricles. (NCI)Ventricular Escape BeatC50799VENTRICULARFIBRILLATIONVentricularfibrillationVentricular Fibrillation is characterized by rapid,usually more than 300 bpm (cycle length: 180 ms orless), grossly irregular ventricular rhythm with markedvariability in QRS cycle length, morphology, andamplitude.Ventricular FibrillationC50800 VENTRICULAR FLUTTER VentricularflutterA ventricular tachyarrhythmia characterized by a highventricular rate (180 and 250 beats per minute) with aregular rhythm. The electrocardiogram shows largeoscillating sine wave-like complexes occurring as aresult of QRS complexes and T waves being merged.The P wave is not visible. (NCI)Ventricular FlutterC71084VENTRICULARHYPERTROPHYVentricularHypertrophyElectrocardiographic findings suggestive of enlargedcardiac ventricles. (NCI)Ventricular HypertrophyC102728VENTRICULARPARASYSTOLEVentricularParasystoleAn electrocardiographic finding of normal sinusrhythm coexisting with a regular ectopic ventricularrhythm.Ventricular ParasystoleC50802VENTRICULARTACHYCARDIAVentriculartachycardiaA tachycardia arising distal to bundle of His, with arate greater than 100 beats per minute. (NCI)Ventricular TachycardiaC71056VENTRICULARTACHYCARDIA STORMVentriculartachycardiastormMultiple, temporally related, episodes of ventriculartachycardia or fibrillation, occurring three or moretimes in a 24 hour period. (NCI)Ventricular Tachycardia StormC62234VENTRICULARTACHYCARDIA,MONOMORPHICVentriculartachycardia,monomorphicA ventricular tachycardia in which the ventricularactivation sequence is constant. The morphology of theelectrocardiographic waveform is unchanging and isreferred to as monomorphic. (NCI)Monomorphic VentricularTachycardiaC62236VENTRICULARTACHYCARDIA,POLYMORPHICVentriculartachycardia,polymorphicA ventricular tachycardia in which the ventricularactivation sequence is variable. The morphology of theelectrocardiographic waveform is variable and isreferred to as polymorphic. (NCI)Polymorphic VentricularTachycardiaC71058VERAPAMIL-SENSITIVEVENTRICULARTACHYCARDIAVerapamil-sensitive ventriculartachycardiaA well-recognized condition that is believed to resultfrom triggered activity. Despite its uniform responseto verapamil, however, this uncommon form ofventricular tachycardia may not be as homogeneous acondition as first believed. (NCI)Verapamil-Sensitive VentricularTachycardiaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 59 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71150 - EGSTRESC - ECG ResultCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62240WANDERING ATRIALPACEMAKERWandering atrialpacemakerA condition characterized by the site of origin ofimpulses, controlling the heart rate, changing withinthe atria, including the sinus node. Variations within Pwaves and PR intervals occur and an irregular rate ofimpulse formation is observed. (NCI)Wandering Atrial PacemakerC71090 WIDE QRS TACHYCARDIA Wide QRStachycardiaAn electrocardiographic finding of a QRS complex.(NCI)Wide QRS TachycardiaC35132WOLFF-PARKINSON-WHITE SYNDROMEWolff-Parkinson-White syndromeA type of ventricular pre-excitation resulting from theactivation of an accessory pathway known as theBundle of Kent. This pathway presents an abnormalelectrical communication from the atria to theventricles. Wolff-Parkinson-White syndrome ischaracterized by a short PR interval and a long QRSinterval with a delta wave. (NCI)Wolff-Parkinson-White SyndromeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 60 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71152 - EGTEST - ECG Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4<strong>12</strong>55 Interpretation Interpretation An act or process of elucidation; explication, orexplanation of the meaning of the event or thing via theassignment of objects from the domain to theconstants of a formal language, truth-values to theproposition symbols, truth-functions to theconnectives, other functions to the function symbols,and extensions to the predicates, if any. Theassignments are result of human logic application andare not native to the symbols of the formal language.InterpretationC62118JTcB - Bazett's CorrectionFormulaJTcB - Bazett'sCorrectionFormulaA formula which takes into account the physiologicshortening of the JT interval which occurs as the heartrate increases, permitting comparison of the JTinterval across a range of rates. It is mathematicallydefined as: JTcB = JT/SqRootRR(seconds) andtheoretically corrects the JT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)JTcB-Bazett's Correction FormulaC62119JTcF - Fridericia's CorrectionFormulaJTcF -Fridericia'sCorrectionFormulaA formula which takes into account the physiologicshortening of the JT interval which occurs as the heartrate increases, permitting comparison of the JTinterval across a range of rates. It is mathematicallydefined as: JTcF = JT/CubeRootRR(seconds) andtheoretically corrects the JT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)JTcF-Fridericia's CorrectionFormulaC62<strong>12</strong>0JTcLC - Linear CorrectionFormulaJTcLC - LinearCorrectionFormulaA formula which takes into account the physiologicshortening of the JT interval which occurs as the heartrate increases, permitting comparison of the JTinterval across a range of rates. The linear correctionformula uses population-based data obtained andvalidated using a large cohort of subjects. Using thismethod, the corrected JT interval is independent of theventricular rate. (NCI)JTcLC-Linear Correction FormulaC100390QRS Duration VentricularPacedQRS DurationVentricularPacedThe average (mean) duration (time) of the QRSinterval, obtained from a set of measurements of theQRS interval while the ventricle rhythm is controlledby an electrical impulse from a mechanical cardiacpacemaker. The QRS interval is defined as the timefrom the beginning of the QRS complex to the end ofthe QRS complex, representing the time it takes forthe ventricles to depolarize.EKG Mean QRS DurationVentricular PacedC100391 QT Interval, Corrected QT Interval,CorrectedThe time interval between the start of the Q wave andthe end of the T wave in the cardiac cycle as correctedwith a non-specified correction formula.Corrected QT IntervalC621<strong>12</strong>QTcB - Bazett's CorrectionFormulaQTcB - Bazett'sCorrectionFormulaA formula which takes into account the physiologicshortening of the QT interval which occurs as the heartrate increases, permitting comparison of the QTinterval across a range of rates. It is mathematicallydefined as: QTcB = QT/SqRootRR(seconds) andtheoretically corrects the QT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)QTcB-Bazett's Correction FormulaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 61 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71152 - EGTEST - ECG Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62113QTcF - Fridericia's CorrectionFormulaQTcF -Fridericia'sCorrectionFormulaA formula which takes into account the physiologicshortening of the QT interval which occurs as the heartrate increases, permitting comparison of the QTinterval across a range of rates. It is mathematicallydefined as: QTcF = QT/CubeRootRR(seconds) andtheoretically corrects the QT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)QTcF-Fridericia's CorrectionFormulaC62114QTcLC - Linear CorrectionFormulaQTcLC - LinearCorrectionFormulaA formula which takes into account the physiologicshortening of the QT interval which occurs as the heartrate increases, permitting comparison of the QTinterval across a range of rates. The linear correctionformula uses population-based data obtained andvalidated using a large cohort of subjects. This methodtheoretically corrects the QT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)QTcLC-Linear Correction FormulaC90442QTcV - Van de Water'sCorrection FormulaQTcV - Van deWater'sCorrectionFormulaA mathematic formula defined as:QTcV = QT-0.087[(60/Heart Rate)-1](seconds). Itpermits comparison of the QT interval across a rangeof rates, while accounting for the progressiveshortening of the QT interval which occurs as the heartrate increases. (NCI)QTcV Van de Water's CorrectionFormulaC39779 Summary (Max) Heart Rate Summary (Max)Heart RateC62117 Summary (Max) JT Interval Summary (Max)JT IntervalC62131 Summary (Max) PR Duration Summary (Max)PR DurationC62135 Summary (Max) QT Duration Summary (Max)QT DurationThe minimum time between successive cycles ofcontraction and subsequent relaxation of the heart,usually expressed as beats per minute, obtained from aset of measurements of the heart rate. (NCI)The maximum duration (time) of the JT interval,obtained from a set of measurements of the JTinterval. The JT interval is defined as the time from theJ point (end of ventricular depolarization, the point atwhich the QRS meets the ST segment) to the end ofthe T wave (representing the end of ventricularrepolarization). (NCI)The maximum duration (time) of the PR interval,obtained from a set of measurements of the PRinterval. The PR interval is defined as the time fromthe beginning of the P wave (representing the onset ofatrial depolarization) to the beginning of the R wave(representing the onset of ventricular depolarization).In some cases, a Q wave will precede the R wave, inwhich case the PR interval is measured from thebeginning of the P wave to the beginning of the Qwave. (NCI)The maximum duration (time) of the QT interval,obtained from a set of measurements of the QTinterval. The QT interval is defined as the time fromthe beginning of the QRS complex to the end of the Twave, representing the time it takes for the ventriclesto depolarize and subsequently repolarize. In somecases, the Q wave will be absent, in which case the QTinterval is measured from the beginning of the R waveto the end of the T wave. (NCI)Maximum Heart RateEKG Maximum JT DurationEKG Maximum PR DurationEKG Maximum QT DurationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 62 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71152 - EGTEST - ECG Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62094 Summary (Max) RR Duration Summary (Max)RR DurationThe maximum duration (time) between successivepeaks of R waves in a particular set of RR intervals.(NCI)EKG Maximum RR DurationC62163Summary (Max) STDepressionSummary (Max)ST DepressionThe maximum depression (negative deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the depressionof the ST segment. (NCI)EKG Maximum ST SegmentDepressionC62157 Summary (Max) ST Deviation Summary (Max)ST DeviationC62160 Summary (Max) ST Elevation Summary (Max)ST ElevationThe maximum deviation (distance from baseline,positive or negative, usually measured in mm) of theST segment, obtained from a set of measurements ofthe deviation of the ST segment. (NCI)The maximum elevation (positive deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the elevationof the ST segment. (NCI)EKG Maximum ST DeviationEKG Maximum ST SegmentElevationC62154Summary (Max) VentricularRateSummary (Max)Ventricular RateThe maximum time between successive cycles ofcontraction and subsequent relaxation of theventricles, usually expressed as beats per minute,obtained from a set of measurements of the ventricularrate. (NCI)Maximum Ventricular Heart RateC62095 Summary (Mean) Heart Rate Summary (Mean)Heart RateC62115 Summary (Mean) JT Interval Summary (Mean)JT IntervalC62<strong>12</strong>1 Summary (Mean) P Axis Summary (Mean)P AxisThe average (mean) number of cycles of contractionand subsequent relaxation of the heart, usuallyexpressed as beats per minute, obtained from a set ofmeasurements of the heart rate. (NCI)The average (mean) duration (time) of the JT interval,obtained from a set of measurements of the JTinterval. The JT interval is defined as the time from theJ point (end of ventricular depolarization, the point atwhich the QRS meets the ST segment) to the end ofthe T wave (representing the end of ventricularrepolarization). (NCI)The mean (average) direction (range -180 degrees to180 degrees) of the electrical potential generated byatrial depolarization in a particular plane (usually thefrontal plane). (NCI)Mean Heart RateEKG Mean JT DurationEKG Mean P AxisC62<strong>12</strong>3Summary (Mean) P WaveDurationSummary (Mean)P Wave DurationThe average (mean) duration (time) from the onset ofatrial depolarization to the completion of atrialdepolarization (length of the P wave), obtained from aset of measurements of the time from beginning to endof atrial depolarization. (NCI)EKG Mean P Wave DurationC62<strong>12</strong>4Summary (Mean) P WaveHeightSummary (Mean)P Wave HeightThe average (mean) height (usually measured in mm)of the maximum deflection from baseline of the Pwave (representing atrial depolarization), obtainedfrom a set of measurements of the P wave, from asingle lead or set of leads. Typically this measurementis obtained by analysis of Lead II. (NCI)EKG Mean P Wave HeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 63 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71152 - EGTEST - ECG Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62086 Summary (Mean) PR Duration Summary (Mean)PR DurationC62132 Summary (Mean) QRS Axis Summary (Mean)QRS AxisThe average (mean) duration (time) of the PR interval,obtained from a set of measurements of the PRinterval. The PR interval is defined as the time fromthe beginning of the P wave (representing the onset ofatrial depolarization) to the beginning of the R wave(representing the onset of ventricular depolarization).In some cases, a Q wave will precede the R wave, inwhich case the PR interval is measured from thebeginning of the P wave to the beginning of the Qwave. (NCI)The mean (average) direction of the electricalpotential generated by ventricular depolarization.(NCI)EKG Mean PR DurationMean QRS AxisC62087Summary (Mean) QRSDurationSummary (Mean)QRS DurationThe average (mean) duration (time) of the QRSinterval, obtained from a set of measurements of theQRS interval. The QRS interval is defined as the timefrom the beginning of the QRS complex to the end ofthe QRS complex, representing the time it takes forthe ventricles to depolarize. (NCI)EKG Mean QRS DurationC62089 Summary (Mean) QT Duration Summary (Mean)QT DurationThe average (mean) duration (time) of the QT interval,obtained from a set of measurements of the QTinterval. The QT interval is defined as the time fromthe beginning of the QRS complex to the end of the Twave, representing the time it takes for the ventriclesto depolarize and subsequently repolarize. In somecases, the Q wave will be absent, in which case the QTinterval is measured from the beginning of the R waveto the end of the T wave. (NCI)EKG Mean QT DurationC62136Summary (Mean) R WaveAmplitudeSummary (Mean)R WaveAmplitudeThe average (mean) amplitude (usually in mm) of the Rwave, obtained from a set of measurements of the Rwave, in a particular lead or set of leads. (NCI)EKG Mean R Wave AmplitudeC62144Summary (Mean) R+SAmplitudeSummary (Mean)R+S AmplitudeThe average (mean) amplitude (usually in mm) of thesummation of the R and S waves, obtained from a setof measurements of the R and S waves, in a particularlead or set of leads. (NCI)EKG Mean R+S AmplitudeC62090 Summary (Mean) RR Duration Summary (Mean)RR DurationThe average (mean) duration (time) of the RR interval,obtained from a set of measurements of the RRinterval. The RR interval is defined as the time betweensuccessive peaks of the R wave and can be used tomeasure the ventricular rate. (NCI)EKG Mean RR DurationC62137Summary (Mean) S WaveAmplitudeSummary (Mean)S WaveAmplitudeThe average (mean) amplitude (usually in mm) of the Swave, obtained from a set of measurements of the Swave, in a particular lead or set of leads. (NCI)EKG Mean S Wave AmplitudeC62161Summary (Mean) STDepressionSummary (Mean)ST DepressionThe average (mean) depression (negative deflectionfrom baseline, usually measured in mm) of the STsegment, obtained from a set of measurements of thedepression of the ST segment. (NCI)EKG Mean ST Segment DepressionC62155Summary (Mean) STDeviationSummary (Mean)ST DeviationThe average (mean) deviation (distance from baseline,positive or negative, usually measured in mm) of theST segment, obtained from a set of measurements ofthe deviation of the ST segment. (NCI)EKG Mean ST DeviationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 64 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71152 - EGTEST - ECG Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62158 Summary (Mean) ST Elevation Summary (Mean)ST ElevationThe average (mean) elevation (positive deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the elevationof the ST segment. (NCI)EKG Mean ST Segment ElevationC62145Summary (Mean) ST SegmentDurationSummary (Mean)ST SegmentDurationThe average (mean) duration (time) from the end ofventricular depolarization (end of QRS complex) tothe end of ventricular repolarization (end of the Twave). (NCI)EKG Mean ST Segment DurationC62148 Summary (Mean) T Wave Area Summary (Mean)T Wave AreaC62146 Summary (Mean) T Wave Axis Summary (Mean)T Wave AxisThe average (mean) area under the curve of thedeflection from baseline of the T wave (representingventricular repolarization), obtained from a set ofmeasurements of the T wave, from a single lead or setof leads. (NCI)The mean (average) direction of the electricalpotential generated by ventricular repolarization. (NCI)EKG Mean T Wave AreaEKG Mean T Wave AxisC62147Summary (Mean) T WaveDurationSummary (Mean)T Wave DurationThe average (mean) duration (time) from the onset ofventricular repolarization to the completion ofventricular repolarization (length of the T wave),obtained from a set of measurements of the time frombeginning to end of ventricular repolarization. (NCI)EKG Mean T Wave DurationC62149Summary (Mean) T WaveHeightSummary (Mean)T Wave HeightThe average (mean) height (usually measured in mm)of the maximum deflection from baseline of the Twave (representing ventricular repolarization),obtained from a set of measurements of the T wave,from a single lead or set of leads. (NCI)EKG Mean T Wave HeightC62152Summary (Mean) VentricularRateSummary (Mean)Ventricular RateThe average (mean) number of cycles of contractionand subsequent relaxation of the ventricles, usuallyexpressed as beats per minute, obtained from a set ofmeasurements of the ventricular rate. (NCI)Mean Ventricular Heart RateC102249 Summary (Median) Heart Rate Summary(Median) HeartRateThe median number of cycles of contraction andsubsequent relaxation of the heart, usually expressedas beats per minute, obtained from a set ofmeasurements of the heart rate.Median Heart RateC102250Summary (Median) PRDurationSummary(Median) PRDurationThe median duration (time) of the PR interval,obtained from a set of measurements of the PRinterval. The PR interval is defined as the time fromthe beginning of the P wave (representing the onset ofatrial depolarization) to the beginning of the R wave(representing the onset of ventricular depolarization).In some cases, a Q wave will precede the R wave, inwhich case the PR interval is measured from thebeginning of the P wave to the beginning of the Qwave.Median PR DurationC102251Summary (Median) QRSDurationSummary(Median) QRSDurationThe median duration (time) of the QRS interval,obtained from a set of measurements of the QRSinterval. The QRS interval is defined as the time fromthe beginning of the QRS complex to the end of theQRS complex, representing the time it takes for theventricles to depolarize.Median QRS DurationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 65 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71152 - EGTEST - ECG Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102252Summary (Median) QTDurationSummary(Median) QTDurationThe median duration (time) of the QT interval,obtained from a set of measurements of the QTinterval. The QT interval is defined as the time fromthe beginning of the QRS complex to the end of the Twave, representing the time it takes for the ventriclesto depolarize and subsequently repolarize. In somecases, the Q wave will be absent, in which case the QTinterval is measured from the beginning of the R waveto the end of the T wave.Median QT DurationC102253 Summary (Median) QTcF Summary(Median) QTcFThe median duration (time) of the QTc interval,obtained from a set of measurements of the QTinterval and corrected using the Fridericia's correctionFormula.Median QTcFC102254Summary (Median) RRDurationSummary(Median) RRDurationThe median duration (time) of the RR interval,obtained from a set of measurements of the RRinterval. The RR interval is defined as the time betweensuccessive peaks of the R wave and can be used tomeasure the ventricular rate.Median RR DurationC62096 Summary (Min) Heart Rate Summary (Min)Heart RateC62116 Summary (Min) JT Interval Summary (Min)JT IntervalC62<strong>12</strong>5 Summary (Min) PR Duration Summary (Min)PR DurationC62133 Summary (Min) QT Duration Summary (Min)QT DurationC62093 Summary (Min) RR Duration Summary (Min)RR DurationThe maximum time between successive cycles ofcontraction and subsequent relaxation of the heart,usually expressed as beats per minute, obtained from aset of measurements of the heart rate. (NCI)The minimum duration (time) of the JT interval,obtained from a set of measurements of the JTinterval. The JT interval is defined as the time from theJ point (end of ventricular depolarization, the point atwhich the QRS meets the ST segment) to the end ofthe T wave (representing the end of ventricularrepolarization). (NCI)The minimum duration (time) of the PR interval,obtained from a set of measurements of the PRinterval. The PR interval is defined as the time fromthe beginning of the P wave (representing the onset ofatrial depolarization) to the beginning of the R wave(representing the onset of ventricular depolarization).In some cases, a Q wave will precede the R wave, inwhich case the PR interval is measured from thebeginning of the P wave to the beginning of the Qwave. (NCI)The minimum duration (time) of the QT interval,obtained from a set of measurements of the QTinterval. The QT interval is defined as the time fromthe beginning of the QRS complex to the end of the Twave, representing the time it takes for the ventriclesto depolarize and subsequently repolarize. In somecases, the Q wave will be absent, in which case the QTinterval is measured from the beginning of the R waveto the end of the T wave. (NCI)The minimum duration (time) between successivepeaks of R waves in a particular set of RR intervals.(NCI)Minimum Heart RateEKG Minimum JT DurationEKG Minimum PR DurationMinimum QT DurationEKG Minimum RR DurationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 66 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71152 - EGTEST - ECG Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62162Summary (Min) STDepressionSummary (Min)ST DepressionThe minimum depression (negative deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the depressionof the ST segment. (NCI)EKG Minimum ST SegmentDepressionC62156 Summary (Min) ST Deviation Summary (Min)ST DeviationC62159 Summary (Min) ST Elevation Summary (Min)ST ElevationThe minimum deviation (distance from baseline,positive or negative, usually measured in mm) of theST segment, obtained from a set of measurements ofthe deviation of the ST segment. (NCI)The minimum elevation (positive deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the elevationof the ST segment. (NCI)EKG Minimum ST DeviationEKG Minimum ST SegmentElevationC62153Summary (Min) VentricularRateSummary (Min)Ventricular RateThe minimum time between successive cycles ofcontraction and subsequent relaxation of theventricles, usually expressed as beats per minute,obtained from a set of measurements of the ventricularrate. (NCI)Minimum Ventricular Heart RateSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 67 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71153 - EGTESTCD - ECG Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC39779 HRMAX Summary (Max)Heart RateC62095 HRMEAN Summary (Mean)Heart RateC102249 HRMED Summary(Median) HeartRateC62096 HRMIN Summary (Min)Heart RateThe minimum time between successive cycles ofcontraction and subsequent relaxation of the heart,usually expressed as beats per minute, obtained from aset of measurements of the heart rate. (NCI)The average (mean) number of cycles of contractionand subsequent relaxation of the heart, usuallyexpressed as beats per minute, obtained from a set ofmeasurements of the heart rate. (NCI)The median number of cycles of contraction andsubsequent relaxation of the heart, usually expressedas beats per minute, obtained from a set ofmeasurements of the heart rate.The maximum time between successive cycles ofcontraction and subsequent relaxation of the heart,usually expressed as beats per minute, obtained from aset of measurements of the heart rate. (NCI)Maximum Heart RateMean Heart RateMedian Heart RateMinimum Heart RateC4<strong>12</strong>55 INTP Interpretation An act or process of elucidation; explication, orexplanation of the meaning of the event or thing via theassignment of objects from the domain to theconstants of a formal language, truth-values to theproposition symbols, truth-functions to theconnectives, other functions to the function symbols,and extensions to the predicates, if any. Theassignments are result of human logic application andare not native to the symbols of the formal language.InterpretationC62118 JTCB JTcB - Bazett'sCorrectionFormulaC62119 JTCF JTcF -Fridericia'sCorrectionFormulaC62<strong>12</strong>0 JTCLC JTcLC - LinearCorrectionFormulaA formula which takes into account the physiologicshortening of the JT interval which occurs as the heartrate increases, permitting comparison of the JTinterval across a range of rates. It is mathematicallydefined as: JTcB = JT/SqRootRR(seconds) andtheoretically corrects the JT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)A formula which takes into account the physiologicshortening of the JT interval which occurs as the heartrate increases, permitting comparison of the JTinterval across a range of rates. It is mathematicallydefined as: JTcF = JT/CubeRootRR(seconds) andtheoretically corrects the JT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)A formula which takes into account the physiologicshortening of the JT interval which occurs as the heartrate increases, permitting comparison of the JTinterval across a range of rates. The linear correctionformula uses population-based data obtained andvalidated using a large cohort of subjects. Using thismethod, the corrected JT interval is independent of theventricular rate. (NCI)JTcB-Bazett's Correction FormulaJTcF-Fridericia's CorrectionFormulaJTcLC-Linear Correction FormulaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 68 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71153 - EGTESTCD - ECG Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62117 JTMAX Summary (Max)JT IntervalC62115 JTMEAN Summary (Mean)JT IntervalC62116 JTMIN Summary (Min)JT IntervalC62<strong>12</strong>1 PAXIS Summary (Mean)P AxisC62131 PRMAX Summary (Max)PR DurationC62086 PRMEAN Summary (Mean)PR DurationC102250 PRMED Summary(Median) PRDurationThe maximum duration (time) of the JT interval,obtained from a set of measurements of the JTinterval. The JT interval is defined as the time from theJ point (end of ventricular depolarization, the point atwhich the QRS meets the ST segment) to the end ofthe T wave (representing the end of ventricularrepolarization). (NCI)The average (mean) duration (time) of the JT interval,obtained from a set of measurements of the JTinterval. The JT interval is defined as the time from theJ point (end of ventricular depolarization, the point atwhich the QRS meets the ST segment) to the end ofthe T wave (representing the end of ventricularrepolarization). (NCI)The minimum duration (time) of the JT interval,obtained from a set of measurements of the JTinterval. The JT interval is defined as the time from theJ point (end of ventricular depolarization, the point atwhich the QRS meets the ST segment) to the end ofthe T wave (representing the end of ventricularrepolarization). (NCI)The mean (average) direction (range -180 degrees to180 degrees) of the electrical potential generated byatrial depolarization in a particular plane (usually thefrontal plane). (NCI)The maximum duration (time) of the PR interval,obtained from a set of measurements of the PRinterval. The PR interval is defined as the time fromthe beginning of the P wave (representing the onset ofatrial depolarization) to the beginning of the R wave(representing the onset of ventricular depolarization).In some cases, a Q wave will precede the R wave, inwhich case the PR interval is measured from thebeginning of the P wave to the beginning of the Qwave. (NCI)The average (mean) duration (time) of the PR interval,obtained from a set of measurements of the PRinterval. The PR interval is defined as the time fromthe beginning of the P wave (representing the onset ofatrial depolarization) to the beginning of the R wave(representing the onset of ventricular depolarization).In some cases, a Q wave will precede the R wave, inwhich case the PR interval is measured from thebeginning of the P wave to the beginning of the Qwave. (NCI)The median duration (time) of the PR interval,obtained from a set of measurements of the PRinterval. The PR interval is defined as the time fromthe beginning of the P wave (representing the onset ofatrial depolarization) to the beginning of the R wave(representing the onset of ventricular depolarization).In some cases, a Q wave will precede the R wave, inwhich case the PR interval is measured from thebeginning of the P wave to the beginning of the Qwave.EKG Maximum JT DurationEKG Mean JT DurationEKG Minimum JT DurationEKG Mean P AxisEKG Maximum PR DurationEKG Mean PR DurationMedian PR DurationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 69 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71153 - EGTESTCD - ECG Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62<strong>12</strong>5 PRMIN Summary (Min)PR DurationC62<strong>12</strong>3 PWAVEDUR Summary (Mean)P Wave DurationC62<strong>12</strong>4 PWAVEHT Summary (Mean)P Wave HeightC62132 QRSAXIS Summary (Mean)QRS AxisC62087 QRSDUR Summary (Mean)QRS DurationC100390 QRSDURVP QRS DurationVentricularPacedC102251 QRSMED Summary(Median) QRSDurationC100391 QTC QT Interval,CorrectedThe minimum duration (time) of the PR interval,obtained from a set of measurements of the PRinterval. The PR interval is defined as the time fromthe beginning of the P wave (representing the onset ofatrial depolarization) to the beginning of the R wave(representing the onset of ventricular depolarization).In some cases, a Q wave will precede the R wave, inwhich case the PR interval is measured from thebeginning of the P wave to the beginning of the Qwave. (NCI)The average (mean) duration (time) from the onset ofatrial depolarization to the completion of atrialdepolarization (length of the P wave), obtained from aset of measurements of the time from beginning to endof atrial depolarization. (NCI)The average (mean) height (usually measured in mm)of the maximum deflection from baseline of the Pwave (representing atrial depolarization), obtainedfrom a set of measurements of the P wave, from asingle lead or set of leads. Typically this measurementis obtained by analysis of Lead II. (NCI)The mean (average) direction of the electricalpotential generated by ventricular depolarization.(NCI)The average (mean) duration (time) of the QRSinterval, obtained from a set of measurements of theQRS interval. The QRS interval is defined as the timefrom the beginning of the QRS complex to the end ofthe QRS complex, representing the time it takes forthe ventricles to depolarize. (NCI)The average (mean) duration (time) of the QRSinterval, obtained from a set of measurements of theQRS interval while the ventricle rhythm is controlledby an electrical impulse from a mechanical cardiacpacemaker. The QRS interval is defined as the timefrom the beginning of the QRS complex to the end ofthe QRS complex, representing the time it takes forthe ventricles to depolarize.The median duration (time) of the QRS interval,obtained from a set of measurements of the QRSinterval. The QRS interval is defined as the time fromthe beginning of the QRS complex to the end of theQRS complex, representing the time it takes for theventricles to depolarize.The time interval between the start of the Q wave andthe end of the T wave in the cardiac cycle as correctedwith a non-specified correction formula.EKG Minimum PR DurationEKG Mean P Wave DurationEKG Mean P Wave HeightMean QRS AxisEKG Mean QRS DurationEKG Mean QRS DurationVentricular PacedMedian QRS DurationCorrected QT IntervalSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 70 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71153 - EGTESTCD - ECG Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC621<strong>12</strong> QTCB QTcB - Bazett'sCorrectionFormulaC62113 QTCF QTcF -Fridericia'sCorrectionFormulaC102253 QTCFMED Summary(Median) QTcFC62114 QTCLC QTcLC - LinearCorrectionFormulaC90442 QTCV QTcV - Van deWater'sCorrectionFormulaC62135 QTMAX Summary (Max)QT DurationC62089 QTMEAN Summary (Mean)QT DurationA formula which takes into account the physiologicshortening of the QT interval which occurs as the heartrate increases, permitting comparison of the QTinterval across a range of rates. It is mathematicallydefined as: QTcB = QT/SqRootRR(seconds) andtheoretically corrects the QT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)A formula which takes into account the physiologicshortening of the QT interval which occurs as the heartrate increases, permitting comparison of the QTinterval across a range of rates. It is mathematicallydefined as: QTcF = QT/CubeRootRR(seconds) andtheoretically corrects the QT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)The median duration (time) of the QTc interval,obtained from a set of measurements of the QTinterval and corrected using the Fridericia's correctionFormula.A formula which takes into account the physiologicshortening of the QT interval which occurs as the heartrate increases, permitting comparison of the QTinterval across a range of rates. The linear correctionformula uses population-based data obtained andvalidated using a large cohort of subjects. This methodtheoretically corrects the QT interval to that whichwould be observed at a heart rate of 1 cycle persecond. (NCI)A mathematic formula defined as:QTcV = QT-0.087[(60/Heart Rate)-1](seconds). Itpermits comparison of the QT interval across a rangeof rates, while accounting for the progressiveshortening of the QT interval which occurs as the heartrate increases. (NCI)The maximum duration (time) of the QT interval,obtained from a set of measurements of the QTinterval. The QT interval is defined as the time fromthe beginning of the QRS complex to the end of the Twave, representing the time it takes for the ventriclesto depolarize and subsequently repolarize. In somecases, the Q wave will be absent, in which case the QTinterval is measured from the beginning of the R waveto the end of the T wave. (NCI)The average (mean) duration (time) of the QT interval,obtained from a set of measurements of the QTinterval. The QT interval is defined as the time fromthe beginning of the QRS complex to the end of the Twave, representing the time it takes for the ventriclesto depolarize and subsequently repolarize. In somecases, the Q wave will be absent, in which case the QTinterval is measured from the beginning of the R waveto the end of the T wave. (NCI)QTcB-Bazett's Correction FormulaQTcF-Fridericia's CorrectionFormulaMedian QTcFQTcLC-Linear Correction FormulaQTcV Van de Water's CorrectionFormulaEKG Maximum QT DurationEKG Mean QT DurationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 71 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71153 - EGTESTCD - ECG Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102252 QTMED Summary(Median) QTDurationC62133 QTMIN Summary (Min)QT DurationC62094 RRMAX Summary (Max)RR DurationC62090 RRMEAN Summary (Mean)RR DurationC102254 RRMED Summary(Median) RRDurationC62093 RRMIN Summary (Min)RR DurationC62144 RSAMP Summary (Mean)R+S AmplitudeC62136 RWAVEAMP Summary (Mean)R WaveAmplitudeC62163 STDPMAX Summary (Max)ST DepressionC62161 STDPMEAN Summary (Mean)ST DepressionC62162 STDPMIN Summary (Min)ST DepressionThe median duration (time) of the QT interval,obtained from a set of measurements of the QTinterval. The QT interval is defined as the time fromthe beginning of the QRS complex to the end of the Twave, representing the time it takes for the ventriclesto depolarize and subsequently repolarize. In somecases, the Q wave will be absent, in which case the QTinterval is measured from the beginning of the R waveto the end of the T wave.The minimum duration (time) of the QT interval,obtained from a set of measurements of the QTinterval. The QT interval is defined as the time fromthe beginning of the QRS complex to the end of the Twave, representing the time it takes for the ventriclesto depolarize and subsequently repolarize. In somecases, the Q wave will be absent, in which case the QTinterval is measured from the beginning of the R waveto the end of the T wave. (NCI)The maximum duration (time) between successivepeaks of R waves in a particular set of RR intervals.(NCI)The average (mean) duration (time) of the RR interval,obtained from a set of measurements of the RRinterval. The RR interval is defined as the time betweensuccessive peaks of the R wave and can be used tomeasure the ventricular rate. (NCI)The median duration (time) of the RR interval,obtained from a set of measurements of the RRinterval. The RR interval is defined as the time betweensuccessive peaks of the R wave and can be used tomeasure the ventricular rate.The minimum duration (time) between successivepeaks of R waves in a particular set of RR intervals.(NCI)The average (mean) amplitude (usually in mm) of thesummation of the R and S waves, obtained from a setof measurements of the R and S waves, in a particularlead or set of leads. (NCI)The average (mean) amplitude (usually in mm) of the Rwave, obtained from a set of measurements of the Rwave, in a particular lead or set of leads. (NCI)The maximum depression (negative deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the depressionof the ST segment. (NCI)The average (mean) depression (negative deflectionfrom baseline, usually measured in mm) of the STsegment, obtained from a set of measurements of thedepression of the ST segment. (NCI)The minimum depression (negative deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the depressionof the ST segment. (NCI)Median QT DurationMinimum QT DurationEKG Maximum RR DurationEKG Mean RR DurationMedian RR DurationEKG Minimum RR DurationEKG Mean R+S AmplitudeEKG Mean R Wave AmplitudeEKG Maximum ST SegmentDepressionEKG Mean ST Segment DepressionEKG Minimum ST SegmentDepressionSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 72 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71153 - EGTESTCD - ECG Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62157 STDVMAX Summary (Max)ST DeviationC62155 STDVMEAN Summary (Mean)ST DeviationC62156 STDVMIN Summary (Min)ST DeviationC62160 STELMAX Summary (Max)ST ElevationC62158 STELMEAN Summary (Mean)ST ElevationC62159 STELMIN Summary (Min)ST ElevationC62145 STSEGDUR Summary (Mean)ST SegmentDurationC62137 SWAVEAMP Summary (Mean)S WaveAmplitudeC62146 TAXIS Summary (Mean)T Wave AxisC62148 TWAVAREA Summary (Mean)T Wave AreaC62147 TWAVEDUR Summary (Mean)T Wave DurationC62149 TWAVEHT Summary (Mean)T Wave HeightThe maximum deviation (distance from baseline,positive or negative, usually measured in mm) of theST segment, obtained from a set of measurements ofthe deviation of the ST segment. (NCI)The average (mean) deviation (distance from baseline,positive or negative, usually measured in mm) of theST segment, obtained from a set of measurements ofthe deviation of the ST segment. (NCI)The minimum deviation (distance from baseline,positive or negative, usually measured in mm) of theST segment, obtained from a set of measurements ofthe deviation of the ST segment. (NCI)The maximum elevation (positive deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the elevationof the ST segment. (NCI)The average (mean) elevation (positive deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the elevationof the ST segment. (NCI)The minimum elevation (positive deflection frombaseline, usually measured in mm) of the ST segment,obtained from a set of measurements of the elevationof the ST segment. (NCI)The duration (time) of the ST segment, measured fromthe J point at the end of the QRS complex to thebeginning of the T wave. (NCI)The average (mean) amplitude (usually in mm) of the Swave, obtained from a set of measurements of the Swave, in a particular lead or set of leads. (NCI)The mean (average) direction of the electricalpotential generated by ventricular repolarization. (NCI)The average (mean) area under the curve of thedeflection from baseline of the T wave (representingventricular repolarization), obtained from a set ofmeasurements of the T wave, from a single lead or setof leads. (NCI)The average (mean) duration (time) from the onset ofventricular repolarization to the completion ofventricular repolarization (length of the T wave),obtained from a set of measurements of the time frombeginning to end of ventricular repolarization. (NCI)The average (mean) height (usually measured in mm)of the maximum deflection from baseline of the Twave (representing ventricular repolarization),obtained from a set of measurements of the T wave,from a single lead or set of leads. (NCI)EKG Maximum ST DeviationEKG Mean ST DeviationEKG Minimum ST DeviationEKG Maximum ST SegmentElevationEKG Mean ST Segment ElevationEKG Minimum ST SegmentElevationEKG Mean ST Segment DurationEKG Mean S Wave AmplitudeEKG Mean T Wave AxisEKG Mean T Wave AreaEKG Mean T Wave DurationEKG Mean T Wave HeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 73 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71153 - EGTESTCD - ECG Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62154 VRMAX Summary (Max)Ventricular RateC62152 VRMEAN Summary (Mean)Ventricular RateC62153 VRMIN Summary (Min)Ventricular RateThe maximum time between successive cycles ofcontraction and subsequent relaxation of theventricles, usually expressed as beats per minute,obtained from a set of measurements of the ventricularrate. (NCI)The average (mean) number of cycles of contractionand subsequent relaxation of the ventricles, usuallyexpressed as beats per minute, obtained from a set ofmeasurements of the ventricular rate. (NCI)The minimum time between successive cycles ofcontraction and subsequent relaxation of theventricles, usually expressed as beats per minute,obtained from a set of measurements of the ventricularrate. (NCI)Maximum Ventricular Heart RateMean Ventricular Heart RateMinimum Ventricular Heart RateSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 74 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71113 - FREQ - FrequencyCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64526 1 TIME PER WEEK One Time PerWeekC64497 2 TIMES PER WEEK Twice per week;BISOne time per week. (NCI)Two times per week. (NCI)Once WeeklyTwice WeeklyC98861 2 TIMES PER YEAR 2 Times Per Year Two times per year. (NCI) Two Times YearlyC98859 3 TIMES PER MONTH 3 Times PerMonthC64528 3 TIMES PER WEEK Three times aweek; TISThree times per month. (NCI)Three times per week. (NCI)Three Times MonthlyThree Times WeeklyC98860 3 TIMES PER YEAR 3 Times Per Year Three times per year. (NCI) Three Times YearlyC98852 4 TIMES PER MONTH 4 Times PerMonthC64531 4 TIMES PER WEEK 4 times perweek; QISFour times per month. (NCI)Four times per week. (NCI)Four Times MonthlyFour Times WeeklyC98853 4 TIMES PER YEAR 4 Times Per Year Four times per year. (NCI) Four Times YearlyC98849 5 TIMES PER DAY 5 Times Daily Five times per day. (NCI) Five Times DailyC98850 5 TIMES PER MONTH 5 Times PerMonthC85552 5 TIMES PER WEEK 5 Times PerWeekFive times per month. (NCI)Five times per week. (NCI)Five Times MonthlyFive Times WeeklyC98851 5 TIMES PER YEAR 5 Times Per Year Five times per year. (NCI) Five Times YearlyC98855 6 TIMES PER DAY 6 Times Daily Six times per day. (NCI) Six Times DailyC98856 6 TIMES PER MONTH 6 Times PerMonthC98857 6 TIMES PER WEEK 6 Times PerWeekSix times per month. (NCI)Six times per week. (NCI)Six Times MonthlySix Times WeeklyC98858 6 TIMES PER YEAR 6 Times Per Year Six times per year. (NCI) Six Times YearlyC98854 7 TIMES PER WEEK 7 Times PerWeekSeven times per week. (NCI)Seven Times WeeklyC64636 AD LIBITUM Ad Libitum As much as desired. As Much as DesiredC64496 BID BD; Twice perdayTwo times per day, at unspecified times. (NCI)Twice DailyC71<strong>12</strong>9 BIM Twice per month Twice per month. (NCI) Twice Per MonthC53279 CONTINUOUS Continuous Remain in force or carry on without letup; keep ormaintain in unaltered condition; exist in time or spacewithout stop or interruption. (NCI)ContinueC71<strong>12</strong>7 EVERY 2 WEEKS Every 2 weeks;Q2SC64535 EVERY 3 WEEKS Every 3 weeks;Q3SC64529 EVERY 4 WEEKS Every 4 weeks;Q4SEvery two weeks. (NCI)Every three weeks. (NCI)Every four weeks. (NCI)Every Two WeeksEvery Three WeeksEvery Four WeeksSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 75 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71113 - FREQ - FrequencyCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103390 EVERY 5 WEEKS Every 5 weeks;Q5SC89788 EVERY 6 WEEKS Every 6 Weeks;Q6SC103389 EVERY 8 WEEKS Every 8 weeks;Q8SC67069 EVERY WEEK Every week; PerWeek; QSEvery five weeks. (NCI)Every six weeks. (NCI)Every eight weeks. (NCI)Every week. (NCI)Every Five WeeksEvery Six WeeksEvery Eight WeeksWeeklyC71325 INTERMITTENT Intermittent Periodically stopping and starting. (NCI) IntermittentC64954 OCCASIONAL Occasional Not occurring regularly or at short intervals. InfrequentC64576 ONCE Once A one time intervention. (NCI) OnceC17649 OTHER Other Different than the one(s) previously specified ormentioned. (NCI)OtherC74924 PA Per Annum; PerYear; Every YearA frequency rate of occurrences of something within aperiod of time equal to three hundred sixty-five days.Per YearC64499 PRN As needed As needed. (NCI) As NecessaryC64500 Q10H Every 10 hours Every ten hours. (NCI) Every Ten HoursC64501 Q11H Every 11 hours Every eleven hours. (NCI) Every Eleven HoursC64502 Q<strong>12</strong>H Every <strong>12</strong> hours Every twelve hours. (NCI) Every Twelve HoursC64503 Q13H Every 13 hours Every thirteen hours. (NCI) Every Thirteen HoursC64504 Q14H Every 14 hours Every fourteen hours. (NCI) Every Fourteen HoursC64505 Q15H Every 15 hours Every fifteen hours. (NCI) Every Fifteen HoursC64506 Q16H Every 16 hours Every sixteen hours. (NCI) Every Sixteen HoursC64507 Q17H Every 17 hours Every seventeen hours. (NCI) Every Seventeen HoursC64508 Q18H Every 18 hours Every eighteen hours. (NCI) Every Eighteen HoursC64509 Q19H Every 19 hours Every nineteen hours. (NCI) Every Nineteen HoursC64511 Q20H Every 20 hours Every twenty hours. (NCI) Every Twenty HoursC645<strong>12</strong> Q21H Every 21 hours Every twenty-one hours. (NCI) Every Twenty-One HoursC64513 Q22H Every 22 hours Every twenty-two hours. (NCI) Every Twenty-Two HoursC64514 Q23H Every 23 hours Every twenty-three hours. (NCI) Every Twenty-Three HoursC64515 Q24H Every 24 hours Every twenty-four hours. (NCI) Every Twenty-Four HoursC64516 Q2H Every 2 hours Every two hours. (NCI) Every Two HoursC64536 Q2M Every twomonthsEvery two months. (NCI)Every Two MonthsC64533 Q3D Every 3 days Every three days. (NCI) Every Three DaysC64517 Q3H Every 3 hours Every three hours. (NCI) Every Three HoursC64537 Q3M Every 3 months Every three months. (NCI) Every Three MonthsC64534 Q4D Every 4 days Every four days. (NCI) Every Four DaysSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 76 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71113 - FREQ - FrequencyCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64518 Q4H Every 4 hours Every four hours. (NCI) Every Four HoursC64538 Q4M Every 4 months Every four months. (NCI) Every Four MonthsC71<strong>12</strong>4 Q5D Every 5 days Every five days. (NCI) Every Five DaysC64519 Q5H Every 5 hours Every five hours. (NCI) Every Five HoursC64520 Q6H Every 6 hours Every six hours. (NCI) Every Six HoursC64521 Q7H Every 7 hours Every seven hours. (NCI) Every Seven HoursC64523 Q8H Every 8 hours Every eight hours. (NCI) Every Eight HoursC64524 Q9H Every 9 hours Every nine hours. (NCI) Every Nine HoursC25473 QD Daily Occurring or done each day. (NCI) DailyC64510 QH Every hour Every hour. (NCI) Every HourC64530 QID 4 times per day Four times per day. (NCI) Four Times DailyC64498 QM Every Month;Per MonthEvery month. (NCI)MonthlyC64525 QOD Every other day Every other day. (NCI) Every Other DayC64527 TID 3 times per day Three times per day. (NCI) Three Times DailyC17998 UNKNOWN U; Unknown Not known, not observed, not recorded, or refused.(NCI)UnknownSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 77 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42887 AEROSOL aer A product that is packaged under pressure and containstherapeutically active ingredients that are releasedupon activation of an appropriate valve system; it isintended for topical application to the skin as well aslocal application into the nose (nasal aerosols), mouth(lingual aerosols), or lungs (inhalation aerosols).(FDA)C42888 AEROSOL, FOAM A dosage form containing one or more activeingredients, surfactants, aqueous or non-aqueousliquids, and the propellants; if the propellant is in theinternal (discontinuous) phase (i.e., of the oil-in-watertype), a stable foam is discharged, and if the propellantis in the external (continuous) phase (i.e., of thewater-in-oil type), a spray or a quick-breaking foam isdischarged. (FDA)C42960 AEROSOL, METERED A pressurized dosage form consisting of metered dosevalves which allow for the delivery of a uniformquantity of spray upon each activation. (NCI)C42971 AEROSOL, POWDER A product that is packaged under pressure and containstherapeutically active ingredients, in the form of apowder, that are released upon activation of anappropriate valve system. (NCI)C42889 AEROSOL, SPRAY An aerosol product which utilizes a compressed gas asthe propellant to provide the force necessary to expelthe product as a wet spray; it is applicable to solutionsof medicinal agents in aqueous solvents. (NCI)C42892 BAR, CHEWABLE A solid dosage form usually in the form of a rectanglethat is meant to be chewed. (NCI)Aerosol Dosage FormAerosol Foam Dosage FormMetered Aerosol Dosage FormPowder Aerosol Dosage FormAerosol Spray Dosage FormChewable Bar Dosage FormC42890 BEAD A solid dosage form in the shape of a small ball. (NCI) Bead Dosage FormC43451BEAD, IMPLANT,EXTENDED RELEASEA small sterile solid mass consisting of a highlypurified drug intended for implantation in the bodywhich would allow at least a reduction in dosingfrequency as compared to that drug presented as aconventional dosage form. (NCI)Extended Release Bead ImplantDosage FormC42891 BLOCK Solid dosage form, usually in the shape of a square orrectangle. (NCI)C97197 CAPLET A solid dosage form in which a tablet has beencompacted into capsule shape.C25158 CAPSULE cap A solid pharmaceutical dosage form that containsmedicinal agent within either a hard or soft solublecontainer or shell, usually used for the oraladministration of medicine. The shells are made of asuitable form of gelatin or other substance. (NCI)C42895 CAPSULE, COATED A solid dosage form in which the drug is enclosedwithin either a hard or soft soluble container or "shell"made from a suitable form of gelatin; additionally, thecapsule is covered in a designated coating. (FDA)Block Dosage FormCaplet Dosage FormCapsule Dosage FormCoated Capsule Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 78 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42896CAPSULE, COATEDPELLETSA solid dosage form in which the drug is enclosedwithin either a hard or soft soluble container or "shell"made from a suitable form of gelatin; the drug itself isin the form of granules to which varying amounts ofcoating have been applied. (NCI)Coated Pellet in Capsule DosageFormC42917CAPSULE, COATED,EXTENDED RELEASEA solid dosage form in which the drug is enclosedwithin either a hard or soft soluble container or "shell"made from a suitable form of gelatin; additionally, thecapsule is covered in a designated coating, and whichreleases a drug (or drugs) in such a manner to allow atleast a reduction in dosing frequency as compared tothat drug (or drugs) presented as a conventional dosageform. (NCI)Extended Release Coated CapsuleDosage FormC42902CAPSULE, DELAYEDRELEASEA solid dosage form in which the drug is enclosedwithin either a hard or soft soluble container madefrom a suitable form of gelatin, and which releases adrug (or drugs) at a time other than promptly afteradministration. Enteric-coated articles are delayedrelease dosage forms. (NCI)Delayed Release Capsule DosageFormC42904CAPSULE, DELAYEDRELEASE PELLETSA solid dosage form in which the drug is enclosedwithin either a hard or soft soluble container or "shell"made from a suitable form of gelatin; the drug itself isin the form of granules to which enteric coating hasbeen applied, thus delaying release of the drug until itspassage into the intestines. (NCI)Delayed Release Pellet in CapsuleDosage FormC42916CAPSULE, EXTENDEDRELEASEA solid dosage form in which the drug is enclosedwithin either a hard or soft soluble container madefrom a suitable form of gelatin, and which releases adrug (or drugs) in such a manner to allow a reductionin dosing frequency as compared to that drug (ordrugs) presented as a conventional dosage form. (NCI)Extended Release Capsule DosageFormC42928CAPSULE, FILM COATED,EXTENDED RELEASEA solid dosage form in which the drug is enclosedwithin either a hard or soft soluble container or "shell"made from a suitable form of gelatin; additionally, thecapsule is covered in a designated film coating, andwhich releases a drug (or drugs) in such a manner toallow at least a reduction in dosing frequency ascompared to that drug (or drugs) presented as aconventional dosage form. (NCI)Extended Release Film CoatedCapsule Dosage FormC42936CAPSULE, GELATINCOATEDA solid dosage form in which the drug is enclosedwithin either a hard or soft soluble container madefrom a suitable form of gelatin; through a bandingprocess, the capsule is coated with additional layers ofgelatin so as to form a complete seal. (NCI)Gelatin Coated Capsule DosageFormC42954 CAPSULE, LIQUID FILLED A solid dosage form in which the drug is enclosedwithin a soluble, gelatin shell which is plasticized bythe addition of a polyol, such as sorbitol or glycerin,and is therefore of a somewhat thicker consistencythan that of a hard shell capsule; typically, the activeingredients are dissolved or suspended in a liquidvehicle. (NCI)C45414 CEMENT A substance that serves to produce solid union betweentwo surfaces. (NCI)Liquid Filled Capsule Dosage FormCement Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 79 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42678 CIGARETTE A narrow tube filled with material that is capable toburn with release of therapeutically-activesubstance(s) during the process of smoking. Cigaretteis a very efficient drug-delivery inhaler system forfast-acting substances. (FDA)C60884 CLOTH A large piece of relatively flat, absorbent material thatcontains a drug. It is typically used for applyingmedication or for cleansing. (FDA)C60891 CONCENTRATE A liquid preparation of increased strength and reducedvolume which is usually diluted prior toadministration. (NCI)C42900 CONE A solid dosage form bounded by a circular base and thesurface formed by line segments joining every point ofthe boundary of the base to a common vertex. A cone(usually containing antibiotics) is normally placedbelow the gingiva after a dental extraction. (NCI)Cigarette Dosage FormCloth Dosage FormConcentrated Dosage FormCone Dosage FormC42919CORE, EXTENDEDRELEASEAn ocular system placed in the eye from which thedrug diffuses through a membrane at a constant rateover a specified period. (NCI)Extended Release Core DosageFormC28944 CREAM A semisolid emulsion of either the oil-in-water or thewater-in-oil type, ordinarily intended for topical use.(NCI)C60897 CREAM, AUGMENTED A cream dosage form that enhances drug delivery.Augmentation does not refer to the strength of thedrug in the dosage form. NOTE: CDER has decided torefrain from expanding the use of this dosage form dueto difficulties in setting specific criteria that must bemet to be considered augmented. (FDA)C42901 CRYSTAL A naturally produced angular solid of definite form inwhich the ultimate units from which it is built up aresystematically arranged; they are usually evenly spacedon a regular space lattice. (FDA)C45415 CULTURE The propagation of microorganisms or of living tissuecells in special media conducive to their growth. (NCI)C47890 DIAPHRAGM A device usually dome-shaped, worn during copulationover the cervical mouth for prevention of conceptionor infection. (NCI)Cream Dosage FormAugmented Cream Dosage FormCrystal Dosage FormCulture Dosage FormVaginal Diaphragm Dosage FormC43525 DISC A circular plate-like organ or structure. (FDA) Disc Dosage FormC42679 DOUCHE A liquid preparation, intended for the irrigativecleansing of the vagina, that is prepared from powders,liquid solutions, or liquid concentrates and containsone or more chemical substances dissolved in asuitable solvent or mutually miscible solvents. (NCI)C42763 DRESSING The application of various materials for protecting awound.(FDA)C17423 DRUG DELIVERY SYSTEM Modern technology, distributed with or as a part of adrug product that allows for the uniform release ortargeting of drugs to the body. (FDA)Douche Dosage FormDressing Dosage FormDrug Delivery SystemSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 80 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC429<strong>12</strong> ELIXIR A clear, pleasantly flavored, sweetened hydroalcoholicliquid containing dissolved medicinal agents; it isintended for oral use. (NCI)C42913 EMULSION A dosage form consisting of a two-phase systemcomprised of at least two immiscible liquids (1), oneof which is dispersed as droplets (internal or dispersedphase) within the other liquid (external or continuousphase), generally stabilized with one or moreemulsifying agents. Note 1: A liquid is pourable; itflows and conforms to its container at roomtemperature. It displays Newtonian or pseudoplasticflow behavior. Note 2: Emulsion is used as a dosageform term unless a more specific term is applicable,e.g. cream, lotion, ointment. (NCI)C42915 ENEMA A rectal preparation for therapeutic, diagnostic, ornutritive purposes. (NCI)C42929 EXTRACT A concentrated preparation of vegetable or animaldrugs obtained by removal of the active constituents ofthe respective drugs with a suitable menstrua,evaporation of all or nearly all of the solvent, andadjustment of the residual masses or powders to theprescribed standards. (NCI)Elixir Dosage FormEmulsion Dosage FormEnema Dosage FormExtract Dosage FormC60926FIBER, EXTENDEDRELEASEA slender and elongated solid thread-like substancethat delivers drug in such a manner to allow a reductionin dosing frequency as compared to that drug (ordrugs) presented as a conventional dosage form. (FDA)Extended Release Fiber DosageFormC42932 FILM A thin layer or coating. (NCI) Film Dosage FormC42920 FILM, EXTENDED RELEASE A drug delivery system in the form of a film thatreleases the drug over an extended period in such a wayas to maintain constant drug levels in the blood ortarget tissue. (NCI)C42984 FILM, SOLUBLE A thin layer or coating which is susceptible to beingdissolved when in contact with a liquid. (NCI)C60927 FOR SOLUTION A product, usually a solid, intended for solution priorto administration. (FDA)C60928 FOR SUSPENSION A product, usually a solid, intended for suspensionprior to administration. (FDA)Extended Release Film Dosage FormSoluble Film Dosage FormDosage Form For SolutionDosage Form For SuspensionC60929FOR SUSPENSION,EXTENDED RELEASEA product, usually a solid, intended for suspensionprior to administration; once the suspension isadministered, the drug will be released at a constantrate over a specified period. (FDA)Extended Release Dosage Form ForSuspensionC42933 GAS Any elastic aeriform fluid in which the molecules areseparated from one another and have free paths. (NCI)Gas Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 81 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42934 GEL A semisolid (1) dosage form that contains a gellingagent to provide stiffness to a solution or a colloidaldispersion (2). A gel may contain suspended particles.Note 1: A semisolid is not pourable; it does not flowor conform to its container at room temperature. Itdoes not flow at low shear stress and generally exhibitsplastic flow behavior. Note 2: A colloidal dispersion isa system in which particles of colloidal dimension(i.e., typically between 1 nm and 1 micrometer) aredistributed uniformly throughout a liquid. (FDA)C42906 GEL, DENTIFRICE A combination of a dentifrice (formulation intended toclean and/or polish the teeth, and which may containcertain additional agents), and a gel. It is used with atoothbrush for the purpose of cleaning and polishingthe teeth. (NCI)C60930 GEL, METERED A gel preparation, with metered dose valves, whichallow for the delivery of a uniform quantity of gel uponeach activation. (FDA)C48193 GENERATOR An apparatus for the formation of vapor or gas from aliquid or solid by heat or chemical action. The termGENERATOR also applies to radioactive columnsfrom which radionuclides are provided. (NCI)C42937 GLOBULE Also called pellets or pilules, are made of puresucrose, lactose, or other polysaccharides. They areformed into small globular masses of various sizes,and are medicated by placing them in a vial and addingthe liquid drug attenuation in the proportion not lessthan one percent (v/w). After shaking, the medicatedglobules are dried at temperatures not to exceed 40degrees Centigrade. (NCI)C45416 GRAFT A slip of skin or of other tissue for implantation.(NCI)Gel Dosage FormDentifrice Gel Dosage FormMetered Gel Dosage FormGenerator Dosage FormGlobule Dosage FormGraft Dosage FormC42938 GRANULE A small particle or grain. (NCI) Granule Dosage FormC42903GRANULE, DELAYEDRELEASEA small medicinal particle or grain to which an entericor other coating has been applied, thus delaying releaseof the drug until its passage into the intestines. (NCI)Delayed Release Granule DosageFormC42909 GRANULE, EFFERVESCENT A small particle or grain containing a medicinal agentin a dry mixture usually composed of sodiumbicarbonate, citric acid, and tartaric acid which, whenin contact with water, has the capability to release gas,resulting in effervescence. (NCI)C42939 GRANULE, FOR SOLUTION A small medicinal particle or grain made available inits more stable dry form, to be reconstituted withsolvent just before dispensing; the granules are soprepared to contain not only the medicinal agent, butthe colorants, flavorants, and any other desiredpharmaceutic ingredient. (NCI)Effervescent Granule Dosage FormGranule For Solution Dosage FormC42940GRANULE, FORSUSPENSIONA small medicinal particle or grain made available inits more stable dry form, to be reconstituted withsolvent just before dispensing to form a suspension;the granules are so prepared to contain not only themedicinal agent, but the colorants, flavorants, and anyother desired pharmaceutic ingredient. (NCI)Granule For Suspension DosageFormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 82 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42921GRANULE, FORSUSPENSION, EXTENDEDRELEASEA small medicinal particle or grain made available inits more stable dry form, to be reconstituted withsolvent just before dispensing to form a suspension;the extended release system achieves slow release ofthe drug over an extended period of time and maintainsconstant drug levels in the blood or target tissue. (NCI)Extended Release Granule ForSuspension Dosage FormC42941 GUM A mucilaginous excretion from various plants. (NCI) Gum Dosage FormC42894 GUM, CHEWING A sweetened and flavored insoluble plastic material ofvarious shapes which when chewed, releases a drugsubstance into the oral cavity. (NCI)C42978 GUM, RESIN Natural mixture of gum and resin, usually obtained asexudations from plants. (NCI)C42942 IMPLANT A material containing drug intended to be insertedsecurely and deeply in a living site for growth, slowrelease, or formation of an organic union. (NCI)C42944 INHALANT A special class of inhalations consisting of a drug orcombination of drugs, that by virtue of their high vaporpressure, can be carried by an air current into the nasalpassage where they exert their effect; the containerfrom which the inhalant generally is administered isknown as an inhaler. (NCI)C60931 INJECTABLE, LIPOSOMAL An injection, which either consists of or formsliposomes (a lipid bilayer vesicle usually composed ofphospholipids which is used to encapsulate an activedrug substance). (FDA)C42946 INJECTION A sterile preparation intended for parenteral use; fivedistinct classes of injections exist as defined by theUSP. (NCI)C42914 INJECTION, EMULSION An emulsion consisting of a sterile, pyrogen-freepreparation intended to be administered parenterally.(FDA)Chewing Gum Dosage FormResin Gum Dosage FormImplant Dosage FormInhalant Dosage FormLiposomal Injection Dosage FormInjectable Dosage FormEmulsion for Injection Dosage FormC42950INJECTION, LIPIDCOMPLEX[definition pending](FDA)Injectable Lipid Complex DosageFormC42974INJECTION, POWDER, FORSOLUTIONA sterile preparation intended for reconstitution toform a solution for parenteral use. (NCI)Powder For Injectable SolutionDosage FormC42976INJECTION, POWDER, FORSUSPENSIONA sterile preparation intended for reconstitution toform a suspension for parenteral use. (NCI)Powder For Injectable SuspensionDosage FormC42977INJECTION, POWDER, FORSUSPENSION, EXTENDEDRELEASEA dried preparation intended for reconstitution to forma suspension for parenteral use which has beenformulated in a manner to allow at least a reduction indosing frequency as compared to that drug presentedas a conventional dosage form (e.g., as a solution).(FDA)Powder For Injectable ExtendedRelease Suspension Dosage FormC42959INJECTION, POWDER,LYOPHILIZED, FORLIPOSOMAL SUSPENSIONA sterile freeze dried preparation intended forreconstitution for parenteral use which has beenformulated in a manner that would allow liposomes (alipid bilayer vesicle usually composed ofphospholipids which is used to encapsulate an activedrug substance, either within a lipid bilayer or in anaqueous space) to be formed upon reconstitution.(NCI)Lyophilized Powder For InjectableLiposomal Suspension Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 83 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42957INJECTION, POWDER,LYOPHILIZED, FORSOLUTIONA dosage form intended for the solution prepared bylyophilization ('freeze drying'), a process whichinvolves the removal of water from products in thefrozen state at extremely low pressures; this isintended for subsequent addition of liquid to create asolution that conforms in all respects to therequirements for Injections. (NCI)Lyophilized Powder For InjectableSolution Dosage FormC42958INJECTION, POWDER,LYOPHILIZED, FORSUSPENSIONA liquid preparation, intended for parenteral use, thatcontains solids suspended in a suitable fluid mediumand conforms in all respects to the requirements forSterile Suspensions; the medicinal agents intended forthe suspension are prepared by lyophilization ("freezedrying"), a process which involves the removal of waterfrom products in the frozen state at extremely lowpressures. (NCI)Lyophilized Powder For InjectableSuspension Dosage FormC42956INJECTION, POWDER,LYOPHILIZED, FORSUSPENSION, EXTENDEDRELEASEA sterile freeze dried preparation intended forreconstitution for parenteral use which has beenformulated in a manner to allow at least a reduction indosing frequency as compared to that drug presentedas a conventional dosage form (e.g., as a solution).(NCI)Lyophilized Powder For ExtendedRelease Injectable SuspensionDosage FormC42945 INJECTION, SOLUTION A liquid preparation containing one or more drugsubstances dissolved in a suitable solvent or mixture ofmutually miscible solvents that is suitable forinjection. (NCI)Injectable Solution Dosage FormC42899INJECTION, SOLUTION,CONCENTRATEA sterile preparation for parenteral use which, upon theaddition of suitable solvents, yields a solutionconforming in all respects to the requirements forInjections. (NCI)Concentrated Injectable SolutionDosage FormC42995 INJECTION, SUSPENSION A liquid preparation, suitable for injection, whichconsists of solid particles dispersed throughout aliquid phase in which the particles are not soluble. Itcan also consist of an oil phase dispersed throughoutan aqueous phase, or vice-versa. (NCI)Injectable Suspension Dosage FormC42926INJECTION, SUSPENSION,EXTENDED RELEASEA sterile preparation intended for parenteral use whichhas been formulated in a manner to allow at least areduction in dosing frequency as compared to that drugpresented as a conventional dosage form (e.g., as asolution or a prompt drug-releasing, conventional soliddosage form). (NCI)Injectable Extended ReleaseSuspension Dosage FormC42951INJECTION, SUSPENSION,LIPOSOMALA liquid parenteral pharmaceutical dosage formstructured as a multilamellar composition ofconcentric phospholipid spheres that encapsulate thedrug (drug delivery systems) separated by layers ofwater. Drug release is facilitated and controlled by invivo erosion of the liposomes. To further increase thein vivo circulation time, liposomes in somepreparations are covalently derivatized with PEG toproduce PEGylated or stealth liposomes. Covalentattachment of drugs to the outer surface of liposomescan potentially serve as a delayed-release product.(NCI)Injectable Liposomal SuspensionDosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 84 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42988INJECTION, SUSPENSION,SONICATEDA liquid preparation, suitable for injection, whichconsists of solid particles dispersed throughout aliquid phase in which the particles are not soluble. Inaddition, the product is sonicated while a gas isbubbled through the suspension, and this results in theformation of microspheres by the solid particles.(NCI)Injectable Sonicated SuspensionDosage FormC60933 INSERT A specially formulated and shaped non-encapsulatedsolid preparation intended to be placed into anon-rectal orifice of the body, where drug is released,generally for localized effects. (FDA)Insert Dosage FormC42922INSERT, EXTENDEDRELEASEA specially formulated and shaped solid preparation(e.g., ring, tablet, or stick) intended to be placed in thevagina by special inserters, where the medication isreleased, generally for localized effects; the extendedrelease preparation is designed to allow a reduction indosing frequency. (NCI)Extended Release Insert DosageFormC47915 INTRAUTERINE DEVICE A device inserted and left in the uterus to preventeffective conception. (NCI)C42947 IRRIGANT A sterile solution intended to bathe or flush openwounds or body cavities; they're used topically, neverparenterally. (NCI)C42948 JELLY A class of gels--semisolid systems which consist ofsuspensions made up of either small inorganicparticles or large organic molecules interpenetrated bya liquid--in which the structural coherent matrixcontains a high portion of liquid, usually water. (NCI)Intrauterine Device Dosage FormIrrigant Dosage FormJelly Dosage FormC47916 KIT A packaged collection of related material. (NCI) Kit Dosage FormC45413 LINER, DENTAL A material applied to the inside of the dental cavity, forprotection or insulation of the surface. (FDA)C42949 LINIMENT A solution or mixture of various substances in oil,alcoholic solutions of soap, or emulsions intended forexternal application. (NCI)C42952 LIPSTICK A waxy solid, usually colored cosmetic, in stick formfor the lips. (NCI)C42953 LIQUID A dosage form consisting of a pure chemical in itsliquid state. This dosage form term should not beapplied to solutions. Note: A liquid is pourable; itflows and conforms to its container at roomtemperature. It displays Newtonian or pseudoplasticflow behavior.(FDA)Dental Liner Dosage FormLiniment Dosage FormLipstick Dosage FormLiquid Dosage FormC60934LIQUID, EXTENDEDRELEASEA liquid that delivers a drug in such a manner to allow areduction in dosing frequency as compared to that drug(or drugs) presented as a conventional dosage form.(FDA)Extended Release Liquid DosageFormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 85 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC29167 LOTION An emulsion, liquid (1) dosage form. This dosage formis generally for external application to the skin (2).Note 1: A liquid is pourable; it flows and conforms toits container at room temperature. It displaysNewtonian or pseudoplastic flow behavior. Note 2:Previously the definition of a lotion was: The termlotion has been used to categorize many topicalsuspensions, solutions, and emulsions intended forapplication to the skin. The current definition of alotion is restricted to an emulsion. (FDA)C60957 LOTION, AUGMENTED A lotion dosage form that enhances drug delivery.Augmentation does not refer to the strength of thedrug in the dosage form. NOTE: CDER has decided torefrain from expanding the use of this dosage form dueto difficulties in setting specific criteria that must bemet to be considered augmented. (FDA)C60958 LOTION/SHAMPOO A lotion dosage form which has a soap or detergentthat is usually used to clean the hair and scalp; it isoften used as a vehicle for dermatologic agents. (FDA)C42955 LOZENGE A solid preparation containing one or moremedicaments, usually in a flavored, sweetened basewhich is intended to dissolve or disintegrate slowly inthe mouth. A lollipop is a lozenge on a stick.(FDA)C29269 MOUTHWASH An aqueous solution which is most often used for itsdeodorant, refreshing, or antiseptic effect. (NCI)C48624 NOT APPLICABLE The use of a dosage form term is not relevant orappropriate. (NCI)C42965 OIL An unctuous, combustible substance which is liquid, oreasily liquefiable, on warming, and is soluble in etherbut insoluble in water. Such substances, depending ontheir origin, are classified as animal, mineral, orvegetable oils. (NCI)C42966 OINTMENT oint A suspension or emulsion, semisolid (1) dosage form,usually containing < 20% water and volatiles (2)and > 50% hydrocarbons, waxes, or polyols as thevehicle. This dosage form is generally for externalapplication to the skin or mucous membranes. Note 1:A semisolid is not pourable; it does not flow orconform to its container at room temperature. It doesnot flow at low shear stress and generally exhibitsplastic flow behavior. Note 2: Percent water andvolatiles are measured by a loss on drying test in whichthe sample is heated at 105 degrees C until constantweight is achieved. (FDA)C60984 OINTMENT, AUGMENTED An ointment dosage form that enhances drug delivery.Augmentation does not refer to the strength of thedrug in the dosage form. NOTE: CDER has decided torefrain from expanding the use of this dosage form dueto difficulties in setting specific criteria that must bemet to be considered augmented. (FDA)C47887 PACKING A material, usually covered by or impregnated with adrug, that is inserted into a body cavity or between thetooth enamel and the gingival margin. (FDA)Lotion Dosage FormAugmented Lotion Dosage FormLotion Shampoo Dosage FormLozenge Dosage FormMouthwash Dosage FormDosage Form Not ApplicableOil Dosage FormOintment Dosage FormAugmented Ointment Dosage FormPacking Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 86 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42967 PASTE A semisolid dosage form, containing a largeproportion (20 - 50%) of solids finely dispersed in afatty vehicle. This dosage form is generally forexternal application to the skin or mucous membranes.Note: A semisolid is not pourable; it does not flow orconform to its container at room temperature. It doesnot flow at low shear stress and generally exhibitsplastic flow behavior. (NCI)C42907 PASTE, DENTIFRICE A paste formulation intended to clean and/or polish theteeth, and which may contain certain additional agents.(NCI)C60985 PASTILLE An aromatic preparation, often with a pleasing flavor,usually intended to dissolve in the mouth. (FDA)C42968 PATCH A drug delivery system that often contains an adhesivebacking that is usually applied to an external site on thebody. Its ingredients either passively diffuse from, orare actively transported from, some portion of thepatch. Depending upon the patch, the ingredients areeither delivered to the outer surface of the body orinto the body. A patch is sometimes synonymous withthe terms Extended Release Film and System. (FDA)Paste Dosage FormDentifrice Paste Dosage FormPastille Dosage FormPatch Dosage FormC42923PATCH, EXTENDEDRELEASEA drug delivery system in the form of a patch thatreleases the drug in such a manner that a reduction indosing frequency compared to that drug presented as aconventional dosage form (e.g., a solution or a promptdrug-releasing, conventional solid dosage form). (NCI)Extended Release Patch DosageFormC42911PATCH, EXTENDEDRELEASE, ELECTRICALLYCONTROLLEDA drug delivery system in the form of a patch which iscontrolled by an electric current that releases the drugin such a manner that a reduction in dosing frequencycompared to that drug presented as a conventionaldosage form (e.g., a solution or a promptdrug-releasing, conventional solid dosage form). (NCI)Electrically Controlled ExtendedRelease Patch Dosage FormC42969 PELLET A small sterile solid mass consisting of a highlypurified drug (with or without excipients) made by theformation of granules, or by compression and molding.(NCI)C42943 PELLET, IMPLANTABLE A small sterile solid mass consisting of a highlypurified drug (with or without excipients) made by theformation of granules, or by compression and molding;they are intended for implantation in the body (usuallysubcutaneously) for the purpose of providingcontinuous release of the drug over long periods oftime. (FDA)Pellet Dosage FormImplantable Pellet Dosage FormC42918PELLETS, COATED,EXTENDED RELEASEA solid dosage form in which the drug itself is in theform of granules to which varying amounts of coatinghave been applied, and which releases a drug (or drugs)in such a manner to allow a reduction in dosingfrequency as compared to that drug (or drugs)presented as a conventional dosage form. (NCI)Extended Release Coated PelletDosage FormC25394 PILL A dose of medicine in the form of a small pellet.(NCI)Pill Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 87 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42970 PLASTER Substance intended for external application made ofsuch materials and of such consistency as to adhere tothe skin and attach to a dressing; plasters are intendedto afford protection and support and/or to furnish anocclusion and macerating action and to bringmedication into close contact with the skin. (FDA)C47913 POULTICE A soft, moist mass of meal, herbs, seed, etc., usuallyapplied hot in cloth that consists of gruel-likeconsistency. (NCI)C42972 POWDER An intimate mixture of dry, finely divided drugs and/orchemicals that may be intended for internal or externaluse. (NCI)C42908 POWDER, DENTIFRICE A powder formulation intended to clean and/or polishthe teeth, and which may contain certain additionalagents. (NCI)C42973 POWDER, FOR SOLUTION An intimate mixture of dry, finely divided drugs and/orchemicals, which, upon the addition of suitablevehicles, yields a solution. (NCI)Plaster Dosage FormPoultice Dosage FormPowder Dosage FormDentifrice Powder Dosage FormPowder for Solution Dosage FormC42975POWDER, FORSUSPENSIONAn intimate mixture of dry, finely divided drugs and/orchemicals, which, upon the addition of suitablevehicles, yields a suspension (a liquid preparationcontaining the solid particles dispersed in the liquidvehicle). (NCI)Powder for Suspension Dosage FormC42961 POWDER, METERED A powder dosage form that is situated inside acontainer that has a mechanism to deliver a specifiedquantity. (NCI)C60988 RING A small circular object with a vacant circular centerthat is usually intended to be placed in the body byspecial inserters, where the medication is released,generally for localized effects. (FDA)Metered Powder Dosage FormRing Dosage FormC42979 RINSE A liquid used to cleanse by flushing. (NCI) Rinse Dosage FormC42980 SALVE A thick ointment or cerate (a fat or wax basedpreparation with a consistency between an ointmentand a plaster). (NCI)C42981 SHAMPOO A liquid soap or detergent used to clean the hair andscalp and is often used as a vehicle for dermatologicagents. (NCI)C42982 SHAMPOO, SUSPENSION A liquid soap or detergent containing one or moresolid, insoluble substances dispersed in a liquidvehicle that is used to clean the hair and scalp and isoften used as a vehicle for dermatologic agents. (NCI)C42983 SOAP Any compound of one or more fatty acids, or theirequivalents, with an alkali; soap is detergent and ismuch employed in liniments, enemas, and in makingpills. It is also a mild aperient, antacid and antiseptic.(NCI)Salve Dosage FormShampoo Dosage FormShampoo Suspension Dosage FormSoap Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 88 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42986 SOLUTION A clear, homogeneous liquid dosage form that containsone or more chemical substances dissolved in asolvent or mixture of mutually miscible solvents.Note: A liquid is pourable; it flows and conforms to itscontainer at room temperature. It displays Newtonianor pseudoplastic flow behavior. (NCI)C42898 SOLUTION, CONCENTRATE A liquid preparation (i.e., a substance that flows readilyin its natural state) that contains a drug dissolved in asuitable solvent or mixture of mutually misciblesolvents; the drug has been strengthened by theevaporation of its non-active parts. (NCI)C42987 SOLUTION, FOR SLUSH A solution for the preparation of an iced saline slush,which is administered by irrigation and used to induceregional hypothermia (in conditions such as certainopen heart and kidney surgical procedures) by itsdirect application. (NCI)Solution Dosage FormConcentrated Solution Dosage FormSolution For Slush Dosage FormC60994SOLUTION, GELFORMING / DROPSA solution, which after usually being administered in adrop-wise fashion, forms a gel. (FDA)Gel Forming Drop Solution DosageFormC42935SOLUTION, GEL FORMING,EXTENDED RELEASEA solution that forms a gel when it comes in contactwith ocular fluid, and which allows at least a reductionin dosing frequency. (FDA)Extended Release Gel FormingSolution Dosage FormC60992 SOLUTION/ DROPS A solution which is usually administered in adrop-wise fashion. (FDA)C479<strong>12</strong> SPONGE A porous, interlacing, absorbent material that containsa drug. It is typically used for applying or introducingmedication, or for cleansing. A sponge usually retainsits shape. (FDA)C42989 SPRAY A liquid minutely divided as by a jet of air or steam.(NCI)C42962 SPRAY, METERED A non-pressurized dosage form consisting of valveswhich allow the dispensing of a specified quantity ofspray upon each activation. (NCI)C42990 SPRAY, SUSPENSION A liquid preparation containing solid particlesdispersed in a liquid vehicle and in the form of coarsedroplets or as finely divided solids to be appliedlocally, most usually to the nasal-pharyngeal tract, ortopically to the skin. (NCI)C42991 STICK A dosage form prepared in a relatively long and slenderoften cylindrical form. (NCI)Drop Solution Dosage FormSponge Dosage FormSpray Dosage FormMetered Spray Dosage FormSpray Suspension Dosage FormStick Dosage FormC47914 STRIP A long narrow piece of material. (FDA) Strip Dosage FormC42993 SUPPOSITORY supp A solid body of various weights and shapes, adapted forintroduction into the rectal, vaginal, or urethral orificeof the human body; they usually melt, soften, ordissolve at body temperature. (FDA)Suppository Dosage FormC42924SUPPOSITORY, EXTENDEDRELEASEA drug delivery system in the form of a suppositorythat allows at least a reduction in dosing frequency.(NCI)Extended Release SuppositoryDosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 89 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42994 SUSPENSION susp A liquid dosage form that contains solid particlesdispersed in a liquid vehicle. Note: A liquid ispourable; it flows and conforms to its container atroom temperature. It displays Newtonian orpseudoplastic flow behavior. (FDA)Suspension Dosage FormC42925SUSPENSION, EXTENDEDRELEASEA liquid preparation consisting of solid particlesdispersed throughout a liquid phase in which theparticles are not soluble; the suspension has beenformulated in a manner to allow at least a reduction indosing frequency as compared to that drug presentedas a conventional dosage form (e.g., as a solution or aprompt drug-releasing, conventional solid dosageform). (NCI)Extended Release SuspensionDosage FormC60995 SUSPENSION/DROPS A suspension which is usually administered in adropwise fashion. (FDA)C47889 SUTURE A strand or fiber used to hold wound edges inapposition during healing. (NCI)C47898 SWAB A small piece of relatively flat absorbent material thatcontains a drug. A swab may also be attached to oneend of a small stick. A swab is typically used forapplying medication or for cleansing. (FDA)C42996 SYRUP An oral solution containing high concentrations ofsucrose or other sugars; the term has also been used toinclude any other liquid dosage form prepared in asweet and viscid vehicle, including oral suspensions.(NCI)C42998 TABLET tab A solid dosage form containing medicinal substanceswith or without suitable diluents. (NCI)C42893 TABLET, CHEWABLE A solid dosage form containing medicinal substanceswith or without suitable diluents that is intended to bechewed, producing a pleasant tasting residue in the oralcavity that is easily swallowed and does not leave abitter or unpleasant after-taste. (NCI)C42897 TABLET, COATED A solid dosage form that contains medicinalsubstances with or without suitable diluents and iscovered with a designated coating. (NCI)Drop Suspension Dosage FormSuture Dosage FormSwab Dosage FormSyrup Dosage FormTablet Dosage FormChewable Tablet Dosage FormCoated Tablet Dosage FormC60997TABLET, COATEDPARTICLESA solid dosage form containing a conglomerate ofmedicinal particles that have each been covered with acoating. (FDA)Tablet Coated Particle Dosage FormC42905TABLET, DELAYEDRELEASEA solid dosage form which releases a drug (or drugs)at a time other than promptly after administration.Enteric-coated articles are delayed release dosageforms. (NCI)Delayed Release Tablet DosageFormC42997TABLET, DELAYEDRELEASE PARTICLESA solid dosage form containing a conglomerate ofmedicinal particles that have been covered with acoating which releases a drug (or drugs) at a time otherthan promptly after administration. Enteric-coatedarticles are delayed release dosage forms. (NCI)Delayed Release Particle TabletDosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 90 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42910 TABLET, EFFERVESCENT A solid dosage form containing mixtures of acids (e.g.,citric acid, tartaric acid) and sodium bicarbonate,which release carbon dioxide when dissolved in water;it is intended to be dissolved or dispersed in waterbefore administration. (FDA)Effervescent Tablet Dosage FormC42927TABLET, EXTENDEDRELEASEA solid dosage form containing a drug which allows atleast a reduction in dosing frequency as compared tothat drug presented in conventional dosage form. (NCI)Extended Release Tablet DosageFormC42931 TABLET, FILM COATED A solid dosage form that contains medicinalsubstances with or without suitable diluents and iscoated with a thin layer of a water-insoluble orwater-soluble polymer. (NCI)Film Coated Tablet Dosage FormC42930TABLET, FILM COATED,EXTENDED RELEASEA solid dosage form that contains medicinalsubstances with or without suitable diluents and iscoated with a thin layer of a water-insoluble orwater-soluble polymer; the tablet is formulated in suchmanner as to make the contained medicament availableover an extended period of time following ingestion.(FDA)Film Coated Extended ReleaseTablet Dosage FormC61004 TABLET, FOR SOLUTION A tablet that forms a solution when placed in a liquid.(FDA)C61005 TABLET, FOR SUSPENSION A tablet that forms a suspension when placed in aliquid (formerly referred to as a Dispersible Tablet).(FDA)C42964 TABLET, MULTILAYER A solid dosage form containing medicinal substancesthat have been compressed to form a multiple-layeredtablet or a tablet-within-a-tablet, the inner tablet beingthe core and the outer portion being the shell. (NCI)Tablet For Solution Dosage FormTablet For Suspension Dosage FormMultilayered Tablet Dosage FormC42963TABLET, MULTILAYER,EXTENDED RELEASEA solid dosage form containing medicinal substancesthat have been compressed to form a multiple-layeredtablet or a tablet-within-a-tablet, the inner tablet beingthe core and the outer portion being the shell, which,additionally, is covered in a designated coating; thetablet is formulated in such manner as to allow at leasta reduction in dosing frequency as compared to thatdrug presented as a conventional dosage form. (NCI)Multilayered Extended ReleaseTablet Dosage FormC42999TABLET, ORALLYDISINTEGRATINGA solid dosage form containing medicinal substanceswhich disintegrates rapidly, usually within a matter ofseconds, when placed upon the tongue. (NCI)Orally Disintegrating Tablet DosageFormC61006TABLET, ORALLYDISINTEGRATING,DELAYED RELEASEA solid dosage form containing medicinal substanceswhich disintegrates rapidly, usually within a matter ofseconds, when placed upon the tongue, but whichreleases a drug (or drugs) at a time other than promptlyafter administration. (FDA)Orally Disintegrating DelayedRelease Tablet Dosage FormC42985 TABLET, SOLUBLE A solid dosage form that contains medicinalsubstances with or without suitable diluents andpossesses the ability to dissolve in fluids. (NCI)C42992 TABLET, SUGAR COATED A solid dosage form that contains medicinalsubstances with or without suitable diluents and iscoated with a colored or an uncolored water-solublesugar. (NCI)Soluble Tablet Dosage FormSugar Coated Tablet Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 91 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66726 - FRM - Pharmaceutical Dosage FormCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC47892 TAMPON A plug made of cotton, sponge, or oakum variouslyused in surgery to plug the nose, vagina, etc., for thecontrol of hemorrhage or the absorption of secretions.(NCI)C47897 TAPE A narrow woven fabric, or a narrow extruded synthetic(such as plastic), usually with an adhesive on one orboth sides. (NCI)C43000 TINCTURE An alcoholic or hydroalcoholic solution prepared fromvegetable materials or from chemical substances.(NCI)C43001 TROCHE A discoid-shaped solid containing the medicinal agentin a suitably flavored base; troches are placed in themouth where they slowly dissolve, liberating the activeingredients. (NCI)Tampon Dosage FormTape Dosage FormTincture Dosage FormTroche Dosage FormC43002 UNASSIGNED A dosage form has yet to be assigned. (NCI) Unassigned Dosage FormC43003 WAFER A thin slice of material containing a medicinal agent.(NCI)Wafer Dosage FormSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 92 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89969 - FWTEST - Food and Water Consumption Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90384 Food Consumption FoodConsumptionA measurement of a subject's nutritional intake. (NCI)Food ConsumptionC90385Food Consumption Relative toBody WtFoodConsumptionRelative to BodyWtThe ratio or percentage of nutritional intake to bodyweight. (NCI)Food Consumption Relative to BodyWeightC90484 Water Consumption WaterConsumptionA measurement of a subject's water intake. (NCI)Water ConsumptionC90485Water Consumption Relativeto Body WtWaterConsumptionRelative to BodyWtThe ratio or percentage of water intake to body weight.(NCI)Water Consumption Relative toBody WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 93 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89970 - FWTESTCD - Food and Water Consumption Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90384 FC FoodConsumptionC90385 FCRELBW FoodConsumptionRelative to BodyWtC90484 WC WaterConsumptionC90485 WCRELBW WaterConsumptionRelative to BodyWtA measurement of a subject's nutritional intake. (NCI)The ratio or percentage of nutritional intake to bodyweight. (NCI)A measurement of a subject's water intake. (NCI)The ratio or percentage of water intake to body weight.(NCI)Food ConsumptionFood Consumption Relative to BodyWeightWater ConsumptionWater Consumption Relative toBody WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 94 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C99073 - LAT - LateralityCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC13332 BILATERAL Affecting both sides of the body, or a pair of organs. BilateralC25307 CONTRALATERAL Having to do with the opposite side of the body, inrelation to a pre-existing reference point.C25308 IPSILATERAL Having to do with the same side of the body, in relationto a pre-existing reference point.ContralateralIpsilateralC25230 LATERAL Situated at or extending to the side. LateralC25229 LEFT Being or located on or directed toward the side of thebody to the west when facing north.C25228 RIGHT Being or located on or directed toward the side of thebody to the east when facing north.C68598 UNILATERAL Affecting one side of the body or one of a pair oforgans.LeftRightUnilateralSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 95 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92267 1, 25-Dihydroxyvitamin D 1,25-Dihydroxyvitamin D; ActiveVitamin DC103344 11-Dehydro-Thromboxane B2 11-Dehydro-Thromboxane B2C92268 25-Hydroxyvitamin D 25-Hydroxyvitamin D; InactiveVitamin DA measurement of the total active Vitamin D in abiological specimen.A measurement of the 11-dehydro-thromboxane B2 ina biological specimen.A measurement of the total inactive Vitamin D in abiological specimen.1, 25-Dihydroxyvitamin DMeasurement11-Dehydro-Thromboxane B2Measurement25-Hydroxyvitamin D MeasurementC1033453,4-DihydroxyphenylaceticAcid3,4-Dihydroxyphenylacetic AcidA measurement of the 3,4-dihydroxyphenylacetic acidin a biological specimen.3,4-Dihydroxyphenylacetic AcidMeasurementC101017 3,4-Dihydroxyphenylglycol 3,4-Dihydroxyphenylglycol; 3.4DihydroxyphenylglycolA measurement of the catecholamine metabolite,3,4-Dihydroxyphenylglycol in a biological specimen.3,4-DihydroxyphenylglycolMeasurementC753593,4-methylenedioxymethamphetamine3,4-methylenedioxymethamphetamine; EcstasyA measurement of the3,4-methylenedioxymethamphetamine (MDMA) in abiological specimen.3,4-Methylenedioxymethamphetamine MeasurementC79437 5 Prime Nucleotidase 5 PrimeNucleotidase;5'-RibonucleotidePhosphohydrolaseC74876 6-Monoacetylmorphine 6-MonoacetylmorphineA measurement of the 5'-nucleotidase in a biologicalspecimen.A measurement of the 6-monoacetylmorphine presentin a biological specimen.5 Prime Nucleotidase Measurement6-MonoacetylmorphineMeasurementC96565A Fetoprotein L3/AFetoproteinA FetoproteinL3/A FetoproteinA relative measurement (ratio or percentage) of alphafetoprotein L3 to total alpha fetoprotein in a biologicalspecimen.Alpha Fetoprotein L3 to Total AlphaFetoprotein Ratio MeasurementC98705 ADV Viral Load ADV Viral Load A measurement of the adenovirus viral load in abiological specimen.C74699 Acanthocytes Acanthocytes A measurement of the acanthocytes in a biologicalspecimen.Adenovirus Viral Load MeasurementAcanthocyte CountC74633 Acanthocytes/Erythrocytes Acanthocytes/ErythrocytesC92247 Acetoacetic Acid AcetoaceticAcid;AcetoacetateA relative measurement (ratio or percentage) ofacanthocytes to all erythrocytes in a biologicalspecimen.A measurement of the acetoacetic acid in a biologicalspecimen.Acanthocyte to Erythrocyte RatioMeasurementAcetoacetic Acid MeasurementC74838 Acetylcholine Acetylcholine A measurement of the acetylcholine hormone in abiological specimen.Acetylcholine MeasurementC96559Acetylcholine ReceptorAntibodyAcetylcholineReceptorAntibodyA measurement of the acetylcholine receptor antibodyin a biological specimen.Acetylcholine Receptor AntibodyMeasurementC96560 Acetylcholinesterase AcetylcholinesteraseC80163 Acid Phosphatase AcidPhosphataseA measurement of the acetylcholinesterase in abiological specimen.A measurement of the acid phosphatase in a biologicalspecimen.Acetylcholinesterase MeasurementAcid Phosphatase MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 96 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC749<strong>12</strong> Acid Urate Crystals Acid UrateCrystalsC103348 Activated Coagulation Time Activated ClotTime; ActivatedClotting Time;CoagulationActivatedC98862 Activated PTT/Standard ActivatedPTT/StandardC100421 Activated PTT/Standard PTT ActivatedPTT/StandardPTT; ActivatedPartialThromboplastinTime/StandardThromboplastinTimeA measurement of the acid urate crystals present in abiological specimen.A measurement of the inhibition of blood coagulationin response to anticoagulant therapies.A relative measurement (ratio or percentage) of thesubject's partial thromboplastin time to a standard orcontrol partial thromboplastin time.A relative measurement (ratio or percentage) of thesubject's activated partial thromboplastin time to astandard or control partial thromboplastin time.Acid Urate Crystal MeasurementActivated Coagulation TimeActivated PTT/Standard RatioMeasurementActivated PTT to Standard PTT RatioMeasurementC38462Activated PartialThromboplastin TimeActivated PartialThromboplastinTimeA measurement of the length of time that it takes forclotting to occur when reagents are added to a plasmaspecimen. The test is partial due to the absence oftissue factor (Factor III) from the reaction mixture.Partial Thromboplastin TimeC100471Activated Protein CResistanceActivated ProteinC Resistance;Factor V LeidenScreenA measurement of the resistance in the anticoagulationresponse to activated protein C in a biologicalspecimen.Activated Protein C ResistanceMeasurementC92286 Acyl Coenzyme A Oxidase Acyl CoenzymeA Oxidase; FattyAcyl CoenzymeA Oxidase; AcylCoA OxidaseC102257 Adenosine Diphosphate AdenosineDiphosphateA measurement of the acyl coenzyme A oxidase in abiological specimen.A measurement of the adenosine diphosphate in abiological specimen.Acyl Coenzyme A OxidaseMeasurementAdenosine DiphosphateMeasurementC74839 Adiponectin Adiponectin A measurement of the adiponectin hormone in abiological specimen.Adiponectin MeasurementC74780AdrenocorticotropicHormoneAdrenocorticotropic HormoneA measurement of the adrenocorticotropic hormone ina biological specimen.Adrenocorticotropic HormoneMeasurementC100430 Alanine Aminopeptidase AlanineAminopeptidaseC64433 Alanine Aminotransferase AlanineAminotransferase; SGPTC64431 Albumin Albumin;MicroalbuminC74761 Albumin/Creatinine Albumin/Creatinine;Microalbumin/Creatinine RatioC74894 Albumin/Globulin Albumin/GlobulinA measurement of the alanine aminopeptidase in abiological specimen.A measurement of the alanine aminotransferase in abiological specimen.A measurement of the albumin protein in a biologicalspecimen.A relative measurement (ratio or percentage) of thealbumin to the creatinine in a biological specimen.The ratio of albumin to globulin in a biologicalspecimen.Alanine AminopeptidaseMeasurementAlanine AminotransferaseMeasurementAlbumin MeasurementAlbumin To Creatinine Protein RatioMeasurementAlbumin to Globulin RatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 97 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103453 Albumin/Total Protein Albumin/TotalProteinA relative measurement (ratio or percentage) of thealbumin to total protein in a biological specimen.Albumin to Total Protein RatioMeasurementC74731 Aldolase Aldolase A measurement of the aldolase enzyme in a biologicalspecimen.C74841 Aldosterone Aldosterone A measurement of the aldosterone hormone in abiological specimen.Aldolase MeasurementAldosterone MeasurementC64432 Alkaline Phosphatase AlkalinePhosphataseA measurement of the alkaline phosphatase in abiological specimen.Alkaline Phosphatase MeasurementC79438AlkalinePhosphatase/CreatinineAlkalinePhosphatase/CreatinineA relative measurement (ratio or percentage) of thealkaline phosphatase to creatinine in a biologicalspecimen.Alkaline Phosphatase to CreatinineRatio MeasurementC74732 Alpha Fetoprotein AlphaFetoproteinC96562 Alpha Fetoprotein L1 AlphaFetoprotein L1C96563 Alpha Fetoprotein L2 AlphaFetoprotein L2C96564 Alpha Fetoprotein L3 AlphaFetoprotein L3A measurement of the alpha fetoprotein in a biologicalspecimen.A measurement of the alpha fetoprotein L1 in abiological specimen.A measurement of the alpha fetoprotein L2 in abiological specimen.A measurement of the alpha fetoprotein L3 in abiological specimen.Alpha-fetoprotein MeasurementAlpha Fetoprotein L1 MeasurementAlpha Fetoprotein L2 MeasurementAlpha Fetoprotein L3 MeasurementC79433AlphaGlutathione-S-TransferaseAlphaGlutathione-S-TransferaseA measurement of the alpha form of glutathioneS-transferase in a biological specimen.Alpha Glutathione-S-TransferaseMeasurementC103349 Alpha Tocopherol AlphaTocopherolC103350 Alpha Tocopherol/Vitamin E Vitamin E AlphaTocopherolC100429 Alpha-1 Acid Glycoprotein Alpha-1 AcidGlycoproteinC80167 Alpha-1 Antitrypsin Alpha-1Antitrypsin;Serum TrypsinInhibitorC92252 Alpha-1 Globulin Alpha-1Globulin;A1-GlobulinA measurement of the alpha tocopherol in a biologicalspecimen.A relative measurement (ratio or percentage) ofalpha-tocopherol to the total vitamin E in a biologicalspecimen.A measurement of the alpha-1 acid glycoprotein in abiological specimen.A measurement of the alpha-1 antitrypsin in abiological specimen.A measurement of the proteins contributing to thealpha 1 fraction in a biological specimen.Alpha Tocopherol MeasurementAlpha Tocopherol to Vitamin E RatioMeasurementAlpha-1 Acid GlycoproteinMeasurementAlpha-1 Antitrypsin MeasurementAlpha-1 Globulin MeasurementC92253Alpha-1 Globulin/TotalProteinAlpha-1Globulin/TotalProteinA relative measurement (ratio or percentage) ofalpha-1-fraction proteins to total proteins in abiological specimen.Alpha-1 Globulin to Total ProteinRatio MeasurementC100461 Alpha-1 Microglobulin Alpha-1Microglobulin;Protein HCA measurement of the alpha-1 microglobulin in abiological specimen.Alpha-1 Microglobulin MeasurementC100462Alpha-1Microglobulin/CreatinineAlpha-1Microglobulin/CreatinineA relative measurement (ratio or percentage) of thealpha-1 microglobulin to creatinine in a biologicalspecimen.Alpha-1 Microglobulin to CreatinineRatio MeasurementC103351 Alpha-2 Antiplasmin Alpha-2 PlasminInhibitorA measurement of the alpha-2 antiplasmin in abiological specimen.Alpha-2 Antiplasmin MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 98 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92254 Alpha-2 Globulin Alpha-2Globulin;A2-GlobulinA measurement of the proteins contributing to thealpha 2 fraction in a biological specimen.Alpha-2 Globulin MeasurementC92255Alpha-2 Globulin/TotalProteinAlpha-2Globulin/TotalProteinA relative measurement (ratio or percentage) ofalpha-2-fraction proteins to total proteins in abiological specimen.Alpha-2 Globulin to Total ProteinRatio MeasurementC80168 Alpha-2 Macroglobulin Alpha-2MacroglobulinA measurement of the alpha-2 macroglobulin in abiological specimen.Alpha-2 MacroglobulinMeasurementC74799 Ammonia Ammonia; NH3 A measurement of the ammonia in a biologicalspecimen.Ammonia MeasurementC74758 Ammonium Biurate Crystals AmmoniumBiurate Crystals;AmmoniumUrate Crystals;Acid AmmoniumUrate CrystalsC74759 Ammonium Oxalate Crystals AmmoniumOxalate CrystalsC74665 Amorphous Crystals AmorphousCrystalsA measurement of the ammonium biurate crystalspresent in a biological specimen.A measurement of the ammonium oxalate crystalspresent in a urine specimen.A measurement of the amorphous (Note: phosphate orurate, depending on pH) crystals present in a biologicalspecimen.Urine Ammonium Biurate CrystalMeasurementUrine Ammonium Oxalate CrystalMeasurementAmorphous Crystal MeasurementC92243Amorphous PhosphateCrystalsAmorphousPhosphateCrystalsA measurement of the amorphous phosphate crystalsin a biological specimen.Amorphous Phosphate CrystalsMeasurementC74666 Amorphous Sediment AmorphousSediment;AmorphousDebrisC92244 Amorphous Urate Crystals AmorphousUrate CrystalsA measurement of the amorphous sediment present ina biological specimen.A measurement of the amorphous urate crystals in abiological specimen.Amorphous Sediment MeasurementAmorphous Urate CrystalsMeasurementC74687 Amphetamine Amphetamine A measurement of any amphetamine class drug presentin a biological specimen.C64434 Amylase Amylase A measurement of the total enzyme amylase in abiological specimen.Amphetamine Drug ClassMeasurementAmylase MeasurementC98767 Amylase, Pancreatic Amylase,PancreaticC98780 Amylase, Salivary Amylase,SalivaryC103352 Amyloid Beta 1-38 Amyloid Beta38; AmyloidBeta 38 Protein;Amyloid Beta1-38C103353 Amyloid Beta 1-40 Amyloid Beta40; AmyloidBeta 40 Protein;Amyloid Beta1-40A measurement of the pancreatic enzyme amylase in abiological specimen.A measurement of the salivary enzyme amylase in abiological specimen.A measurement of amyloid beta protein which iscomposed of peptides 1 to 38 in a biologicalspecimen.A measurement of amyloid beta protein which iscomposed of peptides 1 to 40 in a biologicalspecimen.Pancreatic Amylase MeasurementSalivary Amylase MeasurementAmyloid Beta 1-38 MeasurementAmyloid Beta 1-40 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 99 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC84809 Amyloid Beta 1-42 Amyloid Beta42; AmyloidBeta 42 Protein;Amyloid Beta1-42A measurement of amyloid beta protein which iscomposed of peptides 1 to 42 in a biologicalspecimen.Beta Amyloid 42 MeasurementC81998 Amyloid P Amyloid P A measurement of the total amyloid P in a biologicalspecimen.Amyloid P MeasurementC81999 Amyloid, Beta Amyloid, Beta;Beta AmyloidA measurement of the total amyloid beta in abiological specimen.Beta Amyloid MeasurementC74842 Androstenediol Androstenediol A measurement of the androstenediol metabolite in abiological specimen.Androstenediol MetaboliteMeasurementC74843 Androstenedione Androstenedione;4-AndrostenedioneA measurement of the androstenedione hormone in abiological specimen.Androstenedione MeasurementC80169Angiotensin ConvertingEnzymeAngiotensinConvertingEnzymeA measurement of the angiotensin converting enzymein a biological specimen.Angiotensin Converting EnzymeMeasurementC74844 Angiotensin I Angiotensin I A measurement of the angiotensin I hormone in abiological specimen.C74845 Angiotensin II Angiotensin II A measurement of the angiotensin II hormone in abiological specimen.Angiotensin I MeasurementAngiotensin II MeasurementC74846 Angiotensinogen Angiotensinogen;AngiotensinPrecursorA measurement of the angiotensinogen hormone in abiological specimen.Angiotensinogen MeasurementC74685 Anion Gap Anion Gap A computed estimate of the unmeasured anions (thoseother than the chloride and bicarbonate anions) in abiological specimen.C74797 Anisocytes Anisocytes A measurement of the inequality in the size of the redblood cells in a whole blood specimen.Anion Gap MeasurementAnisocyte MeasurementC81973 Anti-DNA Antibodies Anti-DNAAntibodies;Anti-ds-DNAAntibodiesC74913 Anti-Double Stranded DNA Anti-DoubleStranded DNAC98706 Anti-Factor Xa Activity Anti-Factor XaActivityA measurement of the anti-DNA antibodies in abiological specimen.A measurement of the anti-double stranded DNAantibody in a biological specimen.A measurement of the anti-activated Factor X in abiological specimen. This test is used to monitor lowmolecular weight or unfractionated heparin levels in abiological specimen.Anti-DNA Antibody MeasurementAnti-Double Stranded DNAMeasurementAnti-Factor Xa ActivityMeasurementC81976Anti-Saccharomycescerevisiae AntibodyAnti-Saccharomyces cerevisiaeAntibodyA measurement of the anti-Saccharomyces cerevisiaeantibody in a biological specimen.Anti-Saccharomyces cerevisiaeAntibody MeasurementC92269 Anti-Single Stranded DNA IgG Anti-SingleStranded DNAIgGA measurement of the anti-single stranded DNA IgGantibody in a biological specimen.Anti-Single Stranded DNA IgGMeasurementC74691 Antidepressants Antidepressants A measurement of any antidepressant class drugpresent in a biological specimen.Antidepressant MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 100 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74847 Antidiuretic Hormone AntidiureticHormone;VasopressinC81974 Antiglobulin Test, Direct AntiglobulinTest, Direct;Direct CoombsTestC91372 Antiglobulin Test, Indirect AntiglobulinTest, Indirect;Indirect CoombsTestC81975 Antimitochondrial Antibodies Antimitochondrial Antibodies;MitochondrialAntibodyC74916 Antinuclear Antibodies AntinuclearAntibodiesC102258 Antiphospholipid Antibodies AntiphospholipidAntibodiesC81958 Antithrombin Antithrombin;Antithrombin III;AntithrombinActivity;Antithrombin IIIActivityC81977 Antithrombin Antigen AntithrombinAntigen;Antithrombin IIIAntigenC74733 Apolipoprotein A1 ApolipoproteinA1C103354 Apolipoprotein A4 ApolipoproteinA4C103355 Apolipoprotein A5 ApolipoproteinA5C82000 Apolipoprotein AII ApolipoproteinAIIA measurement of the antidiuretic hormone in abiological specimen.A measurement of the antibody or complement-coatederythrocytes in a biological specimen in vivo.A test that uses Coombs' reagent to detect thepresence of anti-erythrocyte antibodies in a biologicalspecimen.A measurement of the antimitochondrial antibodies ina biological specimen.A measurement of the antinuclear antibodies(antibodies that attack the body's own tissue) in abiological specimen.A measurement of the antiphospholipid antibodies in abiological specimen.A measurement of the antithrombin activity in abiological specimen.A measurement of the antithrombin antigen in abiological specimen.A measurement of the apolipoprotein A1 in the highdensity lipoproteins of a biological specimen.A measurement of the apolipoprotein A4 in abiological specimen.A measurement of the apolipoprotein A5 in abiological specimen.A measurement of the apolipoprotein AII in abiological specimen.Antidiuretic Hormone MeasurementDirect Antiglobulin TestIndirect Antiglobulin TestAntimitochondrial AntibodyMeasurementAntinuclear Antibody MeasurementAntiphospholipid AntibodyMeasurementAntithrombin MeasurementAntithrombin Antigen MeasurementApolipoprotein A1 MeasurementApolipoprotein A4 MeasurementApolipoprotein A5 MeasurementApolipoprotein AII MeasurementC74734 Apolipoprotein B Apolipoprotein B A measurement of the apolipoprotein B in the lowdensity lipoproteins of a biological specimen.Apolipoprotein B MeasurementC103356ApolipoproteinB/Apolipoprotein A1ApolipoproteinB/ApolipoproteinA1A relative measurement (ratio or percentage) of theApolipoprotein B to Apolipoprotein A1 in a biologicalspecimen.Apolipoprotein B to ApolipoproteinA1 Ratio MeasurementC100427 Apolipoprotein C2 ApolipoproteinC2;ApolipoproteinCIIA measurement of the apolipoprotein C2 in abiological specimen.Apolipoprotein C2 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 101 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC82001 Apolipoprotein CIII ApolipoproteinCIIIA measurement of the apolipoprotein CIII in abiological specimen.Apolipoprotein CIII MeasurementC82002 Apolipoprotein E Apolipoprotein E A measurement of the apolipoprotein E in a biologicalspecimen.Apolipoprotein E MeasurementC92293 Apolipoprotein E4 ApolipoproteinE4C82003 Apolipoprotein H ApolipoproteinHC100428 Apolipoprotein J Apolipoprotein J;ClusterinA measurement of the apolipoprotein E4 in abiological specimen.A measurement of the apolipoprotein H in a biologicalspecimen.A measurement of the apolipoprotein J in a biologicalspecimen.Apolipoprotein E4 MeasurementApolipoprotein H MeasurementApolipoprotein J MeasurementC102259 Arachidonic Acid Arachidonic Acid A measurement of the arachidonic acid present in abiological specimen.Arachidonic Acid MeasurementC64467 Aspartate Aminotransferase AspartateAminotransferase; SGOTA measurement of the aspartate aminotransferase in abiological specimen.Aspartate AminotransferaseMeasurementC81978Aspartate AminotransferaseAntigenAspartateAminotransferase Antigen; SGOTAntigenA measurement of the aspartate aminotransferaseantigen in a biological specimen.Aspartate Aminotransferase AntigenMeasurementC74886 Atrial Natriuretic Peptide Atrial NatriureticPeptide;AtriopeptinA measurement of the atrial natriuretic peptide in abiological specimen.Atrial Natriuretic PeptideMeasurementC74657 Auer Rods Auer Rods A measurement of the Auer rods (elongated needlestructures that are found in the cytoplasm of leukemicblasts and are formed by clumps of azurophilicgranular material) in a biological specimen.C98710 BKV Viral Load BKV Viral Load A measurement of the BK virus viral load in abiological specimen.C74753 BUN/Creatinine BUN/Creatinine A relative measurement (ratio or percentage) (ratio) ofthe blood urea nitrogen (BUN) to the creatinine in abiological specimen.C64469 Bacteria Bacteria A measurement of the bacteria in a biologicalspecimen.C74762 Bacterial Casts Bacterial Casts A measurement of the bacterial casts present in abiological specimen.C74688 Barbiturates Barbiturates A measurement of any barbiturate class drug present ina biological specimen.Auer Rod MeasurementBK Virus Viral Load MeasurementBlood Urea Nitrogen To CreatinineRatio MeasurementBacterial CountBacterial Cast MeasurementBarbiturate Drug Class MeasurementC96567 Basophilic Stippling BasophilicStipplingA measurement of the basophilic stippling in abiological specimen.Basophilic Stippling MeasurementC64470 Basophils Basophils A measurement of the basophils in a biologicalspecimen.Total Basophil CountC64471 Basophils/Leukocytes Basophils/LeukocytesC98865 Basophils/Total Cells Basophils/TotalCellsA relative measurement (ratio or percentage) of thebasophils to leukocytes in a biological specimen.A relative measurement (ratio or percentage) of thebasophils to total cells in a biological specimen (forexample a bone marrow specimen).Basophil To Leukocyte RatioBasophil to Total CellRatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 102 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74692 Benzodiazepine Benzodiazepine A measurement of any benzodiazepine class drugpresent in a biological specimen.Benzodiazepine MeasurementC75350 Benzoylecgonine Benzoylecgonine; CocaineMetaboliteC100472 Beta Carotene Beta Carotene;b-Carotene; BetaCarotinA measurement of the benzoylecgonine in a biologicalspecimen.A measurement of the beta carotene in a biologicalspecimen.Benzoylecgonine MeasurementBeta Carotene MeasurementC103357 Beta Catenin Beta Catenin A measurement of the beta catenin in a biologicalspecimen.C92256 Beta Globulin Beta Globulin A measurement of the proteins contributing to the betafraction in a biological specimen.Beta Catenin MeasurementBeta Globulin MeasurementC92294 Beta Globulin/Total Protein BetaGlobulin/TotalProteinA relative measurement (ratio or percentage) of betafraction proteins to total proteins in a biologicalspecimen.Beta Globulin to Total Protein RatioMeasurementC103358Beta-2 Glycoprotein 1 IgGAntibodyBeta-2Glycoprotein 1IgG AntibodyA measurement of the Beta-2 glycoprotein 1 IgGantibodies in a biological specimen.Beta-2 Glycoprotein 1 IgG AntibodyMeasurementC103359Beta-2 Glycoprotein 1 IgMAntibodyBeta-2Glycoprotein 1IgM AntibodyA measurement of the Beta-2 glycoprotein 1 IgMantibodies in a biological specimen.Beta-2 Glycoprotein 1 IgM AntibodyMeasurementC81979 Beta-2 Glycoprotein Antibody Beta-2GlycoproteinAntibodyC81980 Beta-2 Microglobulin Beta-2MicroglobulinC96568 Beta-Hydroxybutyrate Beta-Hydroxybutyrate;B-Hydroxybutyrate;3-HydroxybutyrateC100426 Beta-Trace Protein Beta-TraceProteinC74667 Bicarbonate Bicarbonate;HCO3C74800 Bile Acid Bile Acid; BileSalts; Bile Acids;Bile SaltC38037 Bilirubin Bilirubin; TotalBilirubinC74668 Bilirubin Crystals BilirubinCrystalsA measurement of the beta-2 glycoprotein antibody ina biological specimen.A measurement of the beta-2 microglobulin in abiological specimen.A measurement of the total Beta-hydroxybutyrate in abiological specimen.A measurement of the beta-trace protein in abiological specimen.A measurement of the bicarbonate in a biologicalspecimen.A measurement of the total bile acids in a biologicalspecimen.A measurement of the total bilirubin in a biologicalspecimen.A measurement of the bilirubin crystals present in abiological specimen.Beta-2 Glycoprotein AntibodyMeasurementBeta-2 Microglobulin MeasurementBeta-Hydroxybutyrate MeasurementBeta-Trace Protein MeasurementBicarbonate MeasurementBile Acid MeasurementTotal Bilirubin MeasurementBilirubin Crystal MeasurementC74700 Bite Cells Bite Cells A measurement of the bite cells (erythrocytes with theappearance of a bite having been removed, due tooxidative hemolysis) in a biological specimen .Bite Cell CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 103 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74634 Bite Cells/Erythrocytes BiteCells/ErythrocytesA relative measurement (ratio or percentage) of bitecells (erythrocytes with the appearance of a bite havingbeen removed, due to oxidative hemolysis) to allerythrocytes in a biological specimen .Bite Cell to Erythrocyte RatioMeasurementC74605 Blasts Blasts A measurement of the blast cells in a biologicalspecimen.Blast CountC64487 Blasts/Leukocytes Blasts/LeukocytesC61019 Blood Urea Nitrogen Blood UreaNitrogenA relative measurement (ratio or percentage) of theblasts to leukocytes in a biological specimen.A measurement of the urea nitrogen in a bloodspecimen.Blast To Leukocyte RatioBlood Urea Nitrogen MeasurementC92287Bone Specific AlkalinePhosphataseBone SpecificAlkalinePhosphataseA measurement of the bone specific alkalinephosphatase isoform in a biological specimen.Bone Specific Alkaline PhosphataseMeasurementC74735 Brain Natriuretic Peptide Brain NatriureticPeptide; B-TypeNatriureticPeptideA measurement of the brain (B-type) natriureticpeptide in a biological specimen.Brain Natriuretic PeptideMeasurementC82004Brain-Derived NeurotrophicFactorBrain-DerivedNeurotrophicFactorA measurement of the brain-derived neurotrophicfactor in a biological specimen.Brain-Derived Neurotrophic FactorMeasurementC96588 Broad Casts Broad Casts A measurement of the broad casts in a biologicalspecimen.Broad Casts MeasurementC74701 Burr Cells Burr Cells;KeratocytesC64548 C Reactive Protein C ReactiveProteinA measurement of the Burr cells (erythrocytescharacterized by the presence of small, bluntprojections evenly distributed across the cell surface)in a biological specimen.A measurement of the C reactive protein in abiological specimen.Burr Cell CountC-Reactive Protein MeasurementC74736 C-peptide C-peptide A measurement of the C (connecting) peptide ofinsulin in a biological specimen.C103364 CD1 CD1 A count of the CD1 expressing cells per unit of abiological specimen.C103365 CD14 CD14 A count of the CD14 expressing cells per unit of abiological specimen.C103808 CD19 CD19 A count of the CD19 expressing cells per unit in abiological specimen.C-peptide MeasurementCD1 Expressing Cell MeasurementCD14 Expressing Cell MeasurementCD19 Expressing Cell CountC1038<strong>12</strong> CD19/Lymphocytes CD19/LymphocytesA relative measurement (ratio or percentage) of CD19expressing cells to all lymphocytes in a biologicalspecimen.CD19 Cell to Lymphocyte RatioMeasurementC103366 CD2 CD2 A count of the CD2 expressing cells per unit of abiological specimen.CD2 Expressing Cell MeasurementC103367 CD2/Lymphocytes CD2/LymphocytesA relative measurement (ratio or percentage) of CD2expressing cells to all lymphocytes in a biologicalspecimen.CD2 Expressing Cell to LymphocyteRatio MeasurementC103368 CD20 CD20 A count of the CD20 expressing cells per unit of abiological specimen.CD20 Expressing Cell MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 104 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103809 CD3 CD3 A count of the CD3 expressing cells per unit in abiological specimen.CD3 Expressing Cell CountC103813 CD3/Lymphocytes CD3/LymphocytesA relative measurement (ratio or percentage) of CD3expressing cells to all lymphocytes in a biologicalspecimen.CD3 Cell to Lymphocyte RatioMeasurementC102260 CD34 CD34 A measurement of the CD34 expressing cells per unitin a biological specimen.C103810 CD4 CD4 A count of the CD4 expressing cells per unit in abiological specimen.C103814 CD4/CD8 CD4/CD8 A relative measurement (ratio or percentage) of CD4expressing cells to the CD8 expressing cells in abiological specimen.CD34 MeasurementCD4 Expressing Cell CountCD4 Cell to CD8 Cell RatioMeasurementC103815 CD4/Lymphocytes CD4/LymphocytesA relative measurement (ratio or percentage) of CD4expressing cells to all lymphocytes in a biologicalspecimen.CD4 Cell to Lymphocyte RatioMeasurementC82006 CD40 CD40 A count of the CD40 cells per unit in a biologicalspecimen.C82007 CD40 Ligand CD40 Ligand A count of the CD40 ligand cells per unit in abiological specimen.C103369 CD5 CD5 A count of the CD5 expressing cells per unit of abiological specimen.C103370 CD56 CD56 A count of the CD56 expressing cells per unit of abiological specimen.C103811 CD8 CD8 A count of the CD8 expressing cells per unit in abiological specimen.CD40 MeasurementCD40 Ligand MeasurementCD5 Expressing Cell MeasurementCD56 Expressing Cell MeasurementCD8 Expressing Cell CountC103816 CD8/Lymphocytes CD8/LymphocytesA relative measurement (ratio or percentage) of CD8expressing cells to all lymphocytes in a biologicalspecimen.CD8 Cell to Lymphocyte RatioMeasurementC98716 CMV Viral Load CMV Viral Load A measurement of the cytomegalovirus viral load in abiological specimen.C74702 Cabot Rings Cabot Rings A measurement of the Cabot rings (red-purple staining,threadlike, ring or figure 8 shaped filaments in anerythrocyte) in a biological specimen.C74848 Calcitonin Calcitonin A measurement of the calcitonin hormone in abiological specimen.C74849 Calcitriol Calcitriol A measurement of the calcitriol hormone in abiological specimen.C64488 Calcium Calcium A measurement of the calcium in a biologicalspecimen.Cytomegalovirus Viral LoadMeasurementCabot Ring CountCalcitonin MeasurementCalcitriol MeasurementCalcium MeasurementC103360 Calcium - Phosphorus Product Calcium -PhosphorusProductC74669 Calcium Carbonate Crystals CalciumCarbonateCrystalsA measurement of the product of the calcium andphosphate measurements in a biological specimen.A measurement of the calcium carbonate crystalspresent in a biological specimen.Calcium and Phosphorus ProductMeasurementCalcium Carbonate CrystalMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 105 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96589 Calcium Clearance CalciumClearanceC74670 Calcium Oxalate Crystals Calcium OxalateCrystalsC74671 Calcium Phosphate Crystals CalciumPhosphateCrystalsA measurement of the volume of serum or plasma thatwould be cleared of calcium by excretion of urine fora specified unit of time (e.g. one minute).A measurement of the calcium oxalate crystals presentin a biological specimen.A measurement of the calcium phosphate crystalspresent in a biological specimen.Calcium Clearance MeasurementCalcium Oxalate CrystalMeasurementCalcium Phosphate CrystalMeasurementC96590 Calcium Sulphate Calcium Sulphate A measurement of the calcium sulphate in a biologicalspecimen.C81948 Calcium, Ionized Calcium, Ionized A measurement of the ionized calcium in a biologicalspecimen.Calcium Sulphate MeasurementIonized Calcium MeasurementC79439 Calcium/Creatinine Calcium/CreatinineA relative measurement (ratio or percentage) of thecalcium to creatinine in a biological specimen.Calcium to Creatinine RatioMeasurementC82005 Calprotectin Calprotectin A measurement of the calprotectin in a biologicalspecimen.C103361 Cancer Antigen 1 Cancer Antigen 1 A measurement of the cancer antigen 1 in a biologicalspecimen.Calprotectin MeasurementCancer Antigen 1 MeasurementC81981 Cancer Antigen <strong>12</strong>5 Cancer Antigen<strong>12</strong>5C103362 Cancer Antigen 15-3 Cancer Antigen15-3C81982 Cancer Antigen 19-9 Cancer Antigen19-9A measurement of the cancer antigen <strong>12</strong>5 in abiological specimen.A measurement of the cancer antigen 15-3 in abiological specimen.A measurement of the cancer antigen 19-9 in abiological specimen.Cancer Antigen <strong>12</strong>5 MeasurementCancer Antigen 15-3 MeasurementCancer Antigen 19-9 MeasurementC74689 Cannabinoids Cannabinoids A measurement of any cannabinoid class drug presentin a biological specimen.Cannabinoid Drug ClassMeasurementC101016Carbohydrate-DeficientTransferrinCarbohydrate-DeficientTransferrinA measurement of transferrin with a reduced numberof carbohydrate moieties in a biological specimen.Carbohydrate-Deficient TransferrinMeasurementC64545 Carbon Dioxide Carbon Dioxide A measurement of the carbon dioxide gas in abiological specimen.Carbon Dioxide MeasurementC96591 Carboxyhemoglobin CarboxyhemoglobinC81983 Carcinoembryonic Antigen Carcinoembryonic AntigenC103363 Cardiolipin IgM Antibody Cardiolipin IgMAntibodyA measurement of the carboxyhemoglobin in abiological specimen.A measurement of the carcinoembryonic antigen in abiological specimen.A measurement of the cardiolipin IgM antibodies in abiological specimen.Carboxyhemoglobin MeasurementCarcinoembryonic AntigenMeasurementCardiolipin IgM AntibodyMeasurementC74682 Carnitine Carnitine A measurement of the total carnitine in a biologicalspecimen.Total Carnitine MeasurementC92288 Carnitine Acetyl Transferase Carnitine AcetylTransferaseA measurement of the carnitine acetyl transferase in abiological specimen.Carnitine Acetyl TransferaseMeasurementC74677 Carnitine, Free Carnitine, Free A measurement of the free carnitine in a biologicalspecimen.C74763 Casts Casts A statement that indicates casts were looked for in abiological specimen.Free Carnitine MeasurementCast Present Or AbsentSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 106 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74764 Cellular Casts Cellular Casts A measurement of the cellular (white blood cell, redblood cell, epithelial and bacterial) casts present in abiological specimen.Cellular Cast MeasurementC100432 Ceruloplasmin Ceruloplasmin;CaeruloplasminA measurement of ceruloplasmin in a biologicalspecimen.Ceruloplasmin MeasurementC100431Chemokine (C-X-C Motif)Receptor 3Chemokine(C-X-C Motif)Receptor 3;GPR9, CD183,CXCR3A measurement of the CXCR3, chemokine (C-X-Cmotif) receptor 3, in a biological specimen.Chemokine Receptor CXCR3MeasurementC92432Chlamydia pneumoniae IgAAntibodyChlamydiapneumoniae IgAAntibodyA measurement of the Chlamydia pneumoniae IgAantibody in a biological specimen.Chlamydia pneumoniae IgA AntibodyMeasurementC92433Chlamydia pneumoniae IgMAntibodyChlamydiapneumoniae IgMAntibodyA measurement of the Chlamydia pneumoniae IgM in abiological specimen.Chlamydia pneumoniae IgMAntibody MeasurementC100464Chlamydia trachomatis IgAAntibodyChlamydiatrachomatis IgAAntibodyA measurement of the Chlamydia trachomatis IgAantibody in a biological specimen.Chlamydia trachomatis IgA AntibodyMeasurementC100466Chlamydia trachomatis IgGAntibodyChlamydiatrachomatis IgGAntibodyA measurement of the Chlamydia trachomatis IgGantibody in a biological specimen.Chlamydia trachomatis IgG AntibodyMeasurementC100465Chlamydia trachomatis IgMAntibodyChlamydiatrachomatis IgMAntibodyA measurement of the Chlamydia trachomatis IgMantibody in a biological specimen.Chlamydia trachomatis IgM AntibodyMeasurementC64495 Chloride Chloride A measurement of the chloride in a biologicalspecimen.Chloride MeasurementC79440 Chloride/Creatinine Chloride/CreatinineC74850 Cholecystokinin Cholecystokinin;PancreozyminC61032 Cholesterol Cholesterol;TotalCholesterolC74672 Cholesterol Crystals CholesterolCrystalsC80171 Cholesterol/HDL-Cholesterol Cholesterol/HDL-CholesterolA relative measurement (ratio or percentage) of thechloride to creatinine in a biological specimen.A measurement of the cholecystokinin hormone in abiological specimen.A measurement of the cholesterol in a biologicalspecimen.A measurement of the cholesterol crystals present in abiological specimen.A relative measurement (ratio or percentage) of totalcholesterol to high-density lipoprotein cholesterol(HDL-C) in a biological specimen.Chloride to Creatinine RatioMeasurementCholecystokinin MeasurementSerum Total CholesterolMeasurementCholesterol Crystal MeasurementCholesterol to HDL-CholesterolRatio MeasurementC103380Cholesteryl Ester TransferProtein ActCholesterylEster TransferProtein ActA measurement of the biological activity ofcholesteryl ester transfer protein in a biologicalspecimen.Cholesteryl Ester Transfer ProteinActivity MeasurementC92289 Cholinesterase Cholinesterase A measurement of the cholinesterase in a biologicalspecimen.Cholinesterase MeasurementC64851 Choriogonadotropin Beta Choriogonadotropin Beta;Pregnancy TestA measurement of the Choriogonadotropin Beta in abiological specimen.Choriogonadotropin BetaMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 107 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96592 Circulating Endothelial Cells CirculatingEndothelial CellsC96593 Circulating Tumor Cells CirculatingTumor CellsA measurement of the circulating endothelial cells in abiological specimen.A measurement of the circulating tumor cells in abiological specimen.Circulating Endothelial Cell CountCirculating Tumor Cell CountC92248 Citrate Citrate A measurement of the citrate in a biological specimen. Citrate MeasurementC96594 Clarity Clarity A measurement of the transparency of a biologicalspecimen.Clarity MeasurementC103381 Clostridium difficile Toxin Clostridiumdifficile ToxinA measurement of the Clostridium difficile toxin in abiological specimen.Clostridium difficile ToxinMeasurementC103382Clostridium tetani IgGAntibodyClostridiumtetani IgGAntibodyA measurement of the Clostridium tetani IgG antibodyin a biological specimen.Clostridium tetani IgG AntibodyMeasurementC102261 Clue Cells Clue Cells A measurement of the clue cells in a biologicalspecimen.C74690 Cocaine Cocaine A measurement of the cocaine present in a biologicalspecimen.C74877 Codeine Codeine A measurement of the codeine present in a biologicalspecimen.C103383 Collagen Type IV Collagen Type IV A measurement of the collagen type IV in a biologicalspecimen.Clue Cell CountCocaine MeasurementCodeine MeasurementCollagen Type IV MeasurementC64546 Color Color A measurement of the color of a biological specimen. Color AssessmentC80172 Complement Bb Complement Bb A measurement of the complement Bb in a biologicalspecimen.Complement Bb MeasurementC80173 Complement C1q Antibody ComplementC1q AntibodyA measurement of the complement C1q antibody in abiological specimen.Complement C1q AntibodyMeasurementC80174 Complement C3 Complement C3 A measurement of the complement C3 in a biologicalspecimen.Complement C3 MeasurementC80175 Complement C3a ComplementC3a; ASP,Complement C3DesArg,Acetylation-stimulating ProteinC80176 Complement C3b ComplementC3bA measurement of the complement C3a in a biologicalspecimen.A measurement of the complement C3b in a biologicalspecimen.Complement C3a MeasurementComplement C3b MeasurementC80177 Complement C4 Complement C4 A measurement of the complement C4 in a biologicalspecimen.C80178 Complement C4a Complement C4a A measurement of the complement C4a in a biologicalspecimen.C80179 Complement C5a Complement C5a A measurement of the complement C5a in a biologicalspecimen.Complement C4 MeasurementComplement C4a MeasurementComplement C5a MeasurementC100423 Complement CH50 ComplementCH50; TotalHemolyticComplement;CH50A measurement of the complement required to lyse 50percent of red blood cells in a biological specimen.CH50 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 108 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC80160 Complement Total ComplementTotalA measurement of the total complement in abiological specimen.Complement MeasurementC79434 Corticosterone Corticosterone A measurement of corticosterone in a biologicalspecimen.Corticosterone MeasurementC74851Corticotropin ReleasingHormoneCorticotropinReleasingHormone;CorticotropinReleasing FactorA measurement of the corticotropin releasinghormone in a biological specimen.Corticotropin Releasing HormoneMeasurementC74781 Cortisol Cortisol; TotalCortisolA measurement of the cortisol in a biologicalspecimen.Cortisol MeasurementC88113 Cortisol, Free Cortisol, Free A measurement of the free, unbound cortisol in abiological specimen.C92249 Cotinine Cotinine A measurement of the cotinine in a biologicalspecimen.C64489 Creatine Kinase Creatine Kinase A measurement of the total creatine kinase in abiological specimen.Free Cortisol MeasurementCotinine MeasurementCreatine Kinase MeasurementC64490 Creatine Kinase BB Creatine KinaseBBA measurement of the homozygous B-type creatinekinase in a biological specimen.Creatine Kinase BB MeasurementC79466Creatine Kinase BB/TotalCreatine KinaseCreatine KinaseBB/TotalCreatine KinaseA relative measurement (ratio or percentage) of theBB-type creatine kinase to total creatine kinase in abiological specimen.Creatine Kinase BB to Total CreatineKinase Ratio MeasurementC64491 Creatine Kinase MB Creatine KinaseMBA measurement of the heterozygous MB-type creatinekinase in a biological specimen.Creatine Kinase MB MeasurementC79441Creatine Kinase MB/TotalCreatine KinaseCreatine KinaseMB/TotalCreatine KinaseA relative measurement (ratio or percentage) of theMB-type creatine kinase to total creatine kinase in abiological specimen.Creatine Kinase MB to TotalCreatine Kinase Ratio MeasurementC64494 Creatine Kinase MM Creatine KinaseMMA measurement of the homozygous M-type creatinekinase in a biological specimen.Creatine Kinase MM MeasurementC79442Creatine Kinase MM/TotalCreatine KinaseCreatine KinaseMM/TotalCreatine KinaseA relative measurement (ratio or percentage) of theMM-type creatine kinase to total creatine kinase in abiological specimen.Creatine Kinase MM to TotalCreatine Kinase Ratio MeasurementC64547 Creatinine Creatinine A measurement of the creatinine in a biologicalspecimen.Creatinine MeasurementC25747 Creatinine Clearance CreatinineClearanceC74703 Crenated Cells Crenated Cells;EchinocytesA measurement of the volume of serum or plasma thatwould be cleared of creatinine by excretion of urinefor a specified unit of time (e.g. one minute).A measurement of the Burr cells (erythrocytescharacterized by the presence of multiple small, sharpprojections evenly distributed across the cell surface)in a biological specimen.Creatinine ClearanceCrenated Cell MeasurementC74673 Crystals Crystals A statement that indicates crystals were looked for in abiological specimen.Crystal Present Or AbsentC96595Cyclic Citrullinated PeptideAntibodyCyclicCitrullinatedPeptide AntibodyA measurement of the cyclic citrullinated peptideantibody in a biological specimen.Cyclic Citrullinated PeptideAntibody MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 109 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92290 Cystatin C Cystatin C A measurement of the cystatin C in a biologicalspecimen.C74674 Cystine Crystals Cystine Crystals A measurement of the cystine crystals present in abiological specimen.Cystatin C MeasurementCystine Crystal MeasurementC96596Cytomegalovirus IgGAntibodyCytomegalovirusIgG AntibodyA measurement of the cytomegalovirus IgG antibodyin a biological specimen.Cytomegalovirus IgG AntibodyMeasurementC96597Cytomegalovirus IgMAntibodyCytomegalovirusIgM AntibodyA measurement of the cytomegalovirus IgM antibodyin a biological specimen.Cytomegalovirus IgM AntibodyMeasurementC82621 D-Dimer D-Dimer A measurement of the d-dimers in a biologicalspecimen.D-Dimer MeasurementC100463 DNase-B Antibody DNase-BAntibody;Anti-Dnase BA measurement of Dnase-B antibody in a biologicalspecimen.DNase-B Antibody MeasurementC100441 DTPA Clearance DTPA Clearance A measurement of the volume of serum or plasma thatwould be cleared of Diethylenetriamine pentaacetate(DTPA) through its excretion for a specified unit oftime.Diethylene Triamine PentaaceticAcid ClearanceC64801 Dacryocytes Dacryocytes;Tear ShapedErythrocytes;Teardrop CellsC74852 Dehydroepiandrosterone Dehydroepiandrosterone;DehydroisoandrosteroneA measurement of dacryocytes in a biologicalspecimen.A measurement of the dehydroepiandrosteronehormone in a biological specimen.Dacryocyte AnalysisDehydroepiandrosteroneMeasurementC96629DehydroepiandrosteroneSulfateDehydroepiandrosterone Sulfate;DHEA-S;sDHEA; DHEASulfateA measurement of the sulfatedDehydroepiandrosterone in a biological specimen.Sulfated DHEA MeasurementC79443 Deoxypyridinoline DeoxypyridinolineC79444 Deoxypyridinoline/Creatinine Deoxypyridinoline/CreatinineC102262 Dextroamphetamine Dextroamphetamine;d-amphetamineA measurement of the deoxypyridinoline in abiological specimen.A relative measurement (ratio or percentage) of thedeoxypyridinoline to creatinine in a biologicalspecimen.A measurement of the dextroamphetamine in abiological specimen.Deoxypyridinoline MeasurementDeoxypyridinoline to CreatinineRatio MeasurementDextroamphetamine MeasurementC74878 Dihydrocodeine Dihydrocodeine A measurement of the dihydrocodeine present in abiological specimen.Dihydrocodeine MeasurementC74853 Dihydrotestosterone Dihydrotestosterone;AndrostanaloneA measurement of the dihydrotestosterone hormone ina biological specimen.Dihydrotestosterone MeasurementC96696Dilute Russell's Viper VenomTimeDilute Russell'sViper VenomTime; LupusAnticoagulantTestA measurement of the time it takes a plasma sample toclot after adding dilute Russell's viper venom.Dilute Russell's Viper Venom TimeMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 110 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103386Dilute Russell's Viper VenomTime RatioLupusAnticoagulantRatioA relative measurement (ratio or percentage) of thedilute Russell's viper venom time in a subject sampleto a control sample.Dilute Russell's Viper Venom Timeto Control Ratio MeasurementC103387Dipeptidyl Peptidase-4ActivityDPPIV ActivityA measurement of the biological activity of dipeptidylpeptidase-4 in a biological specimen.Dipeptidyl Peptidase-4 ActivityMeasurementC103388 Diphtheria IgG Antibody Diphtheria IgGAntibodyA measurement of the Diphtheria IgG antibodies in abiological specimen.Diphtheria IgG AntibodyMeasurementC64481 Direct Bilirubin Direct Bilirubin A measurement of the conjugated or water-solublebilirubin in a biological specimen.C74610 Dohle Bodies Dohle Bodies A measurement of the Dohle bodies (blue-gray,basophilic, leukocyte inclusions located in theperipheral cytoplasm of neutrophils) in a biologicalspecimen.C74854 Dopamine Dopamine A measurement of the dopamine hormone in abiological specimen.C78139 Drug Screen Drug Screen An indication of the presence or absence ofrecreational drugs or drugs of abuse in a biologicalspecimen.C98721 EBV Viral Load EBV Viral Load A measurement of the Epstein-Barr virus viral load in abiological specimen.C100440 EDTA Clearance EDTA Clearance A measurement of the volume of serum or plasma thatwould be cleared of Ethylenediamine tetraacetic acid(EDTA) through its excretion for a specified unit oftime.Direct Bilirubin MeasurementDohle Body MeasurementDopamine MeasurementDrug TestEpstein-Barr Virus Viral LoadMeasurementEDTA ClearanceC102263 ETP Area Under Curve ETP Area UnderCurve;EndogenousThrombinPotential AreaUnder CurveC102264 ETP Lag Time ETP Lag Time;EndogenousThrombinPotential LagTimeC102265 ETP Lag Time Relative ETP Lag TimeRelative;EndogenousThrombinPotential LagTime RelativeC102267 ETP Peak Height ETP PeakHeight;EndogenousThrombinPotential PeakHeightA measurement of the area under the thrombingeneration curve.A measurement of time from the start of the thrombingeneration test to the point where a predeterminedamount of thrombin is generated.A relative measurement (ratio or percentage) of timefrom the start of the thrombin generation test to thepoint where a predetermined amount of thrombin isgenerated.A measurement of the maximum concentration ofthrombin generated during a thrombin generation test.Endogenous Thrombin PotentialArea Under Curve MeasurementEndogenous Thrombin Potential LagTime MeasurementEndogenous Thrombin Potential LagTime Relative MeasurementEndogenous Thrombin PotentialPeak Height MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 111 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102268 ETP Peak Height Relative ETP Peak HeightRelative;EndogenousThrombinPotential PeakHeight RelativeC102269 ETP Time to Peak ETP Time toPeak;EndogenousThrombinPotential Time toPeakC102270 ETP Time to Peak Relative ETP Time toPeak Relative;EndogenousThrombinPotential Time toPeak RelativeC100422 Ecarin Clotting Time Ecarin ClottingTimeA relative (ratio or percentage) of the maximumconcentration of thrombin generated during a thrombingeneration test.A measurement of the time it takes to generate themaximum concentration of thrombin.A relative (ratio or percentage) measurement of thetime it takes to generate the maximum concentrationof thrombin.A measurement of the activity of thrombin inhibitorsin a biological specimen based on the generation ofmeizothrombin.Endogenous Thrombin PotentialPeak Height Relative MeasurementEndogenous Thrombin PotentialTime to Peak MeasurementEndogenous Thrombin PotentialTime to Peak Relative MeasurementEcarin Clotting Time MeasurementC96598 Eccentrocytes Eccentrocytes A measurement of the eccentrocytes (erythrocytes inwhich the hemoglobin is localized to a particularportion of the cell, noticeable as localized staining) ina biological specimen.Eccentrocyte CountC64549 Elliptocytes Elliptocytes;OvalocytesA measurement of the elliptically shaped erythrocytesin a biological specimen.Elliptocyte CountC102266Endogenous ThrombinPotentialEndogenousThrombinPotentialA measurement of the total concentration of thrombingenerated in the presence of a substrate in a plasma orblood sample.Endogenous Thrombin PotentialMeasurementC82008 Endothelin-1 Endothelin-1 A measurement of the endothelin-1 in a biologicalspecimen.Endothelin-1 MeasurementC84819 Eosinophilic Metamyelocytes EosinophilicMetamyelocytesC84821 Eosinophilic Myelocytes EosinophilicMyelocytesA measurement of the eosinphilic metamyelocytes in abiological specimen.A measurement of the eosinophilic myelocytes in abiological specimen.Eosinophilic Metamyelocyte CountEosinophilic Myelocyte CountC64550 Eosinophils Eosinophils A measurement of the eosinophils in a biologicalspecimen.Eosinophil CountC64604 Eosinophils/Leukocytes Eosinophils/LeukocytesC98720 Eosinophils/Total Cells Eosinophils/Total CellsC81952 Eotaxin-1 Eotaxin-1;ChemokineLigand 11C81953 Eotaxin-2 Eotaxin-2;ChemokineLigand 24A relative measurement (ratio or percentage) of theeosinophils to leukocytes in a biological specimen.A relative measurement (ratio or percentage) of theeosinophils to total cells in a biological specimen (forexample a bone marrow specimen).A measurement of the eotaxin-1 in a biologicalspecimen.A measurement of the eotaxin-2 in a biologicalspecimen.Eosinophil To Leukocyte RatioEosinophils to Total Cell RatioMeasurementEotaxin-1 MeasurementEotaxin-2 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 1<strong>12</strong> of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC81954 Eotaxin-3 Eotaxin-3;ChemokineLigand 26C82009 Epidermal Growth Factor EpidermalGrowth FactorC79445 Epinephrine Epinephrine;AdrenalineA measurement of the eotaxin-3 in a biologicalspecimen.A measurement of the epidermal growth factor in abiological specimen.A measurement of the epinephrine hormone in abiological specimen.Eotaxin-3 MeasurementEpidermal Growth FactorMeasurementEpinephrine MeasurementC82010Epith Neutrophil-ActivatingPeptide 78EpithNeutrophil-Activating Peptide 78A measurement of the epithelial neutrophil-activatingpeptide in a biological specimen.Epithelial Neutrophil-ActivatingPeptide 78 MeasurementC74779 Epithelial Casts Epithelial Casts A measurement of the epithelial cell casts present in abiological specimen.C64605 Epithelial Cells Epithelial Cells A measurement of the epithelial cells in a biologicalspecimen.Epithelial-Cast MeasurementEpithelial Cell CountC96600Epstein-Barr Capsid IgGAntibodyEpstein-BarrCapsid IgGAntibodyA measurement of the Epstein-Barr capsid IgGantibody in a biological specimen.Epstein-Barr Capsid IgG AntibodyMeasurementC96601Epstein-Barr Capsid IgMAntibodyEpstein-BarrCapsid IgMAntibodyA measurement of the Epstein-Barr capsid IgMantibody in a biological specimen.Epstein-Barr Capsid IgM AntibodyMeasurementC96602 Epstein-Barr Early Antigen Epstein-BarrEarly AntigenA measurement of the Epstein-Barr early antigen in abiological specimen.Epstein-Barr Early AntigenMeasurementC96603Epstein-Barr NuclearAntibodyEpstein-BarrNuclearAntibodyA measurement of the Epstein-Barr nuclear antibody ina biological specimen.Epstein-Barr Nuclear AntibodyMeasurementC96604 Epstein-Barr Nuclear Antigen Epstein-BarrNuclear AntigenA measurement of the Epstein-Barr nuclear antigen ina biological specimen.Epstein-Barr Nuclear AntigenMeasurementC64798Ery. Mean Corpuscular HGBConcentrationEry. MeanCorpuscularHGBConcentrationA quantitative measurement of the mean amount ofhemoglobin per erythrocytes in a specified volume ofa biological specimen.Erythrocyte Mean CorpuscularHemoglobin ConcentrationC64797Ery. Mean CorpuscularHemoglobinEry. MeanCorpuscularHemoglobinA quantitative measurement of the mean amount ofhemoglobin per erythrocyte in a biological specimen.Erythrocyte Mean CorpuscularHemoglobinC64799Ery. Mean CorpuscularVolumeEry. MeanCorpuscularVolumeA quantitative measurement of the mean volume oferythrocytes in a biological specimen.Erythrocyte Mean CorpuscularVolumeC92245 Erythrocyte Cell Clumps Erythrocyte CellClumps; RedBlood CellClumps; RBCClumpsC92296 Erythrocyte Cell Morphology Erythrocyte CellMorphology;Red Blood CellMorphology;RBCMorphologyA measurement of red blood cell clumps in abiological specimen.An examination or assessment of the form andstructure of red blood cells.Erythrocyte Cell ClumpsMeasurementErythrocyte Cell MorphologySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 113 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96605 Erythrocyte Ghosts ErythrocyteGhosts; RBCGhostsA measurement of the erythrocyte ghosts(erythrocytes in which hemoglobin has been removedthrough hemolysis) in a biological specimen.Erythrocyte Ghost CountC74611Erythrocyte SedimentationRateErythrocyteSedimentationRate; BiernackiReactionThe distance (e.g. millimeters) that red blood cellssettle in unclotted blood over a specified unit of time(e.g. one hour).Erythrocyte Sedimentation RateMeasurementC51946 Erythrocytes Erythrocytes;Red Blood CellsA measurement of the total erythrocytes in abiological specimen.Erythrocyte CountC64800Erythrocytes DistributionWidthErythrocytesDistributionWidthA value derived from mean corpuscular volume and thestandard deviation of the red blood cell volume in awhole blood specimen.Erythrocyte Distribution WidthMeasurementC74855 Erythropoietin Erythropoietin;HematopoietinC74782 Estradiol Estradiol;OestradiolA measurement of the erythropoietin hormone in abiological specimen.A measurement of the estradiol in a biologicalspecimen.Erythropoietin MeasurementEstradiol MeasurementC74856 Estriol Estriol; Oestriol A measurement of the estriol hormone in a biologicalspecimen.C81963 Estriol, Free Estriol, Free A measurement of the free estriol in a biologicalspecimen.Estriol MeasurementFree Estriol MeasurementC74857 Estrone Estrone;OestroneA measurement of the estrone hormone in a biologicalspecimen.Estrone MeasurementC74693 Ethanol Ethanol; Alcohol A measurement of the ethanol present in a biologicalspecimen.Ethanol MeasurementC82011Extracell Newly Ident RAGEBind ProteinExtracell NewlyIdent RAGE BindProtein; S100Calcium BindingProtein A<strong>12</strong>A measurement of the extracellular newly identifiedRAGE (receptor for advanced glycation end products)binding protein in a biological specimen.Extracell Newly Ident RAGE BindProtein MeasurementC92270Extractable Nuclear AntigenAntibodyAnti-ENA;ExtractableNuclear AntigenAntibodyA measurement of the extractable nuclear antigenantibody in a biological specimen.Extractable Nuclear AntigenAntibody MeasurementC80180 F2-Isoprostane F2-Isoprostane A measurement of the F2-isoprostane in a biologicalspecimen.F2 Isoprostane MeasurementC96626 Factor II Factor II;ProthrombinC81959 Factor III Factor III; TissueFactor, CD142C98725 Factor IX Factor IX;Christmas FactorC103395 Factor IX Activity Christmas FactorActivityC98726 Factor V Factor V; LabileFactorC103396 Factor V Activity Labile FactorActivityA measurement of the Factor II in a biologicalspecimen.A measurement of the factor III in a biologicalspecimen.A measurement of the factor IX in a biologicalspecimen.A measurement of the biological activity of factor IXin a biological specimen.A measurement of the factor V in a biologicalspecimen.A measurement of the biological activity of factor V ina biological specimen.Prothrombin MeasurementFactor III MeasurementFactor IX MeasurementFactor IX Activity MeasurementFactor V MeasurementFactor V Activity MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 114 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102271 Factor V Leiden Factor V Leiden A measurement of the Factor V Leiden in a biologicalspecimen.Factor V Leiden MeasurementC81960 Factor VII Factor VII;Proconvertin;Stable FactorC103397 Factor VII Activity ProconvertinActivity; StableFactor ActivityC81961 Factor VIII Factor VIII;Anti-hemophilicFactorC103399 Factor VIII Activity Anti-hemophilicFactor ActivityC103398 Factor VIIa Activity Factor VIIaActivityA measurement of the factor VII in a biologicalspecimen.A measurement of the biological activity of factor VIIin a biological specimen.A measurement of the factor VIII in a biologicalspecimen.A measurement of the biological activity of factor VIIIin a biological specimen.A measurement of the biological activity of factor VIIain a biological specimen.Factor VII MeasurementFactor VII Activity MeasurementFactor VIII MeasurementFactor VIII Activity MeasurementFactor VIIa Activity MeasurementC98727 Factor X Factor X A measurement of the factor X in a biologicalspecimen.Factor X MeasurementC102272 Factor XIV Factor XIV;AutoprothrombinIIA; Protein C;Protein CAntigenA measurement of the factor XIV in a biologicalspecimen.Factor XIV MeasurementC96648 Fat Fat A measurement of the fat in a biological specimen. Fat MeasurementC81947 Fat Bodies, Oval Fat Bodies, Oval A measurement of the oval-shaped fat bodies, usuallyrenal proximal tubular cells with lipid aggregates in thecytoplasm, in a biological specimen.C98728 Fat Droplet Fat Droplet A measurement of the triglyceride aggregates within abiological specimen.Oval Fat Body MeasurementFat Droplet MeasurementC8<strong>20<strong>12</strong></strong> Fatty Acid Binding Protein 1 Fatty AcidBinding Protein1A measurement of the fatty acid binding protein 1 in abiological specimen.Fatty Acid Binding Protein 1MeasurementC74766 Fatty Casts Fatty Casts A measurement of the fatty casts present in abiological specimen.C74737 Ferritin Ferritin A measurement of the ferritin in a biologicalspecimen.Fatty Cast MeasurementFerritin MeasurementC82013 Fibrin Degradation Products FibrinDegradationProductsA measurement of the fibrin degradation products in abiological specimen.Fibrin Degradation ProductsMeasurementC64606 Fibrinogen Fibrinogen A measurement of the fibrinogen in a biologicalspecimen.Fibrinogen MeasurementC96650 Fibroblast Growth Factor 23 FibroblastGrowth Factor23; PhosphatoninA measurement of the fibroblast growth factor 23 in abiological specimen.Fibroblast Growth Factor 23MeasurementC82014Fibroblast Growth FactorBasic FormFibroblastGrowth FactorBasic Form;FGF2A measurement of the basic form of fibroblast growthfactor in a biological specimen.Fibroblast Growth Factor BasicForm MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 115 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74783 Follicle Stimulating Hormone FollicleStimulatingHormoneC80200 Free Fatty Acid Free Fatty Acid;Non-EsterifiedFatty Acid, FreeC80206 Free Fatty Acid, Saturated Free Fatty Acid,Saturated;Non-esterifiedFatty Acid,SaturatedC80209 Free Fatty Acid, Unsaturated Free Fatty Acid,Unsaturated;Non-esterifiedFatty Acid,UnsaturatedC100448 Free Glycerol Free Glycerol;Free GlycerinC74678 Fructosamine Fructosamine;Glycated SerumProteinA measurement of the follicle stimulating hormone(FSH) in a biological specimen.A measurement of the total non-esterified fatty acidsin a biological specimen.A measurement of the saturated non-esterified fattyacids in a biological specimen.A measurement of the unsaturated non-esterified fattyacids in a biological specimen.A measurement of the amount of unbound glycerol in abiological specimen.A measurement of the fructosamine in a biologicalspecimen.Follicle Stimulating HormoneMeasurementNon-esterified Fatty AcidsMeasurementSaturated Non-esterified Fatty AcidsMeasurementUnsaturated Non-esterified FattyAcids MeasurementFree Glycerol MeasurementFructosamine MeasurementC100450GFR from B-2 MicroglobulinAdj for BSAGFR from B-2MicroglobulinAdj for BSAA measurement of the glomerular filtration rate (GFR)based on the clearance of beta-2 microglobulin afteradjusting it for the body surface area.Glomerular Filtration Rate from B-2Microglobulin Adjusted for BSAMeasurementC100449GFR from Beta-Trace ProteinAdj for BSAGFR fromBeta-TraceProtein Adj forBSAA measurement of the glomerular filtration rate (GFR)based on the clearance of beta-trace protein afteradjusting it for the body surface area.Glomerular Filtration Rate fromBeta-Trace Protein Adjusted for BSAMeasurementC98735GFR from Creatinine Adjustedfor BSAGFR fromCreatinineAdjusted forBSAAn estimation of the glomerular filtration rate adjustedfor body size based on creatinine.Glomerular Filtration Rate fromCreatinine Adjusted for BSAC98736GFR from Cystatin C Adjustedfor BSAGFR fromCystatin CAdjusted forBSAAn estimation of the glomerular filtration rate adjustedfor body size based on cystatin C.Glomerular Filtration Rate fromCystatin C Adjusted for BSAC80182 Galanin Galanin A measurement of the galanin in a biologicalspecimen.C92257 Gamma Globulin Gamma Globulin A measurement of the proteins contributing to thegamma fraction in a biological specimen.Galanin MeasurementGamma Globulin MeasurementC92295Gamma Globulin/TotalProteinGammaGlobulin/TotalProteinA relative measurement (ratio or percentage) ofgamma fraction proteins to total proteins in abiological specimen.Gamma Globulin to Total ProteinRatio MeasurementC64847 Gamma Glutamyl Transferase Gamma GlutamylTransferaseA measurement of the gamma glutamyl transferase in abiological specimen.Gamma Glutamyl TranspeptidaseMeasurementC79446Gamma GlutamylTransferase/CreatinineGamma GlutamylTransferase/CreatinineA relative measurement (ratio or percentage) of thegamma glutamyl transferase to creatinine in abiological specimen.Gamma Glutamyl Transferase toCreatinine Ratio MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 116 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74858 Gastrin Gastrin A measurement of the gastrin hormone in a biologicalspecimen.Gastrin MeasurementC96651 Giant Neutrophils GiantNeutrophilsA measurement of the giant neutrophils in a biologicalspecimen.Giant Neutrophil CountC74728 Giant Platelets Giant Platelets A measurement of the giant (larger than 7um indiameter) platelets in a biological specimen.C74738 Globulin Globulin A measurement of the globulin protein in a biologicalspecimen.Giant Platelet CountGlobulin Protein MeasurementC90505 Glomerular Filtration Rate GlomerularFiltration RateA kidney function test that measures the fluid volumethat is filtered from the kidney glomeruli to theBowman's capsule per unit of time.Glomerular Filtration RateC98734Glomerular Filtration RateAdj for BSAGlomerularFiltration RateAdj for BSAA measurement of the glomerular filtration rateadjusted for body size.Glomerular Filtration Rate Adjustedfor BSAC74859 Glucagon Glucagon A measurement of the glucagon hormone in abiological specimen.Glucagon MeasurementC80183 Glucagon-Like Peptide-1 Glucagon-LikePeptide-1; TotalGlucagon-LikePeptide-1A measurement of the total glucagon-like peptide-1 ina biological specimen.Glucagon-like Peptide-1MeasurementC80164Glucagon-Like Peptide-1,Active FormGlucagon-LikePeptide-1, ActiveFormA measurement of the active form of glucagon-likepeptide-1 in a biological specimen.Active Glucagon-like Peptide-1MeasurementC41376 Glucose Glucose A measurement of the glucose in a biologicalspecimen.Plasma Glucose MeasurementC96652 Glucose Clearance GlucoseClearanceA measurement of the glucose clearance in abiological specimen.Glucose Clearance MeasurementC80184Glucose-6-PhosphateDehydrogenaseGlucose-6-PhosphateDehydrogenaseA measurement of the glucose-6-phosphatedehydrogenase in a biological specimen.Glucose-6-PhosphateDehydrogenase MeasurementC79447 Glucose/Creatinine Glucose/CreatinineC80165 Glucuronidase, Alpha Glucuronidase,AlphaC80170 Glucuronidase, Beta Glucuronidase,BetaC74739 Glutamate Glutamate;Glutamic AcidC79448 Glutamate Dehydrogenase GlutamateDehydrogenaseA relative measurement (ratio or percentage) of theglucose to creatinine in a biological specimen.A measurement of the alpha glucuronidase in abiological specimen.A measurement of the beta glucuronidase in abiological specimen.A measurement of the glutamate in a biologicalspecimen.A measurement of the glutamate dehydrogenase in abiological specimen.Glucose to Creatinine RatioMeasurementAlpha Glucuronidase MeasurementBeta Glucuronidase MeasurementGlutamate MeasurementGlutamate DehydrogenaseMeasurementC82015Glutamic Acid Decarboxylase1Glutamic AcidDecarboxylase 1;Glutamic AcidDecarboxylase67A measurement of the glutamic acid decarboxylase 1in a biological specimen.Glutamic Acid Decarboxylase 1MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 117 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC82016Glutamic Acid Decarboxylase2Glutamic AcidDecarboxylase 2;Glutamic AcidDecarboxylase65A measurement of the glutamic acid decarboxylase 2in a biological specimen.Glutamic Acid Decarboxylase 2MeasurementC82017Glutamic Acid Decarboxylase2 AntibodyGlutamic AcidDecarboxylase 2Antibody;Glutamic AcidDecarboxylase65 AntibodyA measurement of the glutamic acid decarboxylase 2antibody in a biological specimen.Glutamic Acid Decarboxylase 2Antibody MeasurementC96653Glutamic Acid DecarboxylaseAntibodyGlutamic AcidDecarboxylaseAntibody; GADAntibodyA measurement of the glutamic acid decarboxylaseantibody in a biological specimen.Glutamic Acid DecarboxylaseAntibody MeasurementC80166Glutathione S-Transferase,Alpha/CreatGlutathioneS-Transferase,Alpha/CreatA relative measurement (ratio or percentage) of thealpha glutathione-S-transferase to creatinine in abiological specimen.Alpha Glutathione-S-Transferase toCreatinine Ratio MeasurementC80203 Glutathione S-Transferase, Pi GlutathioneS-Transferase, PiA measurement of the Pi glutathione-s-transferase in abiological specimen.Pi Glutathione S-TransferaseMeasurementC80207Glutathione S-Transferase,ThetaGlutathioneS-Transferase,ThetaA measurement of the theta glutathione-s-transferasein a biological specimen.Theta Glutathione S-TransferaseMeasurementC80185Glutathione S-Transferase,TotalGlutathioneS-Transferase,TotalA measurement of the total glutathione-s-transferasein a biological specimen.Glutathione-S-TransferaseMeasurementC79435Glutathione-S-Transferase/CreatinineGlutathione-S-Transferase/CreatinineA relative measurement (ratio or percentage) of theglutathione S-transferase to creatinine in a biologicalspecimen.Glutathione-S-Transferase toCreatinine Ratio MeasurementC80186 Gold Gold A measurement of the gold in a biological specimen. Gold MeasurementC74860Gonadotropin ReleasingHormoneGonadotropinReleasingHormone;LuteinisingHormoneReleasingHormoneA measurement of the gonadotropin releasinghormone in a biological specimen.Gonadotropin Releasing HormoneMeasurementC74768 Granular Casts Granular Casts A measurement of the granular (coarse and fine) castspresent in a biological specimen.Granular Cast MeasurementC74765 Granular Coarse Casts Granular CoarseCastsC74769 Granular Fine Casts Granular FineCastsA measurement of the coarse granular casts present ina biological specimen.A measurement of the fine granular casts present in abiological specimen.Coarse Granular Cast MeasurementGranular Fine Cast MeasurementC82018Granulocyte ColonyStimulating FactorGranulocyteColonyStimulatingFactorA measurement of the granulocyte colony stimulatingfactor in a biological specimen.Granulocyte Colony StimulatingFactor MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 118 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC82019Granulocyte MacrophageColony Stm FactorGranulocyteMacrophageColony StmFactorA measurement of the granulocyte macrophage colonystimulating factor in a biological specimen.Granulocyte Macrophage ColonyStm Factor MeasurementC96654 Granulocytes Granulocytes A measurement of the granulocytes in a biologicalspecimen.Granulocyte CountC98866 Granulocytes/Total Cells Granulocytes/Total CellsA relative measurement (ratio or percentage) of thegranulocytes to total cells in a biological specimen(for example a bone marrow specimen).Granulocyte to Total CellRatioMeasurementC74861Growth Hormone InhibitingHormoneGrowthHormoneInhibitingHormone;SomatostatinA measurement of the growth hormone inhibitinghormone in a biological specimen.Growth Hormone InhibitingHormone MeasurementC74862Growth Hormone ReleasingHormoneGrowthHormoneReleasingHormone;SomatocrininA measurement of the growth hormone releasinghormone in a biological specimen.Growth Hormone ReleasingHormone MeasurementC92541 HAV Viral Load HAV Viral Load A measurement of the hepatitis A viral load in abiological specimen.C92542 HBV Viral Load HBV Viral Load A measurement of the hepatitis B viral load in abiological specimen.Hepatitis A Viral Load MeasurementHepatitis B Viral Load MeasurementC102274HCT CorrectedReticulocytes/ErythrocytesHCT CorrectedReticulocytes/ErythrocytesA relative measurement (ratio or percentage) of thehematocrit corrected reticulocytes to erythrocytes in abiological specimen.Hematocrit Corrected Reticulocytesto Erythrocytes Ratio MeasurementC92543 HCV Viral Load HCV Viral Load A measurement of the hepatitis C viral load in abiological specimen.C61041 HDL Cholesterol HDL Cholesterol A measurement of the high density lipoproteincholesterol in a biological specimen.Hepatitis C Viral Load MeasurementSerum HDL CholesterolMeasurementC100425HDL Cholesterol/LDLCholesterol RatioHDLCholesterol/LDLCholesterolRatioA relative measurement (ratio or percentage) of theamount of HDL cholesterol compared to LDLcholesterol in a biological specimen.HDL Cholesterol to LDLCholesterol Ratio MeasurementC103402 HDL Particle Size HDL ParticleSizeC80187 HDL-Cholesterol Subclass 2 HDL-Cholesterol Subclass 2C80188 HDL-Cholesterol Subclass 3 HDL-Cholesterol Subclass 3A measurement of the average particle size ofhigh-density lipoprotein in a biological specimen.A measurement of the high-density lipoprotein (HDL)cholesterol subclass 2 in a biological specimen.A measurement of the high-density lipoprotein (HDL)cholesterol subclass 3 in a biological specimen.HDL Particle Size MeasurementHDL-Cholesterol Subclass 2MeasurementHDL-Cholesterol Subclass 3MeasurementC92544 HIV Viral Load HIV Viral Load A measurement of the HIV viral load in a biologicalspecimen.C74713 HIV-1 Antibody HIV-1 Antibody A measurement of the antibody reaction of abiological specimen to the HIV-1 virus.HIV Viral Load MeasurementHIV-1 Antibody MeasurementC92263HIV-1 Group M and ONucleic AcidHIV-1 Group Mand O NucleicAcidA measurement of the HIV-1 group M and O nucleicacids in a biological specimen.HIV-1 Group M and O Nucleic AcidMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 119 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92264 HIV-1 Group O Antibody HIV-1 Group OAntibodyC92265 HIV-1 p24 Antigen HIV-1 p24AntigenC74714 HIV-1/2 Antibody HIV-1/2AntibodyA measurement of the HIV-1 group O antibody in abiological specimen.A measurement of the HIV-1 p24 antigen in abiological specimen.A measurement of the antibody reaction of abiological specimen to the either the HIV-1 or HIV-2virus.HIV-1 Group O AntibodyMeasurementHIV-1 p24 Antigen MeasurementHIV-1 HIV-2 Antibody MeasurementC74715 HIV-2 Antibody HIV-2 Antibody A measurement of the antibody reaction of abiological specimen to the HIV-2 virus.HIV-2 Antibody MeasurementC92266 HIV-2 Nucleic Acid HIV-2 NucleicAcidC100460 HLA-B27 Antigen HLA-B27Antigen; HumanLeukocyteAntigen B27A measurement of the HIV-2 nucleic acids in abiological specimen.A measurement of the human leukocyte antigen B27(HLA-B27) in a biological specimen.HIV-2 Nucleic Acid MeasurementHLA-B27 Antigen MeasurementC74604 Hairy Cells Hairy Cells A measurement of the hairy cells (b-cell lymphocyteswith hairy projections from the cytoplasm) in abiological specimen.Hairy Cell CountC74640 Hairy Cells/Lymphocytes HairyCells/LymphocytesA relative measurement (ratio or percentage) of thehairy cells (b-cell lymphocytes with hairy projectionsfrom the cytoplasm) to all lymphocytes in a biologicalspecimen .Hairy Cell to Lymphocyte RatioMeasurementC74740 Haptoglobin Haptoglobin A measurement of the haptoglobin protein in abiological specimen.Haptoglobin Protein MeasurementC74709 Heinz Bodies Heinz Bodies;Heinz-ErhlichBodiesA measurement of the Heinz bodies (small roundinclusions within the body of a red blood cell) in abiological specimen.Heinz-Ehrlich Body MeasurementC103403Helicobacter pylori IgGAntibodyHelicobacterpylori IgGAntibodyA measurement of the Helicobacter pylori IgGantibody in a biological specimen.Helicobacter pylori IgG AntibodyMeasurementC74658 Helmet Cells Helmet Cells A measurement of the Helmet cells (specializedKeratocytes with two projections on either end that aretapered and hornlike) in a biological specimen.C64796 Hematocrit Hematocrit The percentage of a whole blood specimen that iscomposed of red blood cells (erythrocytes).Helmet Cell CountHematocritC102273Hematocrit CorrectedReticulocytesHematocritCorrectedReticulocytesA measurement of the hematocrit correctedreticulocytes in a biological specimen.Hematocrit Corrected ReticulocyteCountC64848 Hemoglobin Hemoglobin A measurement of the hemoglobin in a biologicalspecimen.C92258 Hemoglobin A Hemoglobin A A measurement of the hemoglobin A in a biologicalspecimen.Hemoglobin MeasurementHemoglobin A MeasurementC64849 Hemoglobin A1C HemoglobinA1CA measurement of the glycosylated hemoglobin in abiological specimen.Glycosylated HemoglobinMeasurementC92259 Hemoglobin A2 Hemoglobin A2 A measurement of the hemoglobin A2 in a biologicalspecimen.Hemoglobin A2 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>0 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92260 Hemoglobin B Hemoglobin B A measurement of the hemoglobin B in a biologicalspecimen.C92261 Hemoglobin C Hemoglobin C A measurement of the hemoglobin C in a biologicalspecimen.Hemoglobin B MeasurementHemoglobin C MeasurementC92262 Hemoglobin F Hemoglobin F;FetalHemoglobinA measurement of the hemoglobin F in a biologicalspecimen.Hemoglobin F MeasurementC96659 Hemosiderin Hemosiderin A measurement of the hemosiderin complex in abiological specimen.Hemosiderin MeasurementC92534 Hepatitis A Virus Antibody Hepatitis A VirusAntibodyA measurement of the antibody reaction of abiological specimen to the Hepatitis A virus.Hepatitis A Antibody MeasurementC92271Hepatitis A Virus AntibodyIgMHepatitis A VirusAntibody IgMA measurement of hepatitis A virus antibody IgM in abiological specimen.Hepatitis A Virus Antibody IgMMeasurementC103404 Hepatitis B DNA Hepatitis B DNA A measurement of hepatitis B virus DNA in abiological specimen.Hepatitis B DNA MeasurementC96660Hepatitis B Virus CoreAntibodyHepatitis B VirusCore AntibodyA measurement of the hepatitis B virus core antibodyin a biological specimen.Hepatitis B Virus Core AntibodyMeasurementC96661Hepatitis B Virus Core IgMAntibodyHepatitis B VirusCore IgMAntibodyA measurement of the hepatitis B virus core IgMantibody in a biological specimen.Hepatitis B Virus Core IgM AntibodyMeasurementC74711Hepatitis B Virus SurfaceAntibodyAnti-HBs;HBsAb; HepatitisB Virus SurfaceAntibodyA measurement of the surface antibody reaction of abiological specimen to the Hepatitis B virus.Hepatitis B Surface AntibodyMeasurementC64850Hepatitis B Virus SurfaceAntigenHBsAg; HepatitisB Virus SurfaceAntigenA measurement of the surface antigen reaction of abiological specimen to the Hepatitis B virus.Hepatitis B Virus Surface AntigenMeasurementC96662 Hepatitis B Virus e Antibody Hepatitis B Viruse AntibodyC96663 Hepatitis B Virus e Antigen Hepatitis B Viruse AntigenC92535 Hepatitis C Virus Antibody Hepatitis C VirusAntibodyC96664 Hepatitis D Virus Antibody Hepatitis D VirusAntibody;Hepatitis DeltaAntibodyA measurement of the hepatitis B e antibody in abiological specimen.A measurement of the hepatitis B e antigen in abiological specimen.A measurement of the antibody reaction of abiological specimen to the Hepatitis C virus.A measurement of the hepatitis D virus antibody in abiological specimen.Hepatitis B Virus e AntibodyMeasurementHepatitis B Virus e AntigenMeasurementHepatitis C Antibody MeasurementHepatitis D Virus AntibodyMeasurementC96665Hepatitis E Virus IgMAntibodyHepatitis E VirusIgM AntibodyA measurement of the hepatitis E virus IgM antibody ina biological specimen.Hepatitis E Virus IgM AntibodyMeasurementC92272 Hepatitis G RNA Hepatitis G RNA A measurement of the Hepatitis G RNA in a biologicalspecimen.Hepatitis G RNA MeasurementC96697Herpes Simplex Virus 1 IgGAntibodyHerpes SimplexVirus 1 IgGAntibody; HSV-1IgG AntibodyA measurement of the herpes simplex virus 1 IgGantibody in a biological specimen.Herpes Simplex Virus 1 IgGAntibody MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>1 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98738Herpes Simplex Virus 1 IgMAntibodyHerpes SimplexVirus 1 IgMAntibodyA measurement of the Herpes Simplex virus 1 IgMantibodies in a biological specimen.Herpes Simplex Virus 1 IgMAntibody MeasurementC96698Herpes Simplex Virus 1/2 IgGAntibodyHerpes SimplexVirus 1/2 IgGAntibody;HSV-1/2 IgG AbA measurement of the herpes simplex virus 1 and 2IgG antibody in a biological specimen.Herpes Simplex Virus 1/2 IgGAntibody MeasurementC96666Herpes Simplex Virus 1/2 IgMAntibodyHerpes SimplexVirus 1/2 IgMAntibody;HSV-1/2 IgM AbA measurement of the herpes simplex virus 1 and 2IgM antibody in a biological specimen.Herpes Simplex Virus 1/2 IgMAntibody MeasurementC96667Herpes Simplex Virus 2 IgGAntibodyHerpes SimplexVirus 2 IgGAntibody; HSV-2IgG AntibodyA measurement of the herpes simplex virus 2 IgGantibody in a biological specimen.Herpes Simplex Virus 2 IgGAntibody MeasurementC98739Herpes Simplex Virus 2 IgMAntibodyHerpes SimplexVirus 2 IgMAntibodyA measurement of the Herpes Simplex virus 2 IgMantibodies in a biological specimen.Herpes Simplex Virus 2 IgMAntibody MeasurementC81984 Heterophile Antibodies HeterophileAntibodiesA measurement of the heterophile antibodies in abiological specimen.Heterophile Antibody MeasurementC96668 Hexokinase Hexokinase A measurement of the hexokinase in a biologicalspecimen.Hexokinase MeasurementC74754 Hippuric Acid Crystals Hippuric AcidCrystalsA measurement of the hippuric acid crystals present ina biological specimen.Hippuric Acid Crystal MeasurementC80189 Histamine Histamine A measurement of the histamine in a biologicalspecimen.C74741 Homocysteine Homocysteine A measurement of the homocysteine amino acid in abiological specimen.Histamine MeasurementHomocysteine Acid MeasurementC100447Homostat Model Assess ofInsulin RstnHomostat ModelAssess of InsulinRstn;HomeostaticModelAssessment ofInsulinResistanceAn assessment of beta-cell function and insulinresistance based on fasting blood glucose and insulinconcentrations.Homeostatic Model Assessment ofInsulin ResistanceC74863 Homovanillic Acid HomovanillicAcidC74704 Howell-Jolly Bodies Howell-JollyBodiesC103405 Human Albumin Antibody Human AlbuminAntibodyA measurement of the homovanillic acid metabolite ina biological specimen.A measurement of the Howell-Jolly bodies (spherical,blue-black condensed DNA inclusions within the bodyof a red blood cell that appear under Wright-stain) in abiological specimen.A measurement of the human albumin antibody in abiological specimen.Homovanillic Acid MeasurementHowell-Jolly Body MeasurementHuman Albumin AntibodyMeasurementC103406 Human Anti-Mouse Antibody HAMA A measurement of the human anti-mouse antibody in abiological specimen.Human Anti-Mouse AntibodyMeasurementC98740Human Anti-Sheep IgEAntibodyHumanAnti-Sheep IgEAntibodyA measurement of the human anti-sheep IgE antibodiesin a biological specimen.Human Anti-Sheep IgE AntibodyMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>2 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98741Human Anti-Sheep IgGAntibodyHumanAnti-Sheep IgGAntibodyA measurement of the human anti-sheep IgGantibodies in a biological specimen.Human Anti-Sheep IgG AntibodyMeasurementC98742Human Anti-Sheep IgMAntibodyHumanAnti-Sheep IgMAntibodyA measurement of the human anti-sheep IgMantibodies in a biological specimen.Human Anti-Sheep IgM AntibodyMeasurementC74770 Hyaline Casts Hyaline Casts A measurement of the hyaline casts present in abiological specimen.C74879 Hydrocodone Hydrocodone A measurement of the hydrocodone present in abiological specimen.C102275 Hydrogen Hydrogen A measurement of the hydrogen in a biologicalspecimen.C74880 Hydromorphone Hydromorphone A measurement of the hydromorphone present in abiological specimen.C80190 Hydroxyproline Hydroxyproline A measurement of the total hydroxyproline in abiological specimen.C96669 Hyperchromia Hyperchromia A measurement of the prevalence of the erthrocyteswith an elevated hemoglobin concentration.Hyaline Cast MeasurementHydrocodone MeasurementHydrogen MeasurementHydromorphone MeasurementHydroxyproline MeasurementHyperchromia MeasurementC746<strong>12</strong> Hypersegmented Cells HypersegmentedCellsA measurement of the hypersegmented (more than fivelobes) neutrophils in a biological specimen.Hypersegmented NeutrophilMeasurementC64802 Hypochromia Hypochromia An observation which indicates that the hemoglobinconcentration in a red blood cell specimen has fallenbelow a specified level.HypochromiaC96670 Immature Basophils ImmatureBasophilsA measurement of the immature basophils in abiological specimen.Immature Basophil CountC96671ImmatureBasophils/LeukocytesImmatureBasophils/LeukocytesA relative measurement (ratio or percentage) ofimmature basophils to total leukocytes in a biologicalspecimen.Immature Basophil to LeukocyteRatio MeasurementC96672 Immature Cells Immature Cells A measurement of the total immature cells in a bloodspecimen.Immature Cell CountC96673 Immature Eosinophils ImmatureEosinophilsA measurement of the immature eosinophils in abiological specimen.Immature Eosinophil CountC96674ImmatureEosinophils/LeukocytesImmatureEosinophils/LeukocytesA relative measurement (ratio or percentage) ofimmature eosinophils to total leukocytes in abiological specimen.Immature Eosinophil to LeukocyteRatio MeasurementC96675 Immature Granulocytes ImmatureGranulocytesA measurement of the total immature granulocytes in abiological specimen.Immature Granulocyte CountC100445ImmatureGranulocytes/LeukocytesImmatureGranulocytes/LeukocytesA relative measurement (ratio or percentage) of theimmature granulocytes to leukocytes in a biologicalspecimen (for example a bone marrow specimen).Immature Granulocytes toLeukocytes Ratio MeasurementC100444 Immature Lymphocytes ImmatureLymphocytesA measurement of the immature lymphocytes in abiological specimen.Immature LymphocytesMeasurementC100443ImmatureLymphocytes/LeukocytesImmatureLymphocytes/LeukocytesA relative measurement (ratio or percentage) of theimmature lymphocytes to leukocytes in a biologicalspecimen.Immature Lymphocytes toLeukocytes Ratio MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>3 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96676 Immature Monocytes ImmatureMonocytesA measurement of the immature monocytes in abiological specimen.Immature Monocyte CountC96677ImmatureMonocytes/LeukocytesImmatureMonocytes/LeukocytesA relative measurement (ratio or percentage) ofimmature monocytes to total leukocytes in abiological specimen.Immature Monocyte to LeukocyteRatio MeasurementC96678 Immature Neutrophils ImmatureNeutrophilsA measurement of the total immature neutrophils in abiological specimen.Immature Neutrophil CountC100442ImmatureNeutrophils/LeukocytesImmatureNeutrophils/LeukocytesA relative measurement (ratio or percentage) of theimmature neutrophils to leukocytes in a biologicalspecimen.Immature Neutrophils to LeukocytesRatio MeasurementC96679 Immature Plasma Cells Immature PlasmaCellsA measurement of the immature plasma cells in abiological specimen.Immature Plasma Cell CountC96680Immature PlasmaCells/LymphocytesImmature PlasmaCells/LymphocytesA relative measurement (ratio or percentage) ofimmature plasma cells to total lymphocytes in abiological specimen.Immature Plasma Cell toLymphocyte Ratio MeasurementC102276Immature ReticulocyteFractionImmatureReticulocyteFractionA measurement of the immature reticulocyte fractionpresent in a biological specimen.Immature Reticulocyte FractionMeasurementC103407 Immunoblasts Lymphocytes,ImmunoblasticC81969 Immunoglobulin A ImmunoglobulinAC98745 Immunoglobulin D ImmunoglobulinDC81970 Immunoglobulin E ImmunoglobulinEC81971 Immunoglobulin G ImmunoglobulinGC81972 Immunoglobulin M ImmunoglobulinMA measurement of the immunoblasts in a biologicalspecimen.A measurement of the Immunoglobulin A in abiological specimen.A measurement of the Immunoglobulin D in abiological specimen.A measurement of the Immunoglobulin E in abiological specimen.A measurement of the Immunoglobulin G in abiological specimen.A measurement of the Immunoglobulin M in abiological specimen.Immunoblast CountImmunoglobulin A MeasurementImmunoglobulin D MeasurementImmunoglobulin E MeasurementImmunoglobulin G MeasurementImmunoglobulin M MeasurementC82044 Indican Indican A measurement of the indican present in a biologicalspecimen.C64483 Indirect Bilirubin Indirect Bilirubin A measurement of the unconjugated ornon-water-soluble bilirubin in a biological specimen.Indican MeasurementIndirect Bilirubin MeasurementC92273 Influenza A H1N1 Viral Load Influenza AH1N1 Viral LoadC103408 Influenza A IgG Antibody Influenza A IgGAntibodyC92274 Influenza A Viral Load Influenza A ViralLoadC103409 Influenza B IgG Antibody Influenza B IgGAntibodyA measurement of the Influenza A H1N1 viral load in abiological specimen.A measurement of the Influenza A IgG antibody in abiological specimen.A measurement of the Influenza A viral load in abiological specimen.A measurement of the Influenza B IgG antibody in abiological specimen.Influenza A H1N1 Viral LoadMeasurementInfluenza A IgG AntibodyMeasurementInfluenza A Viral Load MeasurementInfluenza B IgG AntibodyMeasurementC82020 Inhibin A Inhibin A A measurement of the inhibin A in a biologicalspecimen.Inhibin A MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>4 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96681 Inhibin B Inhibin B A measurement of the inhibin B in a biologicalspecimen.Inhibin B MeasurementC74788 Insulin Insulin A measurement of the insulin in a biological specimen. Insulin MeasurementC74864 Insulin-like Growth Factor-1 Insulin-likeGrowth Factor-1C74865 Insulin-like Growth Factor-2 Insulin-likeGrowth Factor-2A measurement of the insulin-like growth factor-1 in abiological specimen.A measurement of the insulin-like growth factor-2 in abiological specimen.Insulin Like Growth Factor-1MeasurementInsulin Like Growth Factor-2MeasurementC81994 Interferon Alpha Interferon Alpha A measurement of the interferon alpha in a biologicalspecimen.C81995 Interferon Beta Interferon Beta A measurement of the interferon beta in a biologicalspecimen.Interferon Alpha MeasurementInterferon Beta MeasurementC81996 Interferon Gamma InterferonGammaA measurement of the interferon gamma in abiological specimen.Interferon Gamma MeasurementC74805 Interleukin 1 Interleukin 1 A measurement of the interleukin 1 in a biologicalspecimen.C74806 Interleukin 10 Interleukin 10 A measurement of the interleukin 10 in a biologicalspecimen.C74807 Interleukin 11 Interleukin 11 A measurement of the interleukin 11 in a biologicalspecimen.C74808 Interleukin <strong>12</strong> Interleukin <strong>12</strong> A measurement of the interleukin <strong>12</strong> in a biologicalspecimen.C74809 Interleukin 13 Interleukin 13 A measurement of the interleukin 13 in a biologicalspecimen.C74810 Interleukin 14 Interleukin 14 A measurement of the interleukin 14 in a biologicalspecimen.C74811 Interleukin 15 Interleukin 15 A measurement of the interleukin 15 in a biologicalspecimen.C748<strong>12</strong> Interleukin 16 Interleukin 16 A measurement of the interleukin 16 in a biologicalspecimen.C74813 Interleukin 17 Interleukin 17 A measurement of the interleukin 17 in a biologicalspecimen.C74814 Interleukin 18 Interleukin 18 A measurement of the interleukin 18 in a biologicalspecimen.C74815 Interleukin 19 Interleukin 19 A measurement of the interleukin 19 in a biologicalspecimen.C74816 Interleukin 2 Interleukin 2 A measurement of the interleukin 2 in a biologicalspecimen.C74817 Interleukin 20 Interleukin 20 A measurement of the interleukin 20 in a biologicalspecimen.C74818 Interleukin 21 Interleukin 21 A measurement of the interleukin 21 in a biologicalspecimen.C74819 Interleukin 22 Interleukin 22 A measurement of the interleukin 22 in a biologicalspecimen.Interleukin 1 MeasurementInterleukin 10 MeasurementInterleukin 11 MeasurementInterleukin <strong>12</strong> MeasurementInterleukin 13 MeasurementInterleukin 14 MeasurementInterleukin 15 MeasurementInterleukin 16 MeasurementInterleukin 17 MeasurementInterleukin 18 MeasurementInterleukin 19 MeasurementInterleukin 2 MeasurementInterleukin 20 MeasurementInterleukin 21 MeasurementInterleukin 22 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>5 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74820 Interleukin 23 Interleukin 23 A measurement of the interleukin 23 in a biologicalspecimen.C74821 Interleukin 24 Interleukin 24 A measurement of the interleukin 24 in a biologicalspecimen.C74822 Interleukin 25 Interleukin 25 A measurement of the interleukin 25 in a biologicalspecimen.C74823 Interleukin 26 Interleukin 26 A measurement of the interleukin 26 in a biologicalspecimen.C74824 Interleukin 27 Interleukin 27 A measurement of the interleukin 27 in a biologicalspecimen.C74825 Interleukin 28 Interleukin 28 A measurement of the interleukin 28 in a biologicalspecimen.C74826 Interleukin 29 Interleukin 29 A measurement of the interleukin 29 in a biologicalspecimen.C74827 Interleukin 3 Interleukin 3 A measurement of the interleukin 3 in a biologicalspecimen.C74828 Interleukin 30 Interleukin 30 A measurement of the interleukin 30 in a biologicalspecimen.C74829 Interleukin 31 Interleukin 31 A measurement of the interleukin 31 in a biologicalspecimen.C74830 Interleukin 32 Interleukin 32 A measurement of the interleukin 32 in a biologicalspecimen.C74831 Interleukin 33 Interleukin 33 A measurement of the interleukin 33 in a biologicalspecimen.C74832 Interleukin 4 Interleukin 4 A measurement of the interleukin 4 in a biologicalspecimen.C74833 Interleukin 5 Interleukin 5 A measurement of the interleukin 5 in a biologicalspecimen.C74834 Interleukin 6 Interleukin 6 A measurement of the interleukin 6 in a biologicalspecimen.C74835 Interleukin 7 Interleukin 7 A measurement of the interleukin 7 in a biologicalspecimen.C74836 Interleukin 8 Interleukin 8 A measurement of the interleukin 8 in a biologicalspecimen.C74837 Interleukin 9 Interleukin 9 A measurement of the interleukin 9 in a biologicalspecimen.C98748 Inulin Clearance Inulin Clearance A measurement of the volume of serum or plasma thatwould be cleared of inulin by excretion of urine for aspecified unit of time (e.g. one minute).Interleukin 23 MeasurementInterleukin 24 MeasurementInterleukin 25 MeasurementInterleukin 26 MeasurementInterleukin 27 MeasurementInterleukin 28 MeasurementInterleukin 29 MeasurementInterleukin 3 MeasurementInterleukin 30 MeasurementInterleukin 31 MeasurementInterleukin 32 MeasurementInterleukin 33 MeasurementInterleukin 4 MeasurementInterleukin 5 MeasurementInterleukin 6 MeasurementInterleukin 7 MeasurementInterleukin 8 MeasurementInterleukin 9 MeasurementInulin ClearanceC100439 Iohexol Clearance IohexolClearanceC98749 Iothalamate Clearance IothalamateClearanceA measurement of the volume of serum or plasma thatwould be cleared of Iohexol through excretion for aspecified unit of time.A measurement of the volume of serum or plasma thatwould be cleared of iothalamate by excretion of urinefor a specified unit of time (e.g. one minute).Iohexol ClearanceIothalamate ClearanceSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>6 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98750Iothalamate ClearanceAdjusted for BSAIothalamateClearanceAdjusted forBSAA measurement of the volume of serum or plasma thatwould be cleared of iothalamate by excretion of urinefor a specified unit of time (e.g. one minute), adjustedfor body size.Iothalamate Clearance Adjusted forBSAC74679 Iron Iron; FE A measurement of the iron in a biological specimen. Iron MeasurementC81985 Islet Cell 5<strong>12</strong> Antibody Islet Cell 5<strong>12</strong>AntibodyC81986 Islet Cell 5<strong>12</strong> Antigen Islet Cell 5<strong>12</strong>AntigenA measurement of the islet cell 5<strong>12</strong> antibody in abiological specimen.A measurement of the islet cell 5<strong>12</strong> antigen in abiological specimen.Islet Cell 5<strong>12</strong> AntibodyMeasurementIslet Cell 5<strong>12</strong> Antigen MeasurementC81987Islet Neogenesis AssocProtein AntibodyIslet NeogenesisAssoc ProteinAntibodyA measurement of the islet neogenesis associatedprotein antibody in a biological specimen.Islet Neogenesis Associated ProteinAntibody MeasurementC103410 Isoleucine Isoleucine A measurement of the isoleucine in a biologicalspecimen.C100459 Jo-1 Antibody Jo-1 Antibody A measurement of the Jo-1 antibody in a biologicalspecimen.Isoleucine MeasurementJo-1 Antibody MeasurementC98730 Kappa Light Chain, Free Kappa LightChain, FreeA measurement of the free kappa light chain in abiological specimen.Free Kappa Light ChainMeasurementC98731Kappa Lt Chain,Free/LambdaLt Chain,FreeKappa LtChain,Free/Lambda LtChain,FreeA relative measurement (ratio or percentage) of thefree kappa light chain to the free lambda light chain ina biological specimen.Free Kappa Light Chain to FreeLambda Light Chain RatioMeasurementC64854 Ketones Ketones A measurement of the ketones in a biologicalspecimen.Ketone MeasurementC100433 Kidney Injury Molecule-1 Kidney InjuryMolecule-1;KIM-1; HepatitisA Virus CellularReceptor 1A measurement of the kidney injury molecule-1(Kim-1) in a biological specimen.Kidney Injury Molecule-1MeasurementC96682 Kurloff Cells Kurloff Cells A measurement of the large secretorygranule-containing immune cells in a biologicalspecimen taken from members of certain genera of theCaviidae family.Kurloff Cells MeasurementC74887 LDH Isoenzyme 1 LDH Isoenzyme1C79451 LDH Isoenzyme 1/LDH LDH Isoenzyme1/LDHC74888 LDH Isoenzyme 2 LDH Isoenzyme2C79452 LDH Isoenzyme 2/LDH LDH Isoenzyme2/LDHC74889 LDH Isoenzyme 3 LDH Isoenzyme3C79453 LDH Isoenzyme 3/LDH LDH Isoenzyme3/LDHA measurement of the lactate dehydrogenaseisoenzyme 1 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 1 to total lactatedehydrogenase in a biological specimen.A measurement of the lactate dehydrogenaseisoenzyme 2 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 2 to total lactatedehydrogenase in a biological specimen.A measurement of the lactate dehydrogenaseisoenzyme 3 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 3 to total lactatedehydrogenase in a biological specimen.Lactate Dehydrogenase Isoenzyme 1MeasurementLDH Isoenzyme 1 to LDH RatioMeasurementLactate Dehydrogenase Isoenzyme 2MeasurementLDH Isoenzyme 2 to LDH RatioMeasurementLactate Dehydrogenase Isoenzyme 3MeasurementLDH Isoenzyme 3 to LDH RatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>7 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74890 LDH Isoenzyme 4 LDH Isoenzyme4C79454 LDH Isoenzyme 4/LDH LDH Isoenzyme4/LDHC74891 LDH Isoenzyme 5 LDH Isoenzyme5C79455 LDH Isoenzyme 5/LDH LDH Isoenzyme5/LDHA measurement of the lactate dehydrogenaseisoenzyme 4 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 4 to total lactatedehydrogenase in a biological specimen.A measurement of the lactate dehydrogenaseisoenzyme 5 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 5 to total lactatedehydrogenase in a biological specimen.Lactate Dehydrogenase Isoenzyme 4MeasurementLDH Isoenzyme 4 to LDH RatioMeasurementLactate Dehydrogenase Isoenzyme 5MeasurementLDH Isoenzyme 5 to LDH RatioMeasurementC61042 LDL Cholesterol LDL Cholesterol A measurement of the low density lipoproteincholesterol in a biological specimen.Serum LDL CholesterolMeasurementC1034<strong>12</strong> LDL Particle Size LDL ParticleSizeC64855 Lactate Dehydrogenase LactateDehydrogenaseA measurement of the average particle size oflow-density lipoprotein in a biological specimen.A measurement of the lactate dehydrogenase in abiological specimen.LDL Particle Size MeasurementLactate DehydrogenaseMeasurementC79449LactateDehydrogenase/CreatinineLactateDehydrogenase/CreatinineA relative measurement (ratio or percentage) of thelactate dehydrogenase to creatinine in a biologicalspecimen.Lactate Dehydrogenase to CreatinineRatio MeasurementC79450 Lactic Acid Lactic Acid;2-hydroxypropanoic acid; LactateC82021 Lactoferrin Lactoferrin;LactotransferrinC98732 Lambda Light Chain, Free Lambda LightChain, FreeA measurement of the lactic acid in a biologicalspecimen.A measurement of the lactoferrin in a biologicalspecimen.A measurement of the free lambda light chain in abiological specimen.Lactic Acid MeasurementLactoferrin MeasurementFree Lambda Light ChainMeasurementC74729 Large Platelets Large Platelets A measurement of the large (between 4 um and 7um indiameter) platelets in a biological specimen.Large Platelet CountC74659 Large Unstained Cells Large UnstainedCellsA measurement of the large, peroxidase-negative cellswhich cannot be further characterized (i.e. as largelymphocytes, virocytes, or stem cells) present in abiological specimen.Large Unstained Cell CountC79467Large UnstainedCells/LeukocytesLarge UnstainedCells/LeukocytesA relative measure (ratio or percentage) of the largeunstained cells to leukocytes in a biological specimen.Large Unstained Cells to LeukocytesRatio MeasurementC92275Legionella pneumophilaAntigenLegionellapneumophilaAntigenA measurement of the Legionella pneumophila antigenin a biological specimen.Legionella pneumophila AntigenMeasurementC92276Legionella pneumophila IgGAntibodyLegionellapneumophila IgGAntibodyA measurement of the Legionella pneumophila IgGantibody in a biological specimen.Legionella pneumophila IgGAntibody MeasurementC92277Legionella pneumophila IgGIgM AntibodyLegionellapneumophila IgGIgM AntibodyA measurement of the Legionella pneumophila IgG andIgM in a biological specimen.Legionella pneumophila IgG IgMAntibody MeasurementC92278Legionella pneumophila IgMAntibodyLegionellapneumophila IgMAntibodyA measurement of the Legionella pneumophila IgMantibody in a biological specimen.Legionella pneumophila IgMAntibody MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>8 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74866 Leptin Leptin A measurement of the leptin hormone in a biologicalspecimen.C74680 Leucine Crystals Leucine Crystals A measurement of the leucine crystals present in abiological specimen.C74630 Leukemic Blasts Leukemic Blasts A measurement of the leukemic blasts (lymphoblaststhat remain in an immature state even when outside thebone marrow) in a biological specimen.Leptin MeasurementLeucine Crystal MeasurementLeukemic Blast CountC74641LeukemicBlasts/LymphocytesLeukemicBlasts/LymphocytesA relative measurement (ratio or percentage) of theleukemic blasts (immature lymphoblasts) to maturelymphocytes in a biological specimen.Leukemic Blast to LymphocyteRatio MeasurementC92246 Leukocyte Cell Clumps Leukocyte CellClumps; WhiteBlood CellClumps; WBCClumpsC92297 Leukocyte Cell Morphology Leukocyte CellMorphology;White BloodCellMorphology;WBCMorphologyC64856 Leukocyte Esterase LeukocyteEsteraseC51948 Leukocytes Leukocytes;White BloodCellsA measurement of white blood cell clumps in abiological specimen.An examination or assessment of the form andstructure of white blood cells.A measurement of the enzyme which indicates thepresence of white blood cells in a biologicalspecimen.A measurement of the leukocytes in a biologicalspecimen.Leukocyte Cell ClumpsMeasurementLeukocyte Cell MorphologyLeukocyte Esterase MeasurementLeukocyte CountC103413 Leukotriene B4 Leukotriene B4 A measurement of the leukotriene B4 in a biologicalspecimen.C103414 Leukotriene D4 Leukotriene D4 A measurement of the leukotriene D4 in a biologicalspecimen.C103415 Leukotriene E4 Leukotriene E4 A measurement of the leukotriene E4 in a biologicalspecimen.C82022 Lipoprotein-a Lipoprotein-a A measurement of the lipoprotein-a in a biologicalspecimen.Leukotriene B4 MeasurementLeukotriene D4 MeasurementLeukotriene E4 MeasurementLipoprotein a MeasurementC96683Liver Kidney MicrosomalType 1 AntibodyLiver KidneyMicrosomalType 1 Antibody;LKM-1A measurement of the liver kidney microsomal type 1antibody in a biological specimen.Liver Kidney Microsomal Type 1Antibody MeasurementC100456Liver Kidney MicrosomalType 1 IgA AbLiver KidneyMicrosomalType 1 IgA AbA measurement of the liver kidney microsomal type 1IgA antibodies in a biological specimen.Liver Kidney Microsomal Type 1IgA Antibody MeasurementC100454Liver Kidney MicrosomalType 1 IgG AbLiver KidneyMicrosomalType 1 IgG AbA measurement of the liver kidney microsomal type 1IgG antibodies in a biological specimen.Liver Kidney Microsomal Type 1IgG Antibody MeasurementC100455Liver Kidney MicrosomalType 1 IgM AbLiver KidneyMicrosomalType 1 IgM AbA measurement of the liver kidney microsomal type 1IgM antibodies in a biological specimen.Liver Kidney Microsomal Type 1IgM Antibody MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page <strong>12</strong>9 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102277Lupus Anticoagulant SensitiveAPTTLupusAnticoagulantSensitive APTT;APTT-LAA measurement of the length of time that it takes forclotting to occur when a lupus sensitive reagent isadded to a plasma specimen.Lupus Anticoagulant Sensitive APTTMeasurementC74790 Luteinizing Hormone LuteinizingHormoneA measurement of the luteinizing hormone in abiological specimen.Luteinizing Hormone MeasurementC102278 Lymphoblasts Lymphoblasts A measurement of the lymphoblasts (immature cellsthat differentiate to form lymphocytes) in a biologicalspecimen.C51949 Lymphocytes Lymphocytes A measurement of the lymphocytes in a biologicalspecimen.Lymphoblast CountLymphocyte CountC64818 Lymphocytes Atypical LymphocytesAtypicalA measurement of the atypical lymphocytes in abiological specimen.Atypical Lymphocyte CountC64819LymphocytesAtypical/LeukocytesLymphocytesAtypical/LeukocytesA relative measurement (ratio or percentage) of theatypical lymphocytes to leukocytes in a biologicalspecimen.Atypical Lymphocyte To LeukocyteRatioC64820 Lymphocytes/Leukocytes Lymphocytes/LeukocytesC98751 Lymphocytes/Total Cells Lymphocytes/Total CellsA relative measurement (ratio or percentage) of thelymphocytes to leukocytes in a biological specimen.A relative measurement (ratio or percentage) of thelymphocytes to total cells in a biological specimen(for example a bone marrow specimen).Lymphocyte To Leukocyte RatioLymphocyte to Total Cell RatioMeasurementC74613 Lymphoma Cells Lymphoma Cells A measurement of the malignant lymphocytes in abiological specimen.Lymphoma Cell CountC74910LymphomaCells/LymphocytesLymphomaCells/LymphocytesA relative measurement (ratio or percentage) of themalignant lymphocytes to all lymphocytes in abiological specimen.Lymphoma Cell to LymphocyteRatio MeasurementC81955 Lymphotactin Lymphotactin;ChemokineLigand 1C75354 Lysergic Acid Diethylamide Lysergic AcidDiethylamide;AcidA measurement of the lymphotactin in a biologicalspecimen.A measurement of the lysergic acid diethylamine(LSD) in a biological specimen.Lymphotactin MeasurementLysergide MeasurementC92241M. tuberculosis IFN GammaResponseMycobacteriumtuberculosis IFNGammaResponseA measurement of the amount of interferon gammathat is released when Mycobacterium tuberculosisantigen is incubated with a biological specimen,usually blood. Increased amounts of interferon gammasuggests a prior or existing M. tuberculosis infection.Mycobacterium tuberculosisInterferon Gamma ResponseC64821 Macrocytes Macrocytes A measurement of the macrocytes in a biologicalspecimen.Macrocyte CountC80191Macrophage ColonyStimulating FactorMacrophageColonyStimulatingFactorA measurement of the macrophage colony stimulatingfactor in a biological specimen.Macrophage Colony StimulatingFactor MeasurementC82023Macrophage InflammatoryProtein 1 AlphaMacrophageInflammatoryProtein 1 Alpha;ChemokineLigand 3A measurement of the macrophage inflammatoryprotein 1 alpha in a biological specimen.Macrophage Inflammatory Protein 1Alpha MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 130 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC82024Macrophage InflammatoryProtein 1 BetaMacrophageInflammatoryProtein 1 Beta;ChemokineLigand 4A measurement of the macrophage inflammatoryprotein 1 beta in a biological specimen.Macrophage Inflammatory Protein 1Beta MeasurementC81956Macrophage-DerivedChemokineMacrophage-Derived Chemokine;ChemokineLigand 22A measurement of the macrophage-derived chemokinein a biological specimen.Macrophage-Derived ChemokineMeasurementC64840 Magnesium Magnesium A measurement of the magnesium in a biologicalspecimen.Magnesium MeasurementC79456 Magnesium/Creatinine Magnesium/CreatinineC74660 Malignant Cells, NOS Malignant Cells,NOSA relative measurement (ratio or percentage) of themagnesium to creatinine in a biological specimen.A measurement of the malignant cells of all types in abiological specimen.Magnesium to Creatinine RatioMeasurementMalignant Cell CountC74643Malignant Cells, NOS/BloodCellsMalignant Cells,NOS/BloodCellsA relative measurement (ratio or percentage) of themalignant cells of all types to all blood cells in abiological specimen.Malignant Cell to Blood Cell RatioMeasurementC80192 Matrix Metalloproteinase 1 MatrixMetalloproteinase 1; InterstitialCollagenaseC80193 Matrix Metalloproteinase 2 MatrixMetalloproteinase 2; Gelatinase AC80194 Matrix Metalloproteinase 3 MatrixMetalloproteinase 3; Stromelysin1C80195 Matrix Metalloproteinase 7 MatrixMetalloproteinase 7; MatrilysinC80196 Matrix Metalloproteinase 8 MatrixMetalloproteinase 8; NeutrophilCollagenaseC80197 Matrix Metalloproteinase 9 MatrixMetalloproteinase 9; Gelatinase BC74661 Mature Plasma Cells Mature PlasmaCells;PlasmacytesA measurement of the matrix metalloproteinase 1 in abiological specimen.A measurement of the matrix metalloproteinase 2 in abiological specimen.A measurement of the matrix metalloproteinase 3 in abiological specimen.A measurement of the matrix metalloproteinase 7 in abiological specimen.A measurement of the matrix metalloproteinase 8 in abiological specimen.A measurement of the matrix metalloproteinase 9 in abiological specimen.A measurement of the mature plasma cells(plasmacytes) in a biological specimen.Matrix Metalloproteinase 1MeasurementMatrix Metalloproteinase 2MeasurementMatrix Metalloproteinase 3MeasurementMatrix Metalloproteinase 7MeasurementMatrix Metalloproteinase 8MeasurementMatrix Metalloproteinase 9MeasurementMature Plasma Cell CountC74911Mature PlasmaCells/LymphocytesMature PlasmaCells/LymphocytesA relative measurement (ratio or percentage) of themature plasma cells (plasmacytes) to all lymphocytesin a biological specimen.Mature Plasma Cell to LymphocyteRatio MeasurementC74614 May-Hegglin Anomaly May-HegglinAnomalyA measurement of the May-Hegglin anomaly in ablood sample. This anomaly is characterized by large,misshapen platelets and the presence of Dohle bodiesin leukocytes.May-Hegglin Anomaly MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 131 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96686 Mean Platelet Component Mean PlateletComponentC74730 Mean Platelet Volume Mean PlateletVolumeA measurement of the mean platelet component(platelet activity) in a blood specimen.A measurement of the average size of the plateletspresent in a blood sample.Mean Platelet ComponentMeasurementMean Platelet Volume MeasurementC98752 Megakaryoblasts Megakaryoblasts A measurement of the megakaryoblasts in a biologicalspecimen.Megakaryoblast Cell CountC98753 Megakaryoblasts/Total Cells Megakaryoblasts/Total CellsA relative measurement (ratio or percentage) of themegakaryoblasts to total cells in a biological specimen(for example a bone marrow specimen).Megakaryoblast to Total Cell RatioMeasurementC96688 Megakaryocytes Megakaryocytes A measurement of the megakaryocytes per unit of abiological specimen.Megakaryocyte CountC98867 Megakaryocytes/Total Cells Megakaryocytes/Total CellsA relative measurement (ratio or percentage) of themegakaryocytes to total cells in a biological specimen(for example a bone marrow specimen).Megakaryocyte to Total CellRatioMeasurementC74867 Melatonin Melatonin A measurement of the melatonin hormone in abiological specimen.C74615 Metamyelocytes Metamyelocytes A measurement of the metamyelocytes (small,myelocytic neutrophils with an indented nucleus) in abiological specimen.Melatonin MeasurementMetamyelocyte CountC74645 Metamyelocytes/Leukocytes Metamyelocytes/LeukocytesC98754 Metamyelocytes/Total Cells Metamyelocytes/Total CellsA relative measurement (ratio or percentage) of themetamyelocytes (small, myelocytic neutrophils withan indented nucleus) to all leukocytes in a biologicalspecimen.A relative measurement (ratio or percentage ) of themetamyelocytes (small, myelocytic neutrophils withan indented nucleus) to total cells in a biologicalspecimen (for example a bone marrow specimen).Metamyelocyte to Leukocyte RatioMeasurementMetamyelocyte to Total Cell RatioMeasurementC74881 Methadone Methadone A measurement of the methadone present in abiological specimen.Methadone MeasurementC75348 Methamphetamine MethamphetamineA measurement of the methamphetamine drug presentin a biological specimen.Methamphetamine MeasurementC74882 Methaqualone Methaqualone A measurement of the methaqualone present in abiological specimen.C96689 Methemoglobin Methemoglobin A measurement of the methemoglobin in a biologicalspecimen.Methaqualone MeasurementMethemoglobin MeasurementC96690 Methylmalonic Acid MethylmalonicAcidA measurement of the methylmalonic acid in abiological specimen.Methylmalonic Acid MeasurementC64822 Microcytes Microcytes A measurement of the microcytes in a biologicalspecimen.C74771 Mixed Casts Mixed Casts A measurement of the mixed (the cast contains amixture of cell types) casts present in a biologicalspecimen.C74631 Monoblasts Monoblasts A measurement of the monoblast cells in a biologicalspecimen.Microcyte CountMixed Cast CountMonoblast CountC74646 Monoblasts/Leukocytes Monoblasts/LeukocytesA relative measurement (ratio or percentage) of themonoblasts to leukocytes in a biological specimen.Monoblast to Leukocyte RatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 132 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92291 Monoclonal Protein MonoclonalImmunoglobulinProtein;ParaproteinA measurement of homogenous immunoglobulinresulting from the proliferation of a single clone ofplasma cells in a biological specimen.Monoclonal Protein MeasurementC82025Monocyte ChemotacticProtein 1MonocyteChemotacticProtein 1A measurement of the monocyte chemotactic protein1 in a biological specimen.Monocyte Chemotactic Protein 1MeasurementC64823 Monocytes Monocytes A measurement of the monocytes in a biologicalspecimen.Monocyte CountC64824 Monocytes/Leukocytes Monocytes/LeukocytesC98872 Monocytes/Total Cells Monocytes/TotalCellsC74681 Monosodium Urate Crystals MonosodiumUrate CrystalsA relative measurement (ratio or percentage) of themonocytes to leukocytes in a biological specimen.A relative measurement (ratio or percentage) of themonocytes to total cells in a biological specimen (forexample a bone marrow specimen).A measurement of the monosodium urate crystalspresent in a biological specimen.Monocyte To Leukocyte RatioMonocytes to TotalCellRatioMeasurementMonosodium Urate CrystalMeasurementC74883 Morphine Morphine A measurement of the morphine present in a biologicalspecimen.Morphine MeasurementC79457 Mu Glutathione-S-Transferase MuGlutathione-S-TransferaseA measurement of the mu form of glutathioneS-transferase in a biological specimen.Mu Glutathione-S-TransferaseMeasurementC79458MuGlutathione-S-Transferase/CreatinineMuGlutathione-S-Transferase/CreatinineA relative measurement (ratio or percentage) of themu gamma glutamyl transpeptidase to creatinine in abiological specimen.Mu Glutathione-S-Transferase toCreatinine Ratio MeasurementC74721 Mucous Threads Mucous Threads A measurement of the mucous threads present in abiological specimen.Mucous Thread MeasurementC92279Mycobacterium tuberculosisNucleic AcidMycobacteriumtuberculosisNucleic AcidA measurement of the Mycobacterium tuberculosisnucleic acid in a biological specimen.Mycobacterium tuberculosis NucleicAcid MeasurementC103418 Myelin Antibodies MyelinAntibodiesA measurement of the myelin antibodies in abiological specimen.Myelin Antibodies MeasurementC74632 Myeloblasts Myeloblasts A measurement of the myeloblast cells in a biologicalspecimen.Myeloblast CountC64825 Myeloblasts/Leukocytes Myeloblasts/LeukocytesC98761 Myeloblasts/Total Cells Myeloblasts/Total CellsA relative measure (ratio or percentage) of themyeloblasts to leukocytes in a biological specimen.A relative measurement (ratio or percentage) of themyeloblasts to total cells in a biological specimen (forexample a bone marrow specimen).Myeloblast To Leukocyte RatioMyeloblast to Total Cell RatioMeasurementC74662 Myelocytes Myelocytes A measurement of the myelocytes in a biologicalspecimen.Myelocyte CountC64826 Myelocytes/Leukocytes Myelocytes/LeukocytesC98868 Myelocytes/Total Cells Myelocytes/Total CellsA relative measurement (ratio or percentage) of themyelocytes to leukocytes in a biological specimen.A relative measurement (ratio or percentage) of themyelocytes to total cells in a biological specimen (forexample a bone marrow specimen).Myelocyte To Leukocyte RatioMyelocyte to Total CellRatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 133 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92242 Myeloid/Erythroid Ratio Myeloid/Erythroid RatioA relative measurement (ratio or percentage) ofmyeloid progenitor cells to erythrocyte precursorcells in a biological specimen.Myeloid to Erythroid RatioMeasurementC80198 Myeloperoxidase Myeloperoxidase A measurement of the myeloperoxidase in a biologicalspecimen.Myeloperoxidase MeasurementC92280 Myeloperoxidase Antibody MyeloperoxidaseAntibodyA measurement of the myeloperoxidase antibody in abiological specimen.Myeloperoxidase AntibodyMeasurementC79436 Myoglobin Myoglobin A measurement of myoglobin in a biologicalspecimen.Myoglobin MeasurementC79459 N-Acetyl Glucosamide N-AcetylGlucosamide;N-AcetylGlucosamineA measurement of N-acetyl glucosamide in abiological specimen.N-Acetyl Glucosamide MeasurementC79460N-AcetylGlucosamide/CreatinineN-AcetylGlucosamide/CreatinineA relative measurement (ratio or percentage) of theN-acetyl glucosamide to creatinine in a biologicalspecimen.N-Acetyl Glucosamide to CreatinineRatio MeasurementC96610N-Terminal ProB-typeNatriuretic PeptideN-TerminalProB-typeNatriureticPeptide; NTproBNP II;N-terminalpro-BrainNatriureticPeptideA measurement of the N-terminal proB-typenatriuretic peptide in a biological specimen.N-Terminal ProB-type NatriureticPeptide MeasurementC103419N-acetyl-beta-D-glucosaminidaseNAG;Beta-N-acetyl-D-glucosaminidaseA measurement of theN-acetyl-beta-D-glucosaminidase in a biologicalspecimen.N-acetyl-beta-D-glucosaminidaseMeasurementC74743 N-telopeptide N-telopeptide A measurement of the N-telopeptide in a biologicalspecimen.N-telopeptide MeasurementC98762 Natural Killer Cells Natural KillerCellsA measurement of the total natural killer cells in abiological specimen.Natural Killer Cell CountC92298Neisseria gonorrhoeaeScreeningNeisseriagonorrhoeaeScreeningExamination of a biological specimen to detect thepresence of Neisseria gonorrhoeae.Neisseria gonorrhoeae ScreeningC80199 Neopterin Neopterin A measurement of the neopterin in a biologicalspecimen.C74892 Neuropeptide Y Neuropeptide Y A measurement of the neuropeptide Y in a biologicalspecimen.Neopterin MeasurementNeuropeptide Y MeasurementC82026 Neutrophil Elastase NeutrophilElastaseA measurement of the neutrophil elastase in abiological specimen.Neutrophil Elastase MeasurementC82027Neutrophil Elastase,PolymorphonuclearNeutrophilElastase,PolymorphonuclearA measurement of the polymorphonuclear neutrophilelastase in a biological specimen.Polymorphonuclear NeutrophilElastase MeasurementC84822 Neutrophilic Metamyelocytes NeutrophilicMetamyelocytesA measurement of the neutrophilic metamyelocytes ina biological specimen.Neutrophilic Metamyelocyte CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 134 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC84823 Neutrophilic Myelocytes NeutrophilicMyelocytesA measurement of the neutrophilic myelocytes in abiological specimen.Neutrophilic Myelocyte CountC63321 Neutrophils Neutrophils A measurement of the neutrophils in a biologicalspecimen.Absolute Neutrophil CountC64830 Neutrophils Band Form Neutrophils BandFormA measurement of the banded neutrophils in abiological specimen.Neutrophil Band Form CountC64831Neutrophils BandForm/LeukocytesNeutrophils BandForm/LeukocytesA relative measurement (ratio or percentage) of thebanded neutrophils to leukocytes in a biologicalspecimen.Neutrophil Band Form To LeukocyteRatioC81997 Neutrophils, Segmented Neutrophils,SegmentedA measurement of the segmented neutrophils in abiological specimen.Segmented Neutrophil CountC82045Neutrophils,Segmented/LeukocytesNeutrophils,Segmented/LeukocytesA relative measurement (ratio or percentage) ofsegmented neutrophils to leukocytes in a biologicalspecimen.Segmented Neutrophil to LeukocyteRatio MeasurementC64827 Neutrophils/Leukocytes Neutrophils/LeukocytesC98763 Neutrophils/Total Cells Neutrophils/Total CellsC74899 Niacin Niacin; VitaminB3A relative measurement (ratio or percentage) of theneutrophils to leukocytes in a biological specimen.A relative measurement (ratio or percentage) of theneutrophils to total cells in a biological specimen (forexample a bone marrow specimen).A measurement of the niacin in a biological specimen.Neutrophil To Leukocyte RatioNeutrophil to Total Cell RatioMeasurementVitamin B3 MeasurementC64810 Nitrite Nitrite A measurement of the nitrite in a urine specimen. Nitrite MeasurementC84811Non-Phosphorylated TauProteinNon-Phosphorylated Tau ProteinA measurement of the non-phosphorylated Tau proteinin a biological specimen.Nonphosphorylated Tau ProteinMeasurementC100434Non-Prostatic AcidPhosphataseNon-ProstaticAcidPhosphataseA measurement of the non-prostatic acid phosphatasein a biological specimen.Non-Prostatic Acid PhosphataseMeasurementC74868 Norepinephrine Noradrenaline;NorepinephrineC98764 Normoblasts/Total Cells Normoblasts/Total CellsC74705 Nucleated Erythrocytes NucleatedErythrocytes;Nucleated RedBlood CellsA measurement of the norepinephrine hormone in abiological specimen.A relative measurement (ratio or percentage) of thenormoblasts to total cells in a biological specimen(for example a bone marrow specimen).A measurement of the nucleated erythrocytes (large,immature nucleated erythrocytes) in a biologicalspecimen.Noradrenaline MeasurementNormoblast to Total Cell RatioMeasurementNucleated Red Blood Cell CountC74647NucleatedErythrocytes/ErythrocytesNucleatedErythrocytes/Erythrocytes;Nucleated RedBloodCells/ErythrocytesA relative measurement (ratio or percentage) of thenucleated erythrocytes (large, immature nucleatederythrocytes) to all erythrocytes in a biologicalspecimen.Nucleated Red Blood Cell toErythrocyte Ratio MeasurementC82046NucleatedErythrocytes/LeukocytesNucleatedErythrocytes/LeukocytesA relative measurement (ratio or percentage) ofnucleated erythrocytes to leukocytes in a biologicalspecimen.Nucleated Erythrocyte to LeukocyteRatio MeasurementC74686 Occult Blood Occult Blood A measurement of the blood in body products such as astool sample, not detectable on gross examination.Occult Blood MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 135 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74796 Opiate Opiate A measurement of any opiate class drug present in abiological specimen.C74801 Osmolality Osmolality A measurement of the osmoles of solute per unit ofbiological specimen.C74802 Osmolarity Osmolarity A measurement of the osmoles of solute per liter ofsolution.C74744 Osteocalcin Osteocalcin A measurement of the osteocalcin in a biologicalspecimen.C100452 Ova and Parasite Ova and Parasite A measurement of the parasites and ova in a biologicalspecimen.Opiate MeasurementOsmolality MeasurementOsmolarity MeasurementOsteocalcin MeasurementOva and Parasite MeasurementC92250 Oxalate Oxalate;EthanedioateC74884 Oxycodone Oxycodone;OxycontinA measurement of the oxalate in a biologicalspecimen.A measurement of the oxycodone present in abiological specimen.Oxalate MeasurementOxycodone MeasurementC96614 Oxygen Capacity Oxygen Capacity A measurement of the maximum amount of oxygenthat can be combined chemically with hemoglobin in avolume of blood.Oxygen Capacity MeasurementC68032 Oxygen Saturation OxygenSaturationA measurement of the oxygen-hemoglobin saturationof a volume of blood.Oxygen Saturation MeasurementC96616 Oxyhemoglobin Oxyhemoglobin A measurement of the oxyhemoglobin in a biologicalspecimen.Oxyhemoglobin MeasurementC74869 Oxytocin Oxytocin;OxytoxinA measurement of the oxytocin hormone in abiological specimen.Oxytocin MeasurementC102279 P50 Oxygen P50 Oxygen A measurement of the partial pressure of oxygen whenhemoglobin is half saturated in a biological specimen.P50 Oxygen MeasurementC82028 Pancreatic Elastase 1 PancreaticElastase 1A measurement of the pancreatic elastase 1 in abiological specimen.Pancreatic Elastase MeasurementC82029 Pancreatic Elastase 1,PolymorphonuclearPancreaticElastase 1,PolymorphonuclearA measurement of the polymorphonuclear pancreaticelastase 1 in a biological specimen.Polymorphonuclear PancreaticElastase MeasurementC80201 Pancreatic Polypeptide PancreaticPolypeptideC74616 Pappenheimer Bodies PappenheimerBodiesA measurement of the pancreatic polypeptide in abiological specimen.A measurement of the cells containing PappenheimerBodies (violet or blue staining ferritin granules usuallyfound along the periphery of the red blood cells) in abiological specimen.Pancreatic PolypeptideMeasurementPappenheimer Body CountC81964Parathyroid Hormone,C-TerminalParathyroidHormone,C-TerminalA measurement of the C-terminal fragment ofparathyroid hormone in a biological specimen.C-Terminal Parathyroid HormoneMeasurementC74784Parathyroid Hormone,FragmentedParathyroidHormone,FragmentedA measurement of the fragmented parathyroidhormone in a biological specimen.Fragmented Parathyroid HormoneMeasurementC74789 Parathyroid Hormone, Intact ParathyroidHormone, IntactA measurement of the intact parathyroid hormone(consisting of amino acids 1-84 or 7-84) in abiological specimen.Intact Parathyroid HormoneMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 136 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC81965Parathyroid Hormone,Mid-MoleculeParathyroidHormone,Mid-MoleculeA measurement of the mid-molecule fragment ofparathyroid hormone in a biological specimen.Mid-Molecule Parathyroid HormoneMeasurementC81966Parathyroid Hormone,N-TerminalParathyroidHormone,N-TerminalA measurement of the N-terminal fragment ofparathyroid hormone in a biological specimen.N-Terminal Parathyroid HormoneMeasurementC103451 Parathyroid Hormone, Whole ParathyroidHormone, WholeA measurement of the whole parathyroid hormone(consisting of amino acids 1-84) in a biologicalspecimen.Whole Parathyroid HormoneMeasurementC82625Partial Pressure CarbonDioxidePartial PressureCarbon DioxideA measurement of the pressure of carbon dioxide in abiological specimen.Partial Pressure of Carbon DioxideMeasurementC7<strong>12</strong>51 Partial Pressure Oxygen Partial PressureOxygenC96617 Parvovirus B19 IgG Antibody Parvovirus B19IgG AntibodyC96618 Parvovirus B19 IgM Antibody Parvovirus B19IgM AntibodyC74617 Pelger Huet Anomaly Pelger HuetAnomalyC81988 Pemphigoid Antibodies PemphigoidAntibodiesA measurement of the pressure of oxygen in abiological specimen.A measurement of the Parvovirus B19 IgG antibody ina biological specimen.A measurement of the Parvovirus B19 IgM antibody ina biological specimen.A measurement of the Pelger Huet Anomaly (nuclei ofneutrophils and eosinophils appear rod-like, sphericalor dumbbell shaped) in a biological specimen.A measurement of the pemphigoid antibodies in abiological specimen.Partial Pressure of OxygenMeasurementParvovirus B19 IgG AntibodyMeasurementParvovirus B19 IgM AntibodyMeasurementPelger Huet Anomaly MeasurementPemphigoid Antibody MeasurementC100<strong>12</strong>2 Pepsinogen Pepsinogen A measurement of the pepsinogen in a biologicalspecimen.Pepsinogen MeasurementC100469 Pepsinogen A Pepsinogen A;PGAC100470 Pepsinogen C Pepsinogen C;PGCC100467 Pepsinogen I Pepsinogen I;PGIC100468 Pepsinogen II Pepsinogen II;PGIIC80202 Peptide YY Peptide YY;Peptide TyrosineTyrosineC74694 Phencyclidine Phencyclidine;PhenylcyclohexylpiperidineC74695 Phenothiazine Phenothiazine;DibenzothiazineC64857 Phosphate Phosphate;Phosphorus;InorganicPhosphateC79461 Phosphate/Creatinine Phosphate/CreatinineA measurement of the pepsinogen A in a biologicalspecimen.A measurement of the pepsinogen C in a biologicalspecimen.A measurement of the pepsinogen I in a biologicalspecimen.A measurement of the pepsinogen II in a biologicalspecimen.A measurement of the peptide YY in a biologicalspecimen.A measurement of the phencyclidine present in abiological specimen.A measurement of the phenothiazine present in abiological specimen.A measurement of the phosphate in a biologicalspecimen.A relative measurement (ratio or percentage) of thephosphate to creatinine in a biological specimen.Pepsinogen A MeasurementPepsinogen C MeasurementPepsinogen I MeasurementPepsinogen II MeasurementPeptide YY MeasurementPhencyclidine MeasurementPhenothiazine MeasurementPhosphate MeasurementPhosphate to Creatinine RatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 137 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96623 Phospholipid Phospholipid A measurement of the phospholipids in a biologicalspecimen.Phospholipid MeasurementC848<strong>12</strong> Phosphorylated Tau Protein PhosphorylatedTau ProteinC98869 Plasma Cells/Total Cells PlasmaCells/Total CellsC74618 Plasmacytoid Lymphocytes PlasmacytoidLymphocytes;PlymphocytesA measurement of the phosphorylated Tau protein in abiological specimen.A relative measurement (ratio or percentage) of themature plasma cells (plasmacytes) to total cells in abiological specimen (for example a bone marrowspecimen).A measurement of the plasmacytoid lymphocytes(lymphocytes with peripherally clumped chromatin andoften deep blue cytoplasm, and that appear similar toplasma cells) in a biological specimen.Phosphorylated Tau ProteinMeasurementPlasma Cell to Total CellRatioMeasurementPlasmacytoid Lymphocyte CountC74648PlasmacytoidLymphocytes/LymphocytesPlasmacytoidLymphocytes/LymphocytesA relative measurement (ratio or percentage) of theplasmacytoid lymphocytes (lymphocytes withperipherally clumped chromatin and often deep bluecytoplasm, and that appear similar to plasma cells) toall lymphocytes in a biological specimen.Plasmacytoid Lymphocyte toLymphocyte Ratio MeasurementC82030Plasminogen ActivatorInhibitor-1PlasminogenActivatorInhibitor-1A measurement of the plasminogen activatorinhibitor-1 in a biological specimen.Plasminogen Activator Inhibitor-1MeasurementC81989Plasminogen ActivatorInhibitor-1 AGPlasminogenActivatorInhibitor-1 AGA measurement of the plasminogen activatorinhibitor-1 antigen in a biological specimen.Plasminogen Activator Inhibitor-1Antigen MeasurementC100453 Plasmodium Plasmodium Examination of a biological specimen to detect thepresence of any protozoan belonging to thePlasmodium genus.C103427 Platelet Aggregation Platelet Function A measurement of the association of platelets to oneanother via adhesion molecules in a biological sample.Plasmodium MeasurementPlatelet Aggregation MeasurementC96624 Platelet Clumps Platelet Clumps;PLT ClumpsC81962 Platelet Distribution Width PlateletDistributionWidthC100424 Platelet Hematocrit PlateletHematocrit;ThrombocytocritA measurement of the platelet clumps in a biologicalspecimen.A measurement of the range of platelet sizes in abiological specimen.A relative measurement (ratio or percentage) of theproportion of the volume of blood taken up byplatelets.Platelet Clumps CountPlatelet Distribution WidthPlatelet Hematocrit MeasurementC51951 Platelets Platelets A measurement of the platelets in a biologicalspecimen.C79602 Poikilocytes Poikilocytes A measurement of the odd-shaped erythrocytes in awhole blood specimen.Platelet CountPoikilocyte MeasurementC74649 Poikilocytes/Erythrocytes Poikilocytes/ErythrocytesA relative measurement (ratio or percentage) of thepoikilocytes, or irregularly shaped erythrocytes, to allerythrocytes in a biological specimen.Poikilocyte to Erythrocyte RatioMeasurementC64803 Polychromasia Polychromasia A measurement of the blue-staining characteristic ofnewly generated erythrocytes.C64853 Potassium Potassium A measurement of the potassium in a biologicalspecimen.PolychromasiaPotassium MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 138 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC79462 Potassium/Creatinine Potassium/CreatinineA relative measurement (ratio or percentage) of thepotassium to creatinine in a biological specimen.Potassium to Creatinine RatioMeasurementC100435 Prealbumin Prealbumin A measurement of the prealbumin in a biologicalspecimen.Prealbumin MeasurementC74619 Precursor Plasma Cells PrecursorPlasma Cells;PlasmablastA measurement of the precursor (blast stage) plasmacells (antibody secreting cells derived from B cells viaantigen stimulation) in a biological specimen.Precursor Plasma Cell CountC74650Precursor PlasmaCells/LymphocytesPrecursorPlasmaCells/LymphocytesA relative measurement (ratio or percentage) of theprecursor (blast stage) plasma cells (antibodysecreting cells derived from B cells via antigenstimulation) to all lymphocytes in a biologicalspecimen.Precursor Plasma Cell toLymphocyte Ratio MeasurementC82031Pregnancy-Associated PlasmaProtein-APregnancy-Associated PlasmaProtein-AA measurement of the pregnancy-associated plasmaprotein-A in a biological specimen.Pregnancy-Associated PlasmaProtein-A MeasurementC82032 ProB-type Natriuretic Peptide ProB-typeNatriureticPeptide;proBNP;Pro-BrainNatriureticPeptideA measurement of the proB-type natriuretic peptide ina biological specimen.ProB-Type Natriuretic PeptideMeasurementC103430 Procalcitonin Procalcitonin A measurement of the procalcitonin in a biologicalspecimen.Procalcitonin MeasurementC96625Procollagen 1 N-TerminalPropeptideProcollagen 1N-TerminalPropeptide;Amino-terminalpropeptide oftype 1procollagen;P1NPAminoterm Type1A measurement of the procollagen 1 N-terminalpropeptide in a biological specimen.Procollagen 1 N-TerminalPropeptide MeasurementC82033Procollagen Type I CarboxyTerm PeptideProcollagenType I CarboxyTerm PeptideA measurement of the procollagen-1 carboxy-terminalpeptide in a biological specimen.Procollagen Type I CarboxyTerminal Peptide MeasurementC100446 Proerythroblast Proerythroblast A measurement of the proerythroblasts in a biologicalspecimen.C74791 Progesterone Progesterone A measurement of the progesterone hormone in abiological specimen.C81967 Proinsulin Proinsulin A measurement of the proinsulin in a biologicalspecimen.C74870 Prolactin Prolactin A measurement of the prolactin hormone in abiological specimen.C74620 Prolymphocytes Prolymphocytes A measurement of the prolymphocytes in a biologicalspecimen.Proerythroblast MeasurementProgesterone MeasurementProinsulin MeasurementProlactin MeasurementProlymphocyte CountC64829 Prolymphocytes/Leukocytes Prolymphocytes/LeukocytesA relative measurement (ratio or percentage) ofprolymphocytes to leukocytes in a biologicalspecimen.Prolymphocyte To Leukocyte RatioSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 139 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74651 Prolymphocytes/Lymphocytes Prolymphocytes/LymphocytesA relative measurement (ratio or percentage) of theprolymphocytes to all lymphocytes in a biologicalspecimen.Prolymphocyte to Lymphocyte RatioMeasurementC74621 Promonocytes Promonocytes A measurement of the promonocytes in a biologicalspecimen.Promonocyte CountC74652 Promonocytes/Leukocytes Promonocytes/LeukocytesA relative measurement (ratio or percentage) of thepromonocytes to all leukocytes in a biologicalspecimen.Promonocyte to Lymphocyte RatioMeasurementC74622 Promyelocytes Promyelocytes A measurement of the promyelocytes (immaturemyelocytes) in a biological specimen.Promyelocyte CountC74653 Promyelocytes/Leukocytes Promyelocytes/LeukocytesC98773 Promyelocytes/Total Cells Promyelocytes/Total CellsC98870 Pronormoblasts/Total Cells Pronormoblasts/Total CellsA relative measurement (ratio or percentage) of thepromyelocytes (immature myelocytes) to allleukocytes in a biological specimen.A relative measurement (ratio or percentage) of thepromyelocytes (immature myelocytes) to total cells ina biological specimen (for example a bone marrowspecimen).A relative measurement (ratio or percentage) of thepronormoblasts to total cells in a biological specimen(for example a bone marrow specimen).Promyelocyte to Lymphocyte RatioMeasurementPromyelocyte to Total Cell RatioMeasurementPronormoblast to Total CellRatioMeasurementC74885 Propoxyphene Propoxyphene A measurement of the propoxyphene present in abiological specimen.Propoxyphene MeasurementC103343 Prostaglandin TotalProstaglandinA measurement of the total prostaglandin in abiological specimen.Prostaglandin MeasurementC103431 Prostaglandin D2 Prostaglandin D2 A measurement of the prostaglandin D2 in a biologicalspecimen.Prostaglandin D2 MeasurementC103432 Prostaglandin D2 Synthase Prostaglandin D2SynthaseC103433 Prostaglandin E Synthase Prostaglandin ESynthaseA measurement of the prostaglandin D2 synthase in abiological specimen.A measurement of the prostaglandin E synthase in abiological specimen.Prostaglandin D2 SynthaseMeasurementProstaglandin E SynthaseMeasurementC103434 Prostaglandin E1 Prostaglandin E1 A measurement of the prostaglandin E1 in a biologicalspecimen.C103435 Prostaglandin E2 Prostaglandin E2 A measurement of the prostaglandin E2 in a biologicalspecimen.Prostaglandin E1 MeasurementProstaglandin E2 MeasurementC103436 Prostaglandin F1 Alpha Prostaglandin F1AlphaC103437 Prostaglandin F2 Alpha Prostaglandin F2AlphaC17634 Prostate Specific Antigen Prostate SpecificAntigenC80204 Prostatic Acid Phosphatase Prostatic AcidPhosphataseA measurement of the prostaglandin F1 alpha in abiological specimen.A measurement of the prostaglandin F2 alpha in abiological specimen.A measurement of the prostate specific antigen in abiological specimen.A measurement of the prostatic acid phosphatase in abiological specimen.Prostaglandin F1 AlphaMeasurementProstaglandin F2 AlphaMeasurementProstate Specific AntigenMeasurementProstatic Acid PhosphataseMeasurementC64858 Protein Protein A measurement of a group of complex organicmacromolecules composed of one or morealpha-L-amino acid chains in a biological specimen.Total Protein MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 140 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC100436 Protein S Protein S A measurement of the protein S in a biologicalspecimen.Protein S MeasurementC79463 Protein/Creatinine Protein/CreatinineC92240 Protein/Osmolality Protein/Osmolality;Protein/Osmolality RatioC98774 Prothrombin Activity ProthrombinActivity; FactorII ActivityC82034 Prothrombin Fragments 1 + 2 ProthrombinFragments 1 + 2A relative measurement (ratio or percentage) of theprotein to creatinine in a biological specimen.A relative measurement (ratio or percentage) of totalproteins to the osmolality of a biological specimen.A measurement of the biological activity ofprothrombin in a biological specimen.A measurement of the prothrombin fragments 1 and 2in a biological specimen.Protein to Creatinine RatioMeasurementProtein to Osmolality RatioMeasurementProthrombin Activity MeasurementProthrombin Fragments 1 and 2MeasurementC64805Prothrombin Intl. NormalizedRatioProthrombin Intl.NormalizedRatioA ratio that represents the prothrombin time for aplasma specimen, divided by the result for a controlplasma specimen, further standardized for theInternational Sensitivity Index of the tissue factor(thromboplastin) used in the test.International Normalized Ratio ofProthrombin TimeC62656 Prothrombin Time ProthrombinTimeA blood clotting measurement that evaluates theextrinsic pathway of coagulation.Prothrombin TimeC74696 Pseudoephedrine Pseudoephedrine A measurement of the pseudoephedrine present in abiological specimen.C80211 Pyridinoline Pyridinoline A measurement of the pyridinoline in a biologicalspecimen.C74772 RBC Casts RBC Casts A measurement of the red blood cell casts present in abiological specimen.Pseudoephedrine MeasurementPyridinoline MeasurementRed Blood Cell Cast MeasurementC74716 Rapid Plasma Reagin Rapid PlasmaReaginC74629 Reactive Lymphocytes ReactiveLymphocytesA measurement of the antibodies produced by cellulardamage caused by Treponema pallidum (syphilis) in abiological specimen.A measurement of the reactive lymphocytes(lymphocytes which have become large due to anantigen reaction) in a biological specimen.Rapid Plasma Reagin MeasurementReactive Lymphocyte CountMeasurementC74654ReactiveLymphocytes/LymphocytesReactiveLymphocytes/LymphocytesA relative measurement (ratio or percentage) of thereactive lymphocytes (lymphocytes which havebecome large due to an antigen reaction) to alllymphocytes in a biological specimen.Reactive Lymphocyte toLymphocyte Ratio MeasurementC81957Reg upon Act Normal T-cellExprd SecrtdReg upon ActNormal T-cellExprd Secrtd;ChemokineLigand 5A measurement of the RANTES (regulated onactivation, normally, T-cell expressed, and secreted)chemokine in a biological specimen.Reg upon Act Normal T-cell ExprdSecrtd MeasurementC74893 Renin Renin;AngiotensinogenaseA measurement of the renin in a biological specimen.Renin MeasurementC96628 Reptilase Time Reptilase Time A measurement of the time it takes a plasma sample toclot after adding the active enzyme reptilase.C80205 Resistin Resistin A measurement of the resistin in a biologicalspecimen.Reptilase Time MeasurementResistin MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 141 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98776 Reticulocyte Hemoglobin ReticulocyteHemoglobinA measurement of the amount of hemoglobin inreticulocytes.Reticulocyte HemoglobinMeasurementC51947 Reticulocytes Reticulocytes A measurement of the reticulocytes in a biologicalspecimen.Reticulocyte CountC64828 Reticulocytes/Erythrocytes Reticulocytes/ErythrocytesC100437 Retinol Binding Protein Retinol BindingProteinC74717 Rheumatoid Factor RheumatoidFactorC74898 Riboflavin Riboflavin;Vitamin B2C100457 Ribonucleoprotein Antibody Ribonucleoprotein Antibody;RNP AntibodyC100419 Ringed Sideroblasts RingedSideroblastsC74624 Rouleaux Formation RouleauxFormationC74698 Round Epithelial Cells Round EpithelialCellsC103440 Rubella IgG Antibody Rubella IgGAntibodyA relative measurement (ratio or percentage) ofreticulocytes to erythrocytes in a biological specimen.A measurement of the total retinol binding protein in abiological specimen.A measurement of the rheumatoid factor antibody in abiological specimen.A measurement of the riboflavin in a biologicalspecimen.A measurement of the ribonucleoprotein antibody in abiological specimen.A measurement of the ringed sideroblasts (abnormalnucleated erythroblasts with a large number of irondeposits in the perinuclear mitochondria, forming aring around the nucleus) in a biological specimen.A measurement of the number of stacking red bloodcells within a biological specimen.A measurement of the round epithelial cells present ina biological specimen.A measurement of the Rubella IgG antibody in abiological specimen.Reticulocyte To Erythrocyte RatioRetinol Binding ProteinMeasurementRheumatoid Factor MeasurementVitamin B2 MeasurementRibonucleoprotein AntibodyMeasurementRinged Sideroblast MeasurementRouleaux Formation CountRound Epithelial Cell CountRubella IgG Antibody MeasurementC74706 Schistocytes Schistocytes A measurement of the schistocytes (fragmented redblood cells) in a biological specimen.Schistocyte CountC100458 Scl-70 Antibody Scl-70 Antibody;Scleroderma-70AntibodyA measurement of the Scl-70 antibody in a biologicalspecimen.Scl-70 Antibody MeasurementC74871 Secretin Secretin A measurement of the secretin hormone in abiological specimen.C74872 Serotonin Serotonin A measurement of the serotonin hormone in abiological specimen.Secretin MeasurementSerotonin MeasurementC74745Sex Hormone BindingGlobulinSex HormoneBindingGlobulin; SexHormoneBinding ProteinA measurement of the sex hormone binding (globulin)protein in a biological specimen.Sex Hormone Binding ProteinMeasurementC74625 Sezary Cells Sezary Cells A measurement of the Sezary cells (atypicallymphocytes with cerebriform nuclei) in a biologicalspecimen.Sezary Cell CountC74655 Sezary Cells/Lymphocytes SezaryCells/LymphocytesA relative measurement (ratio or percentage of theSezary cells (atypical lymphocytes with cerebriformnuclei) to all lymphocytes in a biological specimen.Sezary Cell to Lymphocyte RatioMeasurementC74626 Sickle Cells Sickle Cells A measurement of the sickle cells (sickle shaped redblood cells) in a biological specimen.Sickle Cell CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 142 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74656 Sickle Cells/Erythrocytes SickleCells/ErythrocytesA relative measurement (ratio or percentage) of thesickle cells (sickle shaped red blood cells) to allerythrocytes in a biological specimen.Sickle Cell to Erythrocyte RatioMeasurementC100418 Sideroblast Sideroblast A measurement of the sideroblasts (nucleatederythroblasts with iron granules in the cytoplasm) in abiological specimen.Sideroblast MeasurementC92236 Sjogrens SS-A Antibody Ro Antibody;Sjogrens SS-AAntibodyC92237 Sjogrens SS-B Antibody La Antibody;Sjogrens SS-BAntibodyA measurement of the Sjogrens SS-A antibody in abiological specimen.A measurement of the Sjogrens SS-B antibody in abiological specimen.Sjogren's SS-A AntibodyMeasurementSjogren's SS-B AntibodyMeasurementC92281 Smith Antibody Smith Antibody A measurement of the Smith antibody in a biologicalspecimen.Smith Antibody MeasurementC74627 Smudge Cells Smudge Cells;Basket CellsA measurement of the smudge cells (the nuclearremnant of a ruptured white blood cell) in a biologicalspecimen.Smudge Cell CountC64809 Sodium Sodium A measurement of the sodium in a biologicalspecimen.Sodium MeasurementC79464 Sodium/Creatinine Sodium/CreatinineA relative measurement (ratio or percentage) of thesodium to creatinine in a biological specimen.Sodium to Creatinine RatioMeasurementC102280 Soluble Interleukin 2Receptor ActivitySolubleInterleukin 2ReceptorActivityA measurement of the soluble interleukin 2 receptoractivity in a biological specimen.Soluble Interleukin 2 ReceptorActivity MeasurementC100438 Soluble Transferrin Receptor SolubleTransferrinReceptorA measurement of the soluble transferrin receptor in abiological specimen.Soluble Transferrin ReceptorMeasurementC92533Soluble Vasc Cell AdhesionMolecule 1Soluble VascularCell AdhesionMolecule 1A measurement of the soluble vascular cell adhesionmolecule 1 in a biological specimen.Soluble Vascular Cell AdhesionMolecule 1C80360 Somatotrophin Somatotrophin;GrowthHormoneC79465 Sorbitol Dehydrogenase SorbitolDehydrogenaseA measurement of the somatotrophin (growth)hormone in a biological specimen.A measurement of the sorbitol dehydrogenase in abiological specimen.Somatotropin MeasurementSorbitol DehydrogenaseMeasurementC64832 Specific Gravity Specific Gravity A ratio of the density of a fluid to the density of water. Specific GravityC25377 Specimen Appearance SpecimenAppearanceThe outward or visible aspect of a specimen.AppearanceC102281 Sperm Motility Sperm Motility A measurement of the sperm capable of forward,progressive movement in a semen specimen.C74663 Spermatozoa Spermatozoa A measurement of the spermatozoa cells present in abiological specimen.C74707 Spherocytes Spherocytes A measurement of the spherocytes (small,sphere-shaped red blood cells) in a biologicalspecimen.Sperm Motility MeasurementSpermatozoa Cell CountSpherocyte CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 143 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74773 Squamous Epithelial Cells SquamousEpithelial CellsA measurement of the squamous epithelial cellspresent in a biological specimen.Squamous Epithelial Cell CountC74774Squamous TransitionalEpithelial CellsSquamousTransitionalEpithelial Cells;Epithelial CellsA measurement of the squamous transitional epithelialcells present in a biological specimen.Squamous Transitional EpithelialCell CountC81951 Starch Crystals Starch Crystals A measurement of the starch crystals in a biologicalspecimen.Starch Crystal MeasurementC82035 Stem Cell Factor Stem CellFactor; KITLigandA measurement of the stem cell factor in a biologicalspecimen.Stem Cell Factor MeasurementC74708 Stomatocytes Stomatocytes A measurement of the stomatocytes (red blood cellswith an oval or rectangular area of central pallor,producing the appearance of a cell mouth) in abiological specimen.Stomatocyte CountC92282 Streptolysin O Antibody Streptolysin OAntibody;AntistreptolysinOA measurement of Streptolysin O Antibody in abiological specimen.Streptolysin O AntibodyMeasurementC74755 Sulfa Crystals Sulfa Crystals A measurement of the sulfa crystals present in abiological specimen.Sulfa Crystal MeasurementC96636 Target Cells Leptocytes;Target Cells;Codocytes;Mexican HatCellsC84810 Tau Protein Tau Protein;Total Tau ProteinC74793 Testosterone Testosterone;TotalTestosteroneC74785 Testosterone, Free Testosterone,FreeC74896 Thiamine Thiamine;Vitamin B1A measurement of the target cells in a biologicalspecimen.A measurement of the total Tau protein in a biologicalspecimen.A measurement of the total (free and bound)testosterone in a biological specimen.A measurement of the free testosterone in a biologicalspecimen.A measurement of the thiamine in a biologicalspecimen.Target Cell CountTau Protein MeasurementTotal Testosterone MeasurementFree Testosterone MeasurementVitamin B1 MeasurementC80365 Thrombin Time Thrombin Time A measurement of the time it takes a plasma sample toclot after adding the active enzyme thrombin. (NCI)C74873 Thrombopoietin Thrombopoietin A measurement of the thrombopoietin hormone in abiological specimen.C103445 Thromboxane B2 Thromboxane B2 A measurement of the thromboxane B2 in a biologicalspecimen.C103446 Thyroglobulin TG A measurement of the thyroglobulin in a biologicalspecimen.Thrombin TimeThrombopoietin MeasurementThromboxane B2 MeasurementThyroglobulin MeasurementC81990 Thyroid Antibodies ThyroidAntibodiesA measurement of the thyroid antibodies in abiological specimen.Thyroid Antibody MeasurementC81991Thyroid AntimicrosomalAntibodiesThyroidAntimicrosomalAntibodiesA measurement of the thyroid antimicrosomalantibodies in a biological specimen.Thyroid Antimicrosomal AntibodyMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 144 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC81992Thyroid AntithyroglobulinAntibodiesThyroidAntithyroglobulin AntibodiesA measurement of the thyroid antithyroglobulinantibodies in a biological specimen.Thyroid Antithyroglobulin AntibodyMeasurementC96639 Thyroperoxidase Thyroperoxidase;ThyroidPeroxidaseC96638 Thyroperoxidase Antibody ThyroperoxidaseAntibodyA measurement of the thyroperoxidase in a biologicalspecimen.A measurement of the thyroperoxidase antibody in abiological specimen.Thyroperoxidase MeasurementThyroperoxidase AntibodyMeasurementC64813 Thyrotropin Thyrotropin A measurement of the thyrotropin in a biologicalspecimen.Thyrotropin MeasurementC74874Thyrotropin ReleasingHormoneThyrotropinReleasingHormone;ThyrotropinReleasing FactorA measurement of the thyrotropin releasing hormonein a biological specimen.Thyrotropin Releasing HormoneMeasurementC74794 Thyroxine Thyroxine; TotalT4C74746 Thyroxine Binding Globulin ThyroxineBinding GlobulinC74786 Thyroxine, Free Thyroxine, Free;Free T4A measurement of the total (free and bound) thyroxinein a biological specimen.A measurement of the thyroxine binding globulinprotein in a biological specimen.A measurement of the free thyroxine in a biologicalspecimen.Total Thyroxine MeasurementThyroxine Binding Globulin ProteinMeasurementFree Thyroxine MeasurementC82036Tissue Inhibitor ofMetalloproteinase 1Tissue InhibitorofMetalloproteinase 1A measurement of the tissue inhibitor ofmetalloproteinase 1 in a biological specimen.Tissue Inhibitor ofMetalloproteinase 1 MeasurementC81993Tissue Plasminogen ActivatorAntigenTissuePlasminogenActivatorAntigenA measurement of the tissue plasminogen activatorantigen in a biological specimen.Tissue Plasminogen ActivatorAntigen MeasurementC74718 Total Iron Binding Capacity Total IronBinding CapacityA measurement of the amount of iron needed to fullysaturate the transferrin in a biological specimen.Total Iron Binding CapacityC80208Total Radical-Trap AntioxidantPotentialTotalRadical-TrapAntioxidantPotentialA measurement of the ability of the antioxidants in abiological specimen to buffer free radicals in asuspension.Total Radical-Trap AntioxidantPotential MeasurementC96641 Toxic Granulation ToxicGranulationA measurement of the toxic granulation in neutrophilicblood cells.Toxic Granulation MeasurementC82037 Transferrin Transferrin A measurement of the transferrin in a biologicalspecimen.Transferrin MeasurementC98792 Transferrin Saturation TransferrinSaturationC92251 Transitional Epithelial Cells TransitionalEpithelial CellsC64807 Triacylglycerol Lipase TriacylglycerolLipaseA measurement of the iron bound to transferrin in abiological specimen.A measurement of the transitional epithelial cellspresent in a biological specimen.A measurement of the pancreatic lipase in a biologicalspecimen.Transferrin Saturation MeasurementTransitional Epithelial CellsMeasurementTriacylglycerol Lipase MeasurementC92238 Trichomonas Trichomonas Examination of a biological specimen to detect thepresence of any protozoan belonging to theTrichomonas genus.Trichomonas ScreeningSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 145 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC100420 Tricyclic Antidepressants TricyclicAntidepressantsA measurement of tricyclic antidepressants in abiological specimen.Tricyclic AntidepressantMeasurementC648<strong>12</strong> Triglycerides Triglycerides A measurement of the triglycerides in a biologicalspecimen.Triglyceride MeasurementC74747 Triiodothyronine Triiodothyronine; Total T3C74748 Triiodothyronine Uptake TriiodothyronineUptakeC74787 Triiodothyronine, Free Triiodothyronine, Free; Free T3C81968 Triiodothyronine, Reverse Triiodothyronine, ReverseC74756 Triple Phosphate Crystals Triple PhosphateCrystalsA measurement of the total (free and bound)triiodothyronine in a biological specimen.A measurement of the binding of triiodothyonine tothyroxine binding globulin protein in a biologicalspecimen.A measurement of the free triiodothyronine in abiological specimen.A measurement of the reverse triiodothyronine in abiological specimen.A measurement of the triple phosphate crystals presentin a biological specimen.Triiodothyronine MeasurementTriiodothyronine UptakeMeasurementFree Triiodothyronine MeasurementReverse TriiodothyronineMeasurementTriple Phosphate CrystalMeasurementC74749 Troponin I Troponin I A measurement of the actin binding troponin in abiological specimen.C74750 Troponin T Troponin T A measurement of the tropomyosin binding troponin ina biological specimen.C92292 Tryptase Tryptase A measurement of the tryptase in a biologicalspecimen.Troponin I MeasurementTroponin T MeasurementTryptase MeasurementC74775 Tubular Epithelial Cells TubularEpithelial Cells;Renal TubularEpithelial CellsC74751 Tumor Necrosis Factor Tumor NecrosisFactor; TumorNecrosis FactoralphaA measurement of the tubular epithelial cells presentin a biological specimen.A measurement of the total tumor necrosis factor(cachexin) cytokine in a biological specimen.Tubular Epithelial Cell CountTumor Necrosis FactorMeasurementC74723 Turbidity Turbidity A measurement of the opacity of a biologicalspecimen.Turbidity MeasurementC82038Type I CollagenC-TelopeptidesType I CollagenC-Telopeptides;Type I CollagenX-linkedC-telopeptideA measurement of the type I collagen C-telopeptidesin a biological specimen.Type I Collagen C-TelopeptideMeasurementC82039Type I CollagenN-TelopeptidesType I CollagenN-TelopeptidesA measurement of the type I collagen N-telopeptidesin a biological specimen.Type I Collagen N-TelopeptideMeasurementC92283 Type I Myeloblasts Type IMyeloblastsA measurement of type I myeloblast cells per unit of abiological specimen.Type I Myeloblasts MeasurementC82040Type II CollagenC-TelopeptidesType II CollagenC-TelopeptidesA measurement of the type II collagen C-telopeptidesin a biological specimen.Type II Collagen C-TelopeptideMeasurementC82041Type II CollagenN-TelopeptidesType II CollagenN-TelopeptidesA measurement of the type II collagen N-telopeptidesin a biological specimen.Type II Collagen N-TelopeptideMeasurementC92284 Type II Myeloblasts Type IIMyeloblastsA measurement of type II myeloblast cells per unit of abiological specimen.Type II Myeloblasts MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 146 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92285 Type III Myeloblasts Type IIIMyeloblastsC74683 Tyrosine Crystals TyrosineCrystalsC74776 Unclassified Casts UnclassifiedCastsC74757 Unclassified Crystals UnclassifiedCrystalsA measurement of type III myeloblast cells per unit ofa biological specimen.A measurement of the triple phosphate crystals presentin a biological specimen.A measurement of the unclassifiable casts present in abiological specimen.A measurement of the unclassifiable crystals presentin a biological specimen.Type III Myeloblasts MeasurementTyrosine Crystal MeasurementUnclassified Cast MeasurementUnclassified Crystal MeasurementC74719Unsaturated Iron BindingCapacityUnsaturated IronBinding CapacityA measurement of the binding capacity of unsaturatediron in a biological specimen.Unsaturated Iron Binding CapacityMeasurementC64814 Urate Urate; Uric Acid A measurement of the urate in a biological specimen. Urate MeasurementC64815 Urea Urea A measurement of the urea in a biological specimen. Urea MeasurementC96645 Urea/Creatinine Urea/Creatinine A relative measurement (ratio or percentage) of theurea to creatinine in a biological specimen.Urea to Creatinine RatioMeasurementC74684 Uric Acid Crystals Uric AcidCrystalsC102282 Urine Conductivity UrineConductivityA measurement of the uric acid crystals present in abiological specimen.A measurement of the urine conductivity which is anon-linear function of the electrolyte concentration inthe urine.Uric Acid Crystal MeasurementUrine ConductivityC64816 Urobilinogen Urobilinogen A measurement of the urobilinogen in a biologicalspecimen.Urobilinogen MeasurementC64806 VLDL Cholesterol VLDLCholesterolC103450 VLDL Particle Size VLDL ParticleSizeC74628 Vacuolated Neutrophils VacuolatedNeutrophilsC74875 Vanillyl Mandelic Acid VanillylMandelic Acid;VanilmandelicAcidA measurement of the very low density lipoproteincholesterol in a biological specimen.A measurement of the average particle size ofvery-low-density lipoprotein in a biological specimen.A measurement of the neutrophils containing smallvacuoles in a biological specimen.A measurement of the vanillyl mandelic acidmetabolite in a biological specimen.Serum VLDL CholesterolMeasurementVLDL Particle Size MeasurementVacuolated Neutrophil CountVanillyl Mandelic AcidMeasurementC98795Varicella Zoster Virus IgAAntibodyVaricella ZosterVirus IgAAntibodyA measurement of the Varicella Zoster virus IgAantibodies in a biological specimen.Varicella Zoster Virus IgA AntibodyMeasurementC98796Varicella Zoster Virus IgGAntibodyVaricella ZosterVirus IgGAntibodyA measurement of the Varicella Zoster virus IgGantibodies in a biological specimen.Varicella Zoster Virus IgG AntibodyMeasurementC98797Varicella Zoster Virus IgMAntibodyVaricella ZosterVirus IgMAntibodyA measurement of the Varicella Zoster virus IgMantibodies in a biological specimen.Varicella Zoster Virus IgM AntibodyMeasurementC82042Vascular Cell AdhesionMolecule 1Vascular CellAdhesionMolecule 1A measurement of the vascular cell adhesion molecule1 in a biological specimen.Vascular Cell Adhesion Molecule 1MeasurementC92514Vascular Endothelial GrowthFactorVascularEndothelialGrowth FactorA measurement of the vascular endothelial growthfactor in a biological specimen.Vascular Endohelial Growth FactorMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 147 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC759<strong>12</strong> Viscosity Viscosity The resistance of a liquid to sheer forces and flow.(NCI)ViscosityC74895 Vitamin A Vitamin A;RetinolA measurement of the Vitamin A in a biologicalspecimen.Vitamin A MeasurementC64817 Vitamin B<strong>12</strong> Vitamin B<strong>12</strong> A measurement of the Vitamin B<strong>12</strong> in a serumspecimen.Vitamin B<strong>12</strong> MeasurementC74897 Vitamin B17 Vitamin B17;AmygdalinC74900 Vitamin B5 Vitamin B5;Pantothenic AcidC74901 Vitamin B6 Vitamin B6;PyridoxineC74902 Vitamin B7 Vitamin B7;BiotinC74676 Vitamin B9 Folic Acid;Vitamin B9C74903 Vitamin C Vitamin C;Ascorbic AcidC84818 Vitamin D Vitamin D; TotalVitamin DC74904 Vitamin D2 Vitamin D2;ErgocalciferolC74905 Vitamin D3 Vitamin D3;CholecalciferolA measurement of the Vitamin B17 in a biologicalspecimen.A measurement of the Vitamin B5 in a biologicalspecimen.A measurement of the Vitamin B6 in a biologicalspecimen.A measurement of the Vitamin B7 in a biologicalspecimen.A measurement of the folic acid in a biologicalspecimen.A measurement of the Vitamin C in a biologicalspecimen.A measurement of the total Vitamin D in a biologicalspecimen.A measurement of the Vitamin D2 in a biologicalspecimen.A measurement of the Vitamin D3 in a biologicalspecimen.Vitamin B17 MeasurementVitamin B5 MeasurementVitamin B6 MeasurementVitamin B7 MeasurementFolic Acid MeasurementVitamin C MeasurementVitamin D MeasurementVitamin D2 MeasurementVitamin D3 MeasurementC74906 Vitamin E Vitamin E A measurement of the Vitamin E in a biologicalspecimen.Vitamin E MeasurementC103448 Vitamin E/Cholesterol VitaminE/CholesterolC74907 Vitamin K Vitamin K;NaphthoquinoneC103449 Vitamin K1 Phylloquinone;PhytomenadioneA relative measurement (ratio or percentage) ofvitamin E to total cholesterol in a biological specimen.A measurement of the total Vitamin K in a biologicalspecimen.A measurement of the Vitamin K1 in a biologicalspecimen.Vitamin E to Cholesterol RatioMeasurementVitamin K MeasurementVitamin K1 MeasurementC74720 Volume Volume A measurement of the volume of a biologicalspecimen.C74778 WBC Casts WBC Casts A measurement of the white blood cell casts present ina biological specimen.C74777 Waxy Casts Waxy Casts A measurement of the waxy casts present in abiological specimen.C74664 Yeast Cells Yeast Cells A measurement of the yeast cells present in abiological specimen.C92239 Yeast Hyphae Yeast Hyphae Examination of a biological specimen to detect thepresence of any yeast cells that are in the long,filamentous and branching phase of growth.Volume MeasurementWhite Blood Cell Cast MeasurementWaxy Cell Cast MeasurementYeast Cell MeasurementYeast Hyphae ScreeningC80210 Zinc Zinc A measurement of the zinc in a biological specimen. Zinc MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 148 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67154 - LBTEST - Laboratory Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC45997 pH pH A measure of the acidity or alkalinity of a fluid on ascale of 0 to 14.pHC98799 von Willebrand Factor von WillebrandFactor; vonWillebrandFactor AntigenA measurement of the von Willebrand factor in abiological specimen.von Willebrand Factor MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 149 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74847 ADH AntidiureticHormone;VasopressinC102257 ADP AdenosineDiphosphateA measurement of the antidiuretic hormone in abiological specimen.A measurement of the adenosine diphosphate in abiological specimen.Antidiuretic Hormone MeasurementAdenosine DiphosphateMeasurementC74839 ADPNCTN Adiponectin A measurement of the adiponectin hormone in abiological specimen.Adiponectin MeasurementC74913 ADSDNA Anti-DoubleStranded DNAA measurement of the anti-double stranded DNAantibody in a biological specimen.Anti-Double Stranded DNAMeasurementC98705 ADVVLD ADV Viral Load A measurement of the adenovirus viral load in abiological specimen.Adenovirus Viral Load MeasurementC98706 AFACTXAA Anti-Factor XaActivityC74732 AFP AlphaFetoproteinC96562 AFPL1 AlphaFetoprotein L1C96563 AFPL2 AlphaFetoprotein L2C96564 AFPL3 AlphaFetoprotein L3C96565 AFPL3AFP A FetoproteinL3/A FetoproteinC64431 ALB Albumin;MicroalbuminC74761 ALBCREAT Albumin/Creatinine;Microalbumin/Creatinine RatioC74894 ALBGLOB Albumin/GlobulinC103453 ALBPROT Albumin/TotalProteinA measurement of the anti-activated Factor X in abiological specimen. This test is used to monitor lowmolecular weight or unfractionated heparin levels in abiological specimen.A measurement of the alpha fetoprotein in a biologicalspecimen.A measurement of the alpha fetoprotein L1 in abiological specimen.A measurement of the alpha fetoprotein L2 in abiological specimen.A measurement of the alpha fetoprotein L3 in abiological specimen.A relative measurement (ratio or percentage) of alphafetoprotein L3 to total alpha fetoprotein in a biologicalspecimen.A measurement of the albumin protein in a biologicalspecimen.A relative measurement (ratio or percentage) of thealbumin to the creatinine in a biological specimen.The ratio of albumin to globulin in a biologicalspecimen.A relative measurement (ratio or percentage) of thealbumin to total protein in a biological specimen.Anti-Factor Xa ActivityMeasurementAlpha-fetoprotein MeasurementAlpha Fetoprotein L1 MeasurementAlpha Fetoprotein L2 MeasurementAlpha Fetoprotein L3 MeasurementAlpha Fetoprotein L3 to Total AlphaFetoprotein Ratio MeasurementAlbumin MeasurementAlbumin To Creatinine Protein RatioMeasurementAlbumin to Globulin RatioMeasurementAlbumin to Total Protein RatioMeasurementC74731 ALDOLASE Aldolase A measurement of the aldolase enzyme in a biologicalspecimen.C74841 ALDSTRN Aldosterone A measurement of the aldosterone hormone in abiological specimen.Aldolase MeasurementAldosterone MeasurementC64432 ALP AlkalinePhosphataseC92287 ALPBS Bone SpecificAlkalinePhosphataseA measurement of the alkaline phosphatase in abiological specimen.A measurement of the bone specific alkalinephosphatase isoform in a biological specimen.Alkaline Phosphatase MeasurementBone Specific Alkaline PhosphataseMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 151 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC79438 ALPCREAT AlkalinePhosphatase/CreatinineC64433 ALT AlanineAminotransferase; SGPTC103349 ALTCPHRL AlphaTocopherolC81975 AMA Antimitochondrial Antibodies;MitochondrialAntibodyA relative measurement (ratio or percentage) of thealkaline phosphatase to creatinine in a biologicalspecimen.A measurement of the alanine aminotransferase in abiological specimen.A measurement of the alpha tocopherol in a biologicalspecimen.A measurement of the antimitochondrial antibodies ina biological specimen.Alkaline Phosphatase to CreatinineRatio MeasurementAlanine AminotransferaseMeasurementAlpha Tocopherol MeasurementAntimitochondrial AntibodyMeasurementC74799 AMMONIA Ammonia; NH3 A measurement of the ammonia in a biologicalspecimen.Ammonia MeasurementC74666 AMORPHSD AmorphousSediment;AmorphousDebrisA measurement of the amorphous sediment present ina biological specimen.Amorphous Sediment MeasurementC74687 AMPHET Amphetamine A measurement of any amphetamine class drug presentin a biological specimen.Amphetamine Drug ClassMeasurementC102262 AMPHETD Dextroamphetamine;d-amphetamineA measurement of the dextroamphetamine in abiological specimen.Dextroamphetamine MeasurementC64434 AMYLASE Amylase A measurement of the total enzyme amylase in abiological specimen.Amylase MeasurementC98767 AMYLASEP Amylase,PancreaticC98780 AMYLASES Amylase,SalivaryC103352 AMYLB38 Amyloid Beta38; AmyloidBeta 38 Protein;Amyloid Beta1-38C103353 AMYLB40 Amyloid Beta40; AmyloidBeta 40 Protein;Amyloid Beta1-40C84809 AMYLB42 Amyloid Beta42; AmyloidBeta 42 Protein;Amyloid Beta1-42C81999 AMYLOIDB Amyloid, Beta;Beta AmyloidA measurement of the pancreatic enzyme amylase in abiological specimen.A measurement of the salivary enzyme amylase in abiological specimen.A measurement of amyloid beta protein which iscomposed of peptides 1 to 38 in a biologicalspecimen.A measurement of amyloid beta protein which iscomposed of peptides 1 to 40 in a biologicalspecimen.A measurement of amyloid beta protein which iscomposed of peptides 1 to 42 in a biologicalspecimen.A measurement of the total amyloid beta in abiological specimen.Pancreatic Amylase MeasurementSalivary Amylase MeasurementAmyloid Beta 1-38 MeasurementAmyloid Beta 1-40 MeasurementBeta Amyloid 42 MeasurementBeta Amyloid MeasurementC81998 AMYLOIDP Amyloid P A measurement of the total amyloid P in a biologicalspecimen.Amyloid P MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 152 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74916 ANA AntinuclearAntibodiesA measurement of the antinuclear antibodies(antibodies that attack the body's own tissue) in abiological specimen.Antinuclear Antibody MeasurementC74842 ANDSTNDL Androstenediol A measurement of the androstenediol metabolite in abiological specimen.Androstenediol MetaboliteMeasurementC74843 ANDSTNDN Androstenedione;4-AndrostenedioneC91372 ANGLBIND AntiglobulinTest, Indirect;Indirect CoombsTestC81974 ANGLOBDR AntiglobulinTest, Direct;Direct CoombsTestA measurement of the androstenedione hormone in abiological specimen.A test that uses Coombs' reagent to detect thepresence of anti-erythrocyte antibodies in a biologicalspecimen.A measurement of the antibody or complement-coatederythrocytes in a biological specimen in vivo.Androstenedione MeasurementIndirect Antiglobulin TestDirect Antiglobulin TestC74844 ANGTNS1 Angiotensin I A measurement of the angiotensin I hormone in abiological specimen.C74845 ANGTNS2 Angiotensin II A measurement of the angiotensin II hormone in abiological specimen.Angiotensin I MeasurementAngiotensin II MeasurementC74846 ANGTNSGN Angiotensinogen;AngiotensinPrecursorA measurement of the angiotensinogen hormone in abiological specimen.Angiotensinogen MeasurementC74685 ANIONG Anion Gap A computed estimate of the unmeasured anions (thoseother than the chloride and bicarbonate anions) in abiological specimen.C74797 ANISO Anisocytes A measurement of the inequality in the size of the redblood cells in a whole blood specimen.Anion Gap MeasurementAnisocyte MeasurementC74886 ANP Atrial NatriureticPeptide;AtriopeptinC81958 ANTHRM Antithrombin;Antithrombin III;AntithrombinActivity;Antithrombin IIIActivityC81977 ANTHRMAG AntithrombinAntigen;Antithrombin IIIAntigenA measurement of the atrial natriuretic peptide in abiological specimen.A measurement of the antithrombin activity in abiological specimen.A measurement of the antithrombin antigen in abiological specimen.Atrial Natriuretic PeptideMeasurementAntithrombin MeasurementAntithrombin Antigen MeasurementC74691 ANTIDPRS Antidepressants A measurement of any antidepressant class drugpresent in a biological specimen.Antidepressant MeasurementC102258 APLAB AntiphospholipidAntibodiesC103351 APLSMA2 Alpha-2 PlasminInhibitorA measurement of the antiphospholipid antibodies in abiological specimen.A measurement of the alpha-2 antiplasmin in abiological specimen.Antiphospholipid AntibodyMeasurementAlpha-2 Antiplasmin MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 153 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74733 APOA1 ApolipoproteinA1C82000 APOA2 ApolipoproteinAIIC103354 APOA4 ApolipoproteinA4C103355 APOA5 ApolipoproteinA5A measurement of the apolipoprotein A1 in the highdensity lipoproteins of a biological specimen.A measurement of the apolipoprotein AII in abiological specimen.A measurement of the apolipoprotein A4 in abiological specimen.A measurement of the apolipoprotein A5 in abiological specimen.Apolipoprotein A1 MeasurementApolipoprotein AII MeasurementApolipoprotein A4 MeasurementApolipoprotein A5 MeasurementC74734 APOB Apolipoprotein B A measurement of the apolipoprotein B in the lowdensity lipoproteins of a biological specimen.Apolipoprotein B MeasurementC103356 APOBAPA1 ApolipoproteinB/ApolipoproteinA1C100427 APOC2 ApolipoproteinC2;ApolipoproteinCIIC82001 APOC3 ApolipoproteinCIIIA relative measurement (ratio or percentage) of theApolipoprotein B to Apolipoprotein A1 in a biologicalspecimen.A measurement of the apolipoprotein C2 in abiological specimen.A measurement of the apolipoprotein CIII in abiological specimen.Apolipoprotein B to ApolipoproteinA1 Ratio MeasurementApolipoprotein C2 MeasurementApolipoprotein CIII MeasurementC82002 APOE Apolipoprotein E A measurement of the apolipoprotein E in a biologicalspecimen.Apolipoprotein E MeasurementC92293 APOE4 ApolipoproteinE4C82003 APOH ApolipoproteinHC100428 APOJ Apolipoprotein J;ClusterinC25377 APPEAR SpecimenAppearanceC102277 APPTLAS LupusAnticoagulantSensitive APTT;APTT-LAC100471 APROTCRS Activated ProteinC Resistance;Factor V LeidenScreenC38462 APTT Activated PartialThromboplastinTimeA measurement of the apolipoprotein E4 in abiological specimen.A measurement of the apolipoprotein H in a biologicalspecimen.A measurement of the apolipoprotein J in a biologicalspecimen.The outward or visible aspect of a specimen.A measurement of the length of time that it takes forclotting to occur when a lupus sensitive reagent isadded to a plasma specimen.A measurement of the resistance in the anticoagulationresponse to activated protein C in a biologicalspecimen.A measurement of the length of time that it takes forclotting to occur when reagents are added to a plasmaspecimen. The test is partial due to the absence oftissue factor (Factor III) from the reaction mixture.Apolipoprotein E4 MeasurementApolipoprotein H MeasurementApolipoprotein J MeasurementAppearanceLupus Anticoagulant Sensitive APTTMeasurementActivated Protein C ResistanceMeasurementPartial Thromboplastin TimeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 154 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC100421 APTTSPTT ActivatedPTT/StandardPTT; ActivatedPartialThromboplastinTime/StandardThromboplastinTimeC98862 APTTSTND ActivatedPTT/StandardA relative measurement (ratio or percentage) of thesubject's activated partial thromboplastin time to astandard or control partial thromboplastin time.A relative measurement (ratio or percentage) of thesubject's partial thromboplastin time to a standard orcontrol partial thromboplastin time.Activated PTT to Standard PTT RatioMeasurementActivated PTT/Standard RatioMeasurementC102259 ARA Arachidonic Acid A measurement of the arachidonic acid present in abiological specimen.Arachidonic Acid MeasurementC81976 ASCAB Anti-Saccharomyces cerevisiaeAntibodyC92269 ASSDNA Anti-SingleStranded DNAIgGC64467 AST AspartateAminotransferase; SGOTC81978 ASTAG AspartateAminotransferase Antigen; SGOTAntigenC103350 ATPVITE Vitamin E AlphaTocopherolA measurement of the anti-Saccharomyces cerevisiaeantibody in a biological specimen.A measurement of the anti-single stranded DNA IgGantibody in a biological specimen.A measurement of the aspartate aminotransferase in abiological specimen.A measurement of the aspartate aminotransferaseantigen in a biological specimen.A relative measurement (ratio or percentage) ofalpha-tocopherol to the total vitamin E in a biologicalspecimen.Anti-Saccharomyces cerevisiaeAntibody MeasurementAnti-Single Stranded DNA IgGMeasurementAspartate AminotransferaseMeasurementAspartate Aminotransferase AntigenMeasurementAlpha Tocopherol to Vitamin E RatioMeasurementC74657 AUERRODS Auer Rods A measurement of the Auer rods (elongated needlestructures that are found in the cytoplasm of leukemicblasts and are formed by clumps of azurophilicgranular material) in a biological specimen.Auer Rod MeasurementC103358 B2G1GGAB Beta-2Glycoprotein 1IgG AntibodyC103359 B2G1GMAB Beta-2Glycoprotein 1IgM AntibodyC81979 B2GLYAB Beta-2GlycoproteinAntibodyC81980 B2MICG Beta-2MicroglobulinA measurement of the Beta-2 glycoprotein 1 IgGantibodies in a biological specimen.A measurement of the Beta-2 glycoprotein 1 IgMantibodies in a biological specimen.A measurement of the beta-2 glycoprotein antibody ina biological specimen.A measurement of the beta-2 microglobulin in abiological specimen.Beta-2 Glycoprotein 1 IgG AntibodyMeasurementBeta-2 Glycoprotein 1 IgM AntibodyMeasurementBeta-2 Glycoprotein AntibodyMeasurementBeta-2 Microglobulin MeasurementC64469 BACT Bacteria A measurement of the bacteria in a biologicalspecimen.C74688 BARB Barbiturates A measurement of any barbiturate class drug present ina biological specimen.Bacterial CountBarbiturate Drug Class MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 155 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64470 BASO Basophils A measurement of the basophils in a biologicalspecimen.Total Basophil CountC98865 BASOCE Basophils/TotalCellsC96670 BASOIM ImmatureBasophilsC96671 BASOIMLE ImmatureBasophils/LeukocytesC64471 BASOLE Basophils/LeukocytesC82004 BDNF Brain-DerivedNeurotrophicFactorC100472 BETACRTN Beta Carotene;b-Carotene; BetaCarotinC96568 BHYXBTR Beta-Hydroxybutyrate;B-Hydroxybutyrate;3-HydroxybutyrateC74667 BICARB Bicarbonate;HCO3A relative measurement (ratio or percentage) of thebasophils to total cells in a biological specimen (forexample a bone marrow specimen).A measurement of the immature basophils in abiological specimen.A relative measurement (ratio or percentage) ofimmature basophils to total leukocytes in a biologicalspecimen.A relative measurement (ratio or percentage) of thebasophils to leukocytes in a biological specimen.A measurement of the brain-derived neurotrophicfactor in a biological specimen.A measurement of the beta carotene in a biologicalspecimen.A measurement of the total Beta-hydroxybutyrate in abiological specimen.A measurement of the bicarbonate in a biologicalspecimen.Basophil to Total CellRatioMeasurementImmature Basophil CountImmature Basophil to LeukocyteRatio MeasurementBasophil To Leukocyte RatioBrain-Derived Neurotrophic FactorMeasurementBeta Carotene MeasurementBeta-Hydroxybutyrate MeasurementBicarbonate MeasurementC64481 BILDIR Direct Bilirubin A measurement of the conjugated or water-solublebilirubin in a biological specimen.Direct Bilirubin MeasurementC74800 BILEAC Bile Acid; BileSalts; Bile Acids;Bile SaltC38037 BILI Bilirubin; TotalBilirubinA measurement of the total bile acids in a biologicalspecimen.A measurement of the total bilirubin in a biologicalspecimen.Bile Acid MeasurementTotal Bilirubin MeasurementC64483 BILIND Indirect Bilirubin A measurement of the unconjugated ornon-water-soluble bilirubin in a biological specimen.C74700 BITECE Bite Cells A measurement of the bite cells (erythrocytes with theappearance of a bite having been removed, due tooxidative hemolysis) in a biological specimen .C98710 BKVVLD BKV Viral Load A measurement of the BK virus viral load in abiological specimen.C74605 BLAST Blasts A measurement of the blast cells in a biologicalspecimen.Indirect Bilirubin MeasurementBite Cell CountBK Virus Viral Load MeasurementBlast CountC103407 BLASTIMM Lymphocytes,ImmunoblasticC64487 BLASTLE Blasts/LeukocytesA measurement of the immunoblasts in a biologicalspecimen.A relative measurement (ratio or percentage) of theblasts to leukocytes in a biological specimen.Immunoblast CountBlast To Leukocyte RatioSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 156 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74630 BLASTLM Leukemic Blasts A measurement of the leukemic blasts (lymphoblaststhat remain in an immature state even when outside thebone marrow) in a biological specimen.Leukemic Blast CountC74641 BLSTLMLY LeukemicBlasts/LymphocytesA relative measurement (ratio or percentage) of theleukemic blasts (immature lymphoblasts) to maturelymphocytes in a biological specimen.Leukemic Blast to LymphocyteRatio MeasurementC102278 BLSTLY Lymphoblasts A measurement of the lymphoblasts (immature cellsthat differentiate to form lymphocytes) in a biologicalspecimen.Lymphoblast CountC98761 BLSTMBCE Myeloblasts/Total CellsA relative measurement (ratio or percentage) of themyeloblasts to total cells in a biological specimen (forexample a bone marrow specimen).Myeloblast to Total Cell RatioMeasurementC98752 BLSTMGK Megakaryoblasts A measurement of the megakaryoblasts in a biologicalspecimen.Megakaryoblast Cell CountC98753 BLSTMKCE Megakaryoblasts/Total CellsC98764 BLSTNMCE Normoblasts/Total CellsC98870 BLSTPNCE Pronormoblasts/Total CellsC100419 BLSTRSID RingedSideroblastsA relative measurement (ratio or percentage) of themegakaryoblasts to total cells in a biological specimen(for example a bone marrow specimen).A relative measurement (ratio or percentage) of thenormoblasts to total cells in a biological specimen(for example a bone marrow specimen).A relative measurement (ratio or percentage) of thepronormoblasts to total cells in a biological specimen(for example a bone marrow specimen).A measurement of the ringed sideroblasts (abnormalnucleated erythroblasts with a large number of irondeposits in the perinuclear mitochondria, forming aring around the nucleus) in a biological specimen.Megakaryoblast to Total Cell RatioMeasurementNormoblast to Total Cell RatioMeasurementPronormoblast to Total CellRatioMeasurementRinged Sideroblast MeasurementC100418 BLSTSID Sideroblast A measurement of the sideroblasts (nucleatederythroblasts with iron granules in the cytoplasm) in abiological specimen.Sideroblast MeasurementC74735 BNP Brain NatriureticPeptide; B-TypeNatriureticPeptideC82032 BNPPRO ProB-typeNatriureticPeptide;proBNP;Pro-BrainNatriureticPeptideC96610 BNPPRONT N-TerminalProB-typeNatriureticPeptide; NTproBNP II;N-terminalpro-BrainNatriureticPeptideA measurement of the brain (B-type) natriureticpeptide in a biological specimen.A measurement of the proB-type natriuretic peptide ina biological specimen.A measurement of the N-terminal proB-typenatriuretic peptide in a biological specimen.Brain Natriuretic PeptideMeasurementProB-Type Natriuretic PeptideMeasurementN-Terminal ProB-type NatriureticPeptide MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 157 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74692 BNZDZPN Benzodiazepine A measurement of any benzodiazepine class drugpresent in a biological specimen.Benzodiazepine MeasurementC75350 BNZLCGN Benzoylecgonine; CocaineMetaboliteC74634 BTECERBC BiteCells/ErythrocytesC100426 BTP Beta-TraceProteinC61019 BUN Blood UreaNitrogenA measurement of the benzoylecgonine in a biologicalspecimen.A relative measurement (ratio or percentage) of bitecells (erythrocytes with the appearance of a bite havingbeen removed, due to oxidative hemolysis) to allerythrocytes in a biological specimen .A measurement of the beta-trace protein in abiological specimen.A measurement of the urea nitrogen in a bloodspecimen.Benzoylecgonine MeasurementBite Cell to Erythrocyte RatioMeasurementBeta-Trace Protein MeasurementBlood Urea Nitrogen MeasurementC74753 BUNCREAT BUN/Creatinine A relative measurement (ratio or percentage) (ratio) ofthe blood urea nitrogen (BUN) to the creatinine in abiological specimen.Blood Urea Nitrogen To CreatinineRatio MeasurementC74701 BURRCE Burr Cells;KeratocytesC80173 C1QAB ComplementC1q AntibodyA measurement of the Burr cells (erythrocytescharacterized by the presence of small, bluntprojections evenly distributed across the cell surface)in a biological specimen.A measurement of the complement C1q antibody in abiological specimen.Burr Cell CountComplement C1q AntibodyMeasurementC80174 C3 Complement C3 A measurement of the complement C3 in a biologicalspecimen.Complement C3 MeasurementC80175 C3A ComplementC3a; ASP,Complement C3DesArg,Acetylation-stimulating ProteinC80176 C3B ComplementC3bA measurement of the complement C3a in a biologicalspecimen.A measurement of the complement C3b in a biologicalspecimen.Complement C3a MeasurementComplement C3b MeasurementC80177 C4 Complement C4 A measurement of the complement C4 in a biologicalspecimen.C80178 C4A Complement C4a A measurement of the complement C4a in a biologicalspecimen.C80179 C5A Complement C5a A measurement of the complement C5a in a biologicalspecimen.C64488 CA Calcium A measurement of the calcium in a biologicalspecimen.Complement C4 MeasurementComplement C4a MeasurementComplement C5a MeasurementCalcium MeasurementC81981 CA<strong>12</strong>5AG Cancer Antigen<strong>12</strong>5C103362 CA15_3AG Cancer Antigen15-3C81982 CA19_9AG Cancer Antigen19-9A measurement of the cancer antigen <strong>12</strong>5 in abiological specimen.A measurement of the cancer antigen 15-3 in abiological specimen.A measurement of the cancer antigen 19-9 in abiological specimen.Cancer Antigen <strong>12</strong>5 MeasurementCancer Antigen 15-3 MeasurementCancer Antigen 19-9 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 158 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103361 CA1AG Cancer Antigen 1 A measurement of the cancer antigen 1 in a biologicalspecimen.C74702 CABOT Cabot Rings A measurement of the Cabot rings (red-purple staining,threadlike, ring or figure 8 shaped filaments in anerythrocyte) in a biological specimen.Cancer Antigen 1 MeasurementCabot Ring CountC96589 CACLR CalciumClearanceC79439 CACREAT Calcium/CreatinineA measurement of the volume of serum or plasma thatwould be cleared of calcium by excretion of urine fora specified unit of time (e.g. one minute).A relative measurement (ratio or percentage) of thecalcium to creatinine in a biological specimen.Calcium Clearance MeasurementCalcium to Creatinine RatioMeasurementC81948 CAION Calcium, Ionized A measurement of the ionized calcium in a biologicalspecimen.C82005 CALPRO Calprotectin A measurement of the calprotectin in a biologicalspecimen.C74689 CANNAB Cannabinoids A measurement of any cannabinoid class drug presentin a biological specimen.Ionized Calcium MeasurementCalprotectin MeasurementCannabinoid Drug ClassMeasurementC103360 CAPHOSPD Calcium -PhosphorusProductC96591 CARBXHGB CarboxyhemoglobinA measurement of the product of the calcium andphosphate measurements in a biological specimen.A measurement of the carboxyhemoglobin in abiological specimen.Calcium and Phosphorus ProductMeasurementCarboxyhemoglobin MeasurementC74682 CARNIT Carnitine A measurement of the total carnitine in a biologicalspecimen.Total Carnitine MeasurementC92288 CARNITAT Carnitine AcetylTransferaseA measurement of the carnitine acetyl transferase in abiological specimen.Carnitine Acetyl TransferaseMeasurementC74677 CARNITF Carnitine, Free A measurement of the free carnitine in a biologicalspecimen.C74763 CASTS Casts A statement that indicates casts were looked for in abiological specimen.C96590 CASULPH Calcium Sulphate A measurement of the calcium sulphate in a biologicalspecimen.C103357 CATNINB Beta Catenin A measurement of the beta catenin in a biologicalspecimen.C80172 CBB Complement Bb A measurement of the complement Bb in a biologicalspecimen.Free Carnitine MeasurementCast Present Or AbsentCalcium Sulphate MeasurementBeta Catenin MeasurementComplement Bb MeasurementC74850 CCK Cholecystokinin;PancreozyminC96595 CCPAB CyclicCitrullinatedPeptide AntibodyA measurement of the cholecystokinin hormone in abiological specimen.A measurement of the cyclic citrullinated peptideantibody in a biological specimen.Cholecystokinin MeasurementCyclic Citrullinated PeptideAntibody MeasurementC103364 CD1 CD1 A count of the CD1 expressing cells per unit of abiological specimen.C103365 CD14 CD14 A count of the CD14 expressing cells per unit of abiological specimen.CD1 Expressing Cell MeasurementCD14 Expressing Cell MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 159 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103808 CD19 CD19 A count of the CD19 expressing cells per unit in abiological specimen.CD19 Expressing Cell CountC1038<strong>12</strong> CD19LY CD19/LymphocytesA relative measurement (ratio or percentage) of CD19expressing cells to all lymphocytes in a biologicalspecimen.CD19 Cell to Lymphocyte RatioMeasurementC103366 CD2 CD2 A count of the CD2 expressing cells per unit of abiological specimen.C103368 CD20 CD20 A count of the CD20 expressing cells per unit of abiological specimen.CD2 Expressing Cell MeasurementCD20 Expressing Cell MeasurementC103367 CD2LY CD2/LymphocytesA relative measurement (ratio or percentage) of CD2expressing cells to all lymphocytes in a biologicalspecimen.CD2 Expressing Cell to LymphocyteRatio MeasurementC103809 CD3 CD3 A count of the CD3 expressing cells per unit in abiological specimen.C102260 CD34 CD34 A measurement of the CD34 expressing cells per unitin a biological specimen.CD3 Expressing Cell CountCD34 MeasurementC103813 CD3LY CD3/LymphocytesA relative measurement (ratio or percentage) of CD3expressing cells to all lymphocytes in a biologicalspecimen.CD3 Cell to Lymphocyte RatioMeasurementC103810 CD4 CD4 A count of the CD4 expressing cells per unit in abiological specimen.C82006 CD40 CD40 A count of the CD40 cells per unit in a biologicalspecimen.C82007 CD40L CD40 Ligand A count of the CD40 ligand cells per unit in abiological specimen.C103814 CD4CD8 CD4/CD8 A relative measurement (ratio or percentage) of CD4expressing cells to the CD8 expressing cells in abiological specimen.CD4 Expressing Cell CountCD40 MeasurementCD40 Ligand MeasurementCD4 Cell to CD8 Cell RatioMeasurementC103815 CD4LY CD4/LymphocytesA relative measurement (ratio or percentage) of CD4expressing cells to all lymphocytes in a biologicalspecimen.CD4 Cell to Lymphocyte RatioMeasurementC103369 CD5 CD5 A count of the CD5 expressing cells per unit of abiological specimen.C103370 CD56 CD56 A count of the CD56 expressing cells per unit of abiological specimen.C103811 CD8 CD8 A count of the CD8 expressing cells per unit in abiological specimen.CD5 Expressing Cell MeasurementCD56 Expressing Cell MeasurementCD8 Expressing Cell CountC103816 CD8LY CD8/LymphocytesC103381 CDFTXN Clostridiumdifficile ToxinC101016 CDT Carbohydrate-DeficientTransferrinA relative measurement (ratio or percentage) of CD8expressing cells to all lymphocytes in a biologicalspecimen.A measurement of the Clostridium difficile toxin in abiological specimen.A measurement of transferrin with a reduced numberof carbohydrate moieties in a biological specimen.CD8 Cell to Lymphocyte RatioMeasurementClostridium difficile ToxinMeasurementCarbohydrate-Deficient TransferrinMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 160 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC81983 CEA Carcinoembryonic AntigenC96592 CEC CirculatingEndothelial CellsA measurement of the carcinoembryonic antigen in abiological specimen.A measurement of the circulating endothelial cells in abiological specimen.Carcinoembryonic AntigenMeasurementCirculating Endothelial Cell CountC96672 CELLSIM Immature Cells A measurement of the total immature cells in a bloodspecimen.Immature Cell CountC103380 CETPA CholesterylEster TransferProtein ActC100423 CH50 ComplementCH50; TotalHemolyticComplement;CH50C61032 CHOL Cholesterol;TotalCholesterolC80171 CHOLHDL Cholesterol/HDL-CholesterolA measurement of the biological activity ofcholesteryl ester transfer protein in a biologicalspecimen.A measurement of the complement required to lyse 50percent of red blood cells in a biological specimen.A measurement of the cholesterol in a biologicalspecimen.A relative measurement (ratio or percentage) of totalcholesterol to high-density lipoprotein cholesterol(HDL-C) in a biological specimen.Cholesteryl Ester Transfer ProteinActivity MeasurementCH50 MeasurementSerum Total CholesterolMeasurementCholesterol to HDL-CholesterolRatio MeasurementC92289 CHOLINES Cholinesterase A measurement of the cholinesterase in a biologicalspecimen.Cholinesterase MeasurementC92248 CITRATE Citrate A measurement of the citrate in a biological specimen. Citrate MeasurementC64489 CK Creatine Kinase A measurement of the total creatine kinase in abiological specimen.Creatine Kinase MeasurementC64490 CKBB Creatine KinaseBBC79466 CKBBCK Creatine KinaseBB/TotalCreatine KinaseC64491 CKMB Creatine KinaseMBC79441 CKMBCK Creatine KinaseMB/TotalCreatine KinaseC64494 CKMM Creatine KinaseMMC79442 CKMMCK Creatine KinaseMM/TotalCreatine KinaseA measurement of the homozygous B-type creatinekinase in a biological specimen.A relative measurement (ratio or percentage) of theBB-type creatine kinase to total creatine kinase in abiological specimen.A measurement of the heterozygous MB-type creatinekinase in a biological specimen.A relative measurement (ratio or percentage) of theMB-type creatine kinase to total creatine kinase in abiological specimen.A measurement of the homozygous M-type creatinekinase in a biological specimen.A relative measurement (ratio or percentage) of theMM-type creatine kinase to total creatine kinase in abiological specimen.Creatine Kinase BB MeasurementCreatine Kinase BB to Total CreatineKinase Ratio MeasurementCreatine Kinase MB MeasurementCreatine Kinase MB to TotalCreatine Kinase Ratio MeasurementCreatine Kinase MM MeasurementCreatine Kinase MM to TotalCreatine Kinase Ratio MeasurementC64495 CL Chloride A measurement of the chloride in a biologicalspecimen.C96594 CLARITY Clarity A measurement of the transparency of a biologicalspecimen.Chloride MeasurementClarity MeasurementC79440 CLCREAT Chloride/CreatinineA relative measurement (ratio or percentage) of thechloride to creatinine in a biological specimen.Chloride to Creatinine RatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 161 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74848 CLCTONN Calcitonin A measurement of the calcitonin hormone in abiological specimen.C74849 CLCTRIOL Calcitriol A measurement of the calcitriol hormone in abiological specimen.C102261 CLUECE Clue Cells A measurement of the clue cells in a biologicalspecimen.Calcitonin MeasurementCalcitriol MeasurementClue Cell CountC96596 CMVIGGAB CytomegalovirusIgG AntibodyC96597 CMVIGMAB CytomegalovirusIgM AntibodyA measurement of the cytomegalovirus IgG antibodyin a biological specimen.A measurement of the cytomegalovirus IgM antibodyin a biological specimen.Cytomegalovirus IgG AntibodyMeasurementCytomegalovirus IgM AntibodyMeasurementC98716 CMVVLD CMV Viral Load A measurement of the cytomegalovirus viral load in abiological specimen.C64545 CO2 Carbon Dioxide A measurement of the carbon dioxide gas in abiological specimen.C74690 COCAINE Cocaine A measurement of the cocaine present in a biologicalspecimen.C74877 CODEINE Codeine A measurement of the codeine present in a biologicalspecimen.C103383 COL4 Collagen Type IV A measurement of the collagen type IV in a biologicalspecimen.Cytomegalovirus Viral LoadMeasurementCarbon Dioxide MeasurementCocaine MeasurementCodeine MeasurementCollagen Type IV MeasurementC64546 COLOR Color A measurement of the color of a biological specimen. Color AssessmentC102282 CONDUCTU UrineConductivityA measurement of the urine conductivity which is anon-linear function of the electrolyte concentration inthe urine.Urine ConductivityC88113 CORTFR Cortisol, Free A measurement of the free, unbound cortisol in abiological specimen.Free Cortisol MeasurementC74781 CORTISOL Cortisol; TotalCortisolA measurement of the cortisol in a biologicalspecimen.Cortisol MeasurementC92249 COTININE Cotinine A measurement of the cotinine in a biologicalspecimen.C74736 CPEPTIDE C-peptide A measurement of the C (connecting) peptide ofinsulin in a biological specimen.Cotinine MeasurementC-peptide MeasurementC92432 CPNIGAAB Chlamydiapneumoniae IgAAntibodyC92433 CPNIGMAB Chlamydiapneumoniae IgMAntibodyC103363 CRDIGMAB Cardiolipin IgMAntibodyA measurement of the Chlamydia pneumoniae IgAantibody in a biological specimen.A measurement of the Chlamydia pneumoniae IgM in abiological specimen.A measurement of the cardiolipin IgM antibodies in abiological specimen.Chlamydia pneumoniae IgA AntibodyMeasurementChlamydia pneumoniae IgMAntibody MeasurementCardiolipin IgM AntibodyMeasurementC64547 CREAT Creatinine A measurement of the creatinine in a biologicalspecimen.Creatinine MeasurementC25747 CREATCLR CreatinineClearanceA measurement of the volume of serum or plasma thatwould be cleared of creatinine by excretion of urinefor a specified unit of time (e.g. one minute).Creatinine ClearanceSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 162 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74703 CRENCE Crenated Cells;EchinocytesC74851 CRH CorticotropinReleasingHormone;CorticotropinReleasing FactorC100432 CRLPLSMN Ceruloplasmin;CaeruloplasminC64548 CRP C ReactiveProteinA measurement of the Burr cells (erythrocytescharacterized by the presence of multiple small, sharpprojections evenly distributed across the cell surface)in a biological specimen.A measurement of the corticotropin releasinghormone in a biological specimen.A measurement of ceruloplasmin in a biologicalspecimen.A measurement of the C reactive protein in abiological specimen.Crenated Cell MeasurementCorticotropin Releasing HormoneMeasurementCeruloplasmin MeasurementC-Reactive Protein MeasurementC79434 CRTRONE Corticosterone A measurement of corticosterone in a biologicalspecimen.C74673 CRYSTALS Crystals A statement that indicates crystals were looked for in abiological specimen.C74762 CSBACT Bacterial Casts A measurement of the bacterial casts present in abiological specimen.C96588 CSBROAD Broad Casts A measurement of the broad casts in a biologicalspecimen.C74764 CSCELL Cellular Casts A measurement of the cellular (white blood cell, redblood cell, epithelial and bacterial) casts present in abiological specimen.C74779 CSEPI Epithelial Casts A measurement of the epithelial cell casts present in abiological specimen.C74766 CSFAT Fatty Casts A measurement of the fatty casts present in abiological specimen.C74768 CSGRAN Granular Casts A measurement of the granular (coarse and fine) castspresent in a biological specimen.Corticosterone MeasurementCrystal Present Or AbsentBacterial Cast MeasurementBroad Casts MeasurementCellular Cast MeasurementEpithelial-Cast MeasurementFatty Cast MeasurementGranular Cast MeasurementC74765 CSGRANC Granular CoarseCastsC74769 CSGRANF Granular FineCastsA measurement of the coarse granular casts present ina biological specimen.A measurement of the fine granular casts present in abiological specimen.Coarse Granular Cast MeasurementGranular Fine Cast MeasurementC74770 CSHYAL Hyaline Casts A measurement of the hyaline casts present in abiological specimen.C74771 CSMIX Mixed Casts A measurement of the mixed (the cast contains amixture of cell types) casts present in a biologicalspecimen.C74772 CSRBC RBC Casts A measurement of the red blood cell casts present in abiological specimen.Hyaline Cast MeasurementMixed Cast CountRed Blood Cell Cast MeasurementC74776 CSUNCLA UnclassifiedCastsA measurement of the unclassifiable casts present in abiological specimen.Unclassified Cast MeasurementC74777 CSWAX Waxy Casts A measurement of the waxy casts present in abiological specimen.Waxy Cell Cast MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 163 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74778 CSWBC WBC Casts A measurement of the white blood cell casts present ina biological specimen.White Blood Cell Cast MeasurementC96593 CTC CirculatingTumor CellsC80160 CTOT ComplementTotalC100464 CTRIGAAB Chlamydiatrachomatis IgAAntibodyC100466 CTRIGGAB Chlamydiatrachomatis IgGAntibodyC100465 CTRIGMAB Chlamydiatrachomatis IgMAntibodyC103382 CTTIGGAB Clostridiumtetani IgGAntibodyC82038 CTXI Type I CollagenC-Telopeptides;Type I CollagenX-linkedC-telopeptideC82040 CTXII Type II CollagenC-TelopeptidesC100431 CXCR3 Chemokine(C-X-C Motif)Receptor 3;GPR9, CD183,CXCR3C749<strong>12</strong> CYACIDU Acid UrateCrystalsC74758 CYAMMBIU AmmoniumBiurate Crystals;AmmoniumUrate Crystals;Acid AmmoniumUrate CrystalsC74759 CYAMMOX AmmoniumOxalate CrystalsC74665 CYAMORPH AmorphousCrystalsC92243 CYAMPPH AmorphousPhosphateCrystalsC92244 CYAMPURT AmorphousUrate CrystalsA measurement of the circulating tumor cells in abiological specimen.A measurement of the total complement in abiological specimen.A measurement of the Chlamydia trachomatis IgAantibody in a biological specimen.A measurement of the Chlamydia trachomatis IgGantibody in a biological specimen.A measurement of the Chlamydia trachomatis IgMantibody in a biological specimen.A measurement of the Clostridium tetani IgG antibodyin a biological specimen.A measurement of the type I collagen C-telopeptidesin a biological specimen.A measurement of the type II collagen C-telopeptidesin a biological specimen.A measurement of the CXCR3, chemokine (C-X-Cmotif) receptor 3, in a biological specimen.A measurement of the acid urate crystals present in abiological specimen.A measurement of the ammonium biurate crystalspresent in a biological specimen.A measurement of the ammonium oxalate crystalspresent in a urine specimen.A measurement of the amorphous (Note: phosphate orurate, depending on pH) crystals present in a biologicalspecimen.A measurement of the amorphous phosphate crystalsin a biological specimen.A measurement of the amorphous urate crystals in abiological specimen.Circulating Tumor Cell CountComplement MeasurementChlamydia trachomatis IgA AntibodyMeasurementChlamydia trachomatis IgG AntibodyMeasurementChlamydia trachomatis IgM AntibodyMeasurementClostridium tetani IgG AntibodyMeasurementType I Collagen C-TelopeptideMeasurementType II Collagen C-TelopeptideMeasurementChemokine Receptor CXCR3MeasurementAcid Urate Crystal MeasurementUrine Ammonium Biurate CrystalMeasurementUrine Ammonium Oxalate CrystalMeasurementAmorphous Crystal MeasurementAmorphous Phosphate CrystalsMeasurementAmorphous Urate CrystalsMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 164 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74668 CYBILI BilirubinCrystalsC74669 CYCACAR CalciumCarbonateCrystalsC74670 CYCAOXA Calcium OxalateCrystalsC74671 CYCAPHOS CalciumPhosphateCrystalsC74672 CYCHOL CholesterolCrystalsA measurement of the bilirubin crystals present in abiological specimen.A measurement of the calcium carbonate crystalspresent in a biological specimen.A measurement of the calcium oxalate crystals presentin a biological specimen.A measurement of the calcium phosphate crystalspresent in a biological specimen.A measurement of the cholesterol crystals present in abiological specimen.Bilirubin Crystal MeasurementCalcium Carbonate CrystalMeasurementCalcium Oxalate CrystalMeasurementCalcium Phosphate CrystalMeasurementCholesterol Crystal MeasurementC74674 CYCYSTIN Cystine Crystals A measurement of the cystine crystals present in abiological specimen.Cystine Crystal MeasurementC74754 CYHIPPAC Hippuric AcidCrystalsA measurement of the hippuric acid crystals present ina biological specimen.Hippuric Acid Crystal MeasurementC74680 CYLEUC Leucine Crystals A measurement of the leucine crystals present in abiological specimen.Leucine Crystal MeasurementC74681 CYMSU MonosodiumUrate CrystalsA measurement of the monosodium urate crystalspresent in a biological specimen.Monosodium Urate CrystalMeasurementC81951 CYSTARCH Starch Crystals A measurement of the starch crystals in a biologicalspecimen.C92290 CYSTATC Cystatin C A measurement of the cystatin C in a biologicalspecimen.C74755 CYSULFA Sulfa Crystals A measurement of the sulfa crystals present in abiological specimen.Starch Crystal MeasurementCystatin C MeasurementSulfa Crystal MeasurementC74756 CYTRPHOS Triple PhosphateCrystalsC74683 CYTYRO TyrosineCrystalsC74757 CYUNCLA UnclassifiedCrystalsC74684 CYURIAC Uric AcidCrystalsA measurement of the triple phosphate crystals presentin a biological specimen.A measurement of the triple phosphate crystals presentin a biological specimen.A measurement of the unclassifiable crystals presentin a biological specimen.A measurement of the uric acid crystals present in abiological specimen.Triple Phosphate CrystalMeasurementTyrosine Crystal MeasurementUnclassified Crystal MeasurementUric Acid Crystal MeasurementC82621 DDIMER D-Dimer A measurement of the d-dimers in a biologicalspecimen.D-Dimer MeasurementC74852 DHEA Dehydroepiandrosterone;DehydroisoandrosteroneC96629 DHEAS Dehydroepiandrosterone Sulfate;DHEA-S;sDHEA; DHEASulfateA measurement of the dehydroepiandrosteronehormone in a biological specimen.A measurement of the sulfatedDehydroepiandrosterone in a biological specimen.DehydroepiandrosteroneMeasurementSulfated DHEA MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 165 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC101017 DHPG 3,4-Dihydroxyphenylglycol; 3.4DihydroxyphenylglycolC74853 DHT Dihydrotestosterone;AndrostanaloneA measurement of the catecholamine metabolite,3,4-Dihydroxyphenylglycol in a biological specimen.A measurement of the dihydrotestosterone hormone ina biological specimen.3,4-DihydroxyphenylglycolMeasurementDihydrotestosterone MeasurementC74878 DIHYDCDN Dihydrocodeine A measurement of the dihydrocodeine present in abiological specimen.Dihydrocodeine MeasurementC81973 DNAAB Anti-DNAAntibodies;Anti-ds-DNAAntibodiesC100463 DNASEBAB DNase-BAntibody;Anti-Dnase BA measurement of the anti-DNA antibodies in abiological specimen.A measurement of Dnase-B antibody in a biologicalspecimen.Anti-DNA Antibody MeasurementDNase-B Antibody MeasurementC74610 DOHLE Dohle Bodies A measurement of the Dohle bodies (blue-gray,basophilic, leukocyte inclusions located in theperipheral cytoplasm of neutrophils) in a biologicalspecimen.Dohle Body MeasurementC103345 DOPAC 3,4-Dihydroxyphenylacetic AcidA measurement of the 3,4-dihydroxyphenylacetic acidin a biological specimen.3,4-Dihydroxyphenylacetic AcidMeasurementC74854 DOPAMINE Dopamine A measurement of the dopamine hormone in abiological specimen.Dopamine MeasurementC79443 DPD DeoxypyridinolineC79444 DPDCREAT Deoxypyridinoline/CreatinineA measurement of the deoxypyridinoline in abiological specimen.A relative measurement (ratio or percentage) of thedeoxypyridinoline to creatinine in a biologicalspecimen.Deoxypyridinoline MeasurementDeoxypyridinoline to CreatinineRatio MeasurementC103387 DPPIVA DPPIV Activity A measurement of the biological activity of dipeptidylpeptidase-4 in a biological specimen.Dipeptidyl Peptidase-4 ActivityMeasurementC103388 DPTIGGAB Diphtheria IgGAntibodyA measurement of the Diphtheria IgG antibodies in abiological specimen.Diphtheria IgG AntibodyMeasurementC78139 DRUGSCR Drug Screen An indication of the presence or absence ofrecreational drugs or drugs of abuse in a biologicalspecimen.Drug TestC96696 DRVVT Dilute Russell'sViper VenomTime; LupusAnticoagulantTestC103386 DRVVTRT LupusAnticoagulantRatioA measurement of the time it takes a plasma sample toclot after adding dilute Russell's viper venom.A relative measurement (ratio or percentage) of thedilute Russell's viper venom time in a subject sampleto a control sample.Dilute Russell's Viper Venom TimeMeasurementDilute Russell's Viper Venom Timeto Control Ratio MeasurementC100441 DTPACLR DTPA Clearance A measurement of the volume of serum or plasma thatwould be cleared of Diethylenetriamine pentaacetate(DTPA) through its excretion for a specified unit oftime.Diethylene Triamine PentaaceticAcid ClearanceSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 166 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96600 EBCIGGAB Epstein-BarrCapsid IgGAntibodyC96601 EBCIGMAB Epstein-BarrCapsid IgMAntibodyC96602 EBEAG Epstein-BarrEarly AntigenC96603 EBNAB Epstein-BarrNuclearAntibodyC96604 EBNAG Epstein-BarrNuclear AntigenA measurement of the Epstein-Barr capsid IgGantibody in a biological specimen.A measurement of the Epstein-Barr capsid IgMantibody in a biological specimen.A measurement of the Epstein-Barr early antigen in abiological specimen.A measurement of the Epstein-Barr nuclear antibody ina biological specimen.A measurement of the Epstein-Barr nuclear antigen ina biological specimen.Epstein-Barr Capsid IgG AntibodyMeasurementEpstein-Barr Capsid IgM AntibodyMeasurementEpstein-Barr Early AntigenMeasurementEpstein-Barr Nuclear AntibodyMeasurementEpstein-Barr Nuclear AntigenMeasurementC98721 EBVVLD EBV Viral Load A measurement of the Epstein-Barr virus viral load in abiological specimen.C96598 ECCENTCY Eccentrocytes A measurement of the eccentrocytes (erythrocytes inwhich the hemoglobin is localized to a particularportion of the cell, noticeable as localized staining) ina biological specimen.Epstein-Barr Virus Viral LoadMeasurementEccentrocyte CountC100422 ECT Ecarin ClottingTimeA measurement of the activity of thrombin inhibitorsin a biological specimen based on the generation ofmeizothrombin.Ecarin Clotting Time MeasurementC100440 EDTACLR EDTA Clearance A measurement of the volume of serum or plasma thatwould be cleared of Ethylenediamine tetraacetic acid(EDTA) through its excretion for a specified unit oftime.EDTA ClearanceC82009 EGF EpidermalGrowth FactorC82028 ELA1 PancreaticElastase 1C82029 ELA1PMN PancreaticElastase 1,PolymorphonuclearC82026 ELA2 NeutrophilElastaseC82027 ELA2PMN NeutrophilElastase,PolymorphonuclearC64549 ELLIPCY Elliptocytes;OvalocytesC82010 ENA78 EpithNeutrophil-Activating Peptide 78A measurement of the epidermal growth factor in abiological specimen.A measurement of the pancreatic elastase 1 in abiological specimen.A measurement of the polymorphonuclear pancreaticelastase 1 in a biological specimen.A measurement of the neutrophil elastase in abiological specimen.A measurement of the polymorphonuclear neutrophilelastase in a biological specimen.A measurement of the elliptically shaped erythrocytesin a biological specimen.A measurement of the epithelial neutrophil-activatingpeptide in a biological specimen.Epidermal Growth FactorMeasurementPancreatic Elastase MeasurementPolymorphonuclear PancreaticElastase MeasurementNeutrophil Elastase MeasurementPolymorphonuclear NeutrophilElastase MeasurementElliptocyte CountEpithelial Neutrophil-ActivatingPeptide 78 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 167 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92270 ENAAB Anti-ENA;ExtractableNuclear AntigenAntibodyA measurement of the extractable nuclear antigenantibody in a biological specimen.Extractable Nuclear AntigenAntibody MeasurementC82008 ENDOTH1 Endothelin-1 A measurement of the endothelin-1 in a biologicalspecimen.C82011 ENRAGE Extracell NewlyIdent RAGE BindProtein; S100Calcium BindingProtein A<strong>12</strong>A measurement of the extracellular newly identifiedRAGE (receptor for advanced glycation end products)binding protein in a biological specimen.C64550 EOS Eosinophils A measurement of the eosinophils in a biologicalspecimen.Endothelin-1 MeasurementExtracell Newly Ident RAGE BindProtein MeasurementEosinophil CountC98720 EOSCE Eosinophils/Total CellsC96673 EOSIM ImmatureEosinophilsC96674 EOSIMLE ImmatureEosinophils/LeukocytesC64604 EOSLE Eosinophils/LeukocytesC84819 EOSMM EosinophilicMetamyelocytesC84821 EOSMYL EosinophilicMyelocytesC81952 EOTAXIN1 Eotaxin-1;ChemokineLigand 11C81953 EOTAXIN2 Eotaxin-2;ChemokineLigand 24C81954 EOTAXIN3 Eotaxin-3;ChemokineLigand 26A relative measurement (ratio or percentage) of theeosinophils to total cells in a biological specimen (forexample a bone marrow specimen).A measurement of the immature eosinophils in abiological specimen.A relative measurement (ratio or percentage) ofimmature eosinophils to total leukocytes in abiological specimen.A relative measurement (ratio or percentage) of theeosinophils to leukocytes in a biological specimen.A measurement of the eosinphilic metamyelocytes in abiological specimen.A measurement of the eosinophilic myelocytes in abiological specimen.A measurement of the eotaxin-1 in a biologicalspecimen.A measurement of the eotaxin-2 in a biologicalspecimen.A measurement of the eotaxin-3 in a biologicalspecimen.Eosinophils to Total Cell RatioMeasurementImmature Eosinophil CountImmature Eosinophil to LeukocyteRatio MeasurementEosinophil To Leukocyte RatioEosinophilic Metamyelocyte CountEosinophilic Myelocyte CountEotaxin-1 MeasurementEotaxin-2 MeasurementEotaxin-3 MeasurementC64605 EPIC Epithelial Cells A measurement of the epithelial cells in a biologicalspecimen.Epithelial Cell CountC79445 EPIN Epinephrine;AdrenalineC74698 EPIROCE Round EpithelialCellsC74773 EPISQCE SquamousEpithelial CellsC74774 EPISQTCE SquamousTransitionalEpithelial Cells;Epithelial CellsA measurement of the epinephrine hormone in abiological specimen.A measurement of the round epithelial cells present ina biological specimen.A measurement of the squamous epithelial cellspresent in a biological specimen.A measurement of the squamous transitional epithelialcells present in a biological specimen.Epinephrine MeasurementRound Epithelial Cell CountSquamous Epithelial Cell CountSquamous Transitional EpithelialCell CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 168 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92251 EPITCE TransitionalEpithelial CellsC74775 EPITUCE TubularEpithelial Cells;Renal TubularEpithelial CellsC74855 EPO Erythropoietin;HematopoietinC74611 ESR ErythrocyteSedimentationRate; BiernackiReactionC74782 ESTRDIOL Estradiol;OestradiolA measurement of the transitional epithelial cellspresent in a biological specimen.A measurement of the tubular epithelial cells presentin a biological specimen.A measurement of the erythropoietin hormone in abiological specimen.The distance (e.g. millimeters) that red blood cellssettle in unclotted blood over a specified unit of time(e.g. one hour).A measurement of the estradiol in a biologicalspecimen.Transitional Epithelial CellsMeasurementTubular Epithelial Cell CountErythropoietin MeasurementErythrocyte Sedimentation RateMeasurementEstradiol MeasurementC74856 ESTRIOL Estriol; Oestriol A measurement of the estriol hormone in a biologicalspecimen.C81963 ESTRIOLF Estriol, Free A measurement of the free estriol in a biologicalspecimen.Estriol MeasurementFree Estriol MeasurementC74857 ESTRONE Estrone;OestroneA measurement of the estrone hormone in a biologicalspecimen.Estrone MeasurementC74693 ETHANOL Ethanol; Alcohol A measurement of the ethanol present in a biologicalspecimen.Ethanol MeasurementC102266 ETP EndogenousThrombinPotentialC102263 ETPAUC ETP Area UnderCurve;EndogenousThrombinPotential AreaUnder CurveC102264 ETPLT ETP Lag Time;EndogenousThrombinPotential LagTimeC102265 ETPLTR ETP Lag TimeRelative;EndogenousThrombinPotential LagTime RelativeC102267 ETPPH ETP PeakHeight;EndogenousThrombinPotential PeakHeightA measurement of the total concentration of thrombingenerated in the presence of a substrate in a plasma orblood sample.A measurement of the area under the thrombingeneration curve.A measurement of time from the start of the thrombingeneration test to the point where a predeterminedamount of thrombin is generated.A relative measurement (ratio or percentage) of timefrom the start of the thrombin generation test to thepoint where a predetermined amount of thrombin isgenerated.A measurement of the maximum concentration ofthrombin generated during a thrombin generation test.Endogenous Thrombin PotentialMeasurementEndogenous Thrombin PotentialArea Under Curve MeasurementEndogenous Thrombin Potential LagTime MeasurementEndogenous Thrombin Potential LagTime Relative MeasurementEndogenous Thrombin PotentialPeak Height MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 169 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102268 ETPPHR ETP Peak HeightRelative;EndogenousThrombinPotential PeakHeight RelativeC102269 ETPTP ETP Time toPeak;EndogenousThrombinPotential Time toPeakC102270 ETPTPR ETP Time toPeak Relative;EndogenousThrombinPotential Time toPeak RelativeC8<strong>20<strong>12</strong></strong> FABP1 Fatty AcidBinding Protein1C96626 FACTII Factor II;ProthrombinC81959 FACTIII Factor III; TissueFactor, CD142C98725 FACTIX Factor IX;Christmas FactorC103395 FACTIXA Christmas FactorActivityC98726 FACTV Factor V; LabileFactorC103396 FACTVA Labile FactorActivityC81960 FACTVII Factor VII;Proconvertin;Stable FactorC103397 FACTVIIA ProconvertinActivity; StableFactor ActivityC81961 FACTVIII Factor VIII;Anti-hemophilicFactorA relative (ratio or percentage) of the maximumconcentration of thrombin generated during a thrombingeneration test.A measurement of the time it takes to generate themaximum concentration of thrombin.A relative (ratio or percentage) measurement of thetime it takes to generate the maximum concentrationof thrombin.A measurement of the fatty acid binding protein 1 in abiological specimen.A measurement of the Factor II in a biologicalspecimen.A measurement of the factor III in a biologicalspecimen.A measurement of the factor IX in a biologicalspecimen.A measurement of the biological activity of factor IXin a biological specimen.A measurement of the factor V in a biologicalspecimen.A measurement of the biological activity of factor V ina biological specimen.A measurement of the factor VII in a biologicalspecimen.A measurement of the biological activity of factor VIIin a biological specimen.A measurement of the factor VIII in a biologicalspecimen.Endogenous Thrombin PotentialPeak Height Relative MeasurementEndogenous Thrombin PotentialTime to Peak MeasurementEndogenous Thrombin PotentialTime to Peak Relative MeasurementFatty Acid Binding Protein 1MeasurementProthrombin MeasurementFactor III MeasurementFactor IX MeasurementFactor IX Activity MeasurementFactor V MeasurementFactor V Activity MeasurementFactor VII MeasurementFactor VII Activity MeasurementFactor VIII MeasurementC102271 FACTVL Factor V Leiden A measurement of the Factor V Leiden in a biologicalspecimen.Factor V Leiden MeasurementC98799 FACTVW von WillebrandFactor; vonWillebrandFactor AntigenA measurement of the von Willebrand factor in abiological specimen.von Willebrand Factor MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 170 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98727 FACTX Factor X A measurement of the factor X in a biologicalspecimen.Factor X MeasurementC102272 FACTXIV Factor XIV;AutoprothrombinIIA; Protein C;Protein CAntigenA measurement of the factor XIV in a biologicalspecimen.Factor XIV MeasurementC96648 FAT Fat A measurement of the fat in a biological specimen. Fat MeasurementC80200 FATACFR Free Fatty Acid;Non-EsterifiedFatty Acid, FreeC80206 FATACFRS Free Fatty Acid,Saturated;Non-esterifiedFatty Acid,SaturatedC80209 FATACFRU Free Fatty Acid,Unsaturated;Non-esterifiedFatty Acid,UnsaturatedA measurement of the total non-esterified fatty acidsin a biological specimen.A measurement of the saturated non-esterified fattyacids in a biological specimen.A measurement of the unsaturated non-esterified fattyacids in a biological specimen.Non-esterified Fatty AcidsMeasurementSaturated Non-esterified Fatty AcidsMeasurementUnsaturated Non-esterified FattyAcids MeasurementC81947 FATBODOV Fat Bodies, Oval A measurement of the oval-shaped fat bodies, usuallyrenal proximal tubular cells with lipid aggregates in thecytoplasm, in a biological specimen.C98728 FATDROP Fat Droplet A measurement of the triglyceride aggregates within abiological specimen.Oval Fat Body MeasurementFat Droplet MeasurementC103398 FCTVIIAA Factor VIIaActivityC103399 FCTVIIIA Anti-hemophilicFactor ActivityC82013 FDP FibrinDegradationProductsA measurement of the biological activity of factor VIIain a biological specimen.A measurement of the biological activity of factor VIIIin a biological specimen.A measurement of the fibrin degradation products in abiological specimen.Factor VIIa Activity MeasurementFactor VIII Activity MeasurementFibrin Degradation ProductsMeasurementC74737 FERRITIN Ferritin A measurement of the ferritin in a biologicalspecimen.Ferritin MeasurementC96650 FGF23 FibroblastGrowth Factor23; PhosphatoninC82014 FGFBF FibroblastGrowth FactorBasic Form;FGF2A measurement of the fibroblast growth factor 23 in abiological specimen.A measurement of the basic form of fibroblast growthfactor in a biological specimen.Fibroblast Growth Factor 23MeasurementFibroblast Growth Factor BasicForm MeasurementC64606 FIBRINO Fibrinogen A measurement of the fibrinogen in a biologicalspecimen.Fibrinogen MeasurementC74678 FRUCT Fructosamine;Glycated SerumProteinA measurement of the fructosamine in a biologicalspecimen.Fructosamine MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 171 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74783 FSH FollicleStimulatingHormoneC80184 G6PD Glucose-6-PhosphateDehydrogenaseC82015 GAD1 Glutamic AcidDecarboxylase 1;Glutamic AcidDecarboxylase67C82016 GAD2 Glutamic AcidDecarboxylase 2;Glutamic AcidDecarboxylase65C82017 GAD2AB Glutamic AcidDecarboxylase 2Antibody;Glutamic AcidDecarboxylase65 AntibodyC96653 GADAB Glutamic AcidDecarboxylaseAntibody; GADAntibodyA measurement of the follicle stimulating hormone(FSH) in a biological specimen.A measurement of the glucose-6-phosphatedehydrogenase in a biological specimen.A measurement of the glutamic acid decarboxylase 1in a biological specimen.A measurement of the glutamic acid decarboxylase 2in a biological specimen.A measurement of the glutamic acid decarboxylase 2antibody in a biological specimen.A measurement of the glutamic acid decarboxylaseantibody in a biological specimen.Follicle Stimulating HormoneMeasurementGlucose-6-PhosphateDehydrogenase MeasurementGlutamic Acid Decarboxylase 1MeasurementGlutamic Acid Decarboxylase 2MeasurementGlutamic Acid Decarboxylase 2Antibody MeasurementGlutamic Acid DecarboxylaseAntibody MeasurementC80182 GALANIN Galanin A measurement of the galanin in a biologicalspecimen.C74858 GASTRIN Gastrin A measurement of the gastrin hormone in a biologicalspecimen.Galanin MeasurementGastrin MeasurementC82018 GCSF GranulocyteColonyStimulatingFactorC90505 GFR GlomerularFiltration RateC98734 GFRBSA GlomerularFiltration RateAdj for BSAC100450 GFRBSB2M GFR from B-2MicroglobulinAdj for BSAC100449 GFRBSBTP GFR fromBeta-TraceProtein Adj forBSAC98735 GFRBSCRT GFR fromCreatinineAdjusted forBSAA measurement of the granulocyte colony stimulatingfactor in a biological specimen.A kidney function test that measures the fluid volumethat is filtered from the kidney glomeruli to theBowman's capsule per unit of time.A measurement of the glomerular filtration rateadjusted for body size.A measurement of the glomerular filtration rate (GFR)based on the clearance of beta-2 microglobulin afteradjusting it for the body surface area.A measurement of the glomerular filtration rate (GFR)based on the clearance of beta-trace protein afteradjusting it for the body surface area.An estimation of the glomerular filtration rate adjustedfor body size based on creatinine.Granulocyte Colony StimulatingFactor MeasurementGlomerular Filtration RateGlomerular Filtration Rate Adjustedfor BSAGlomerular Filtration Rate from B-2Microglobulin Adjusted for BSAMeasurementGlomerular Filtration Rate fromBeta-Trace Protein Adjusted for BSAMeasurementGlomerular Filtration Rate fromCreatinine Adjusted for BSASource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 172 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98736 GFRBSCYC GFR fromCystatin CAdjusted forBSAC64847 GGT Gamma GlutamylTransferaseC79446 GGTCREAT Gamma GlutamylTransferase/CreatinineC79448 GLDH GlutamateDehydrogenaseC92252 GLOBA1 Alpha-1Globulin;A1-GlobulinC92253 GLOBA1PT Alpha-1Globulin/TotalProteinC92254 GLOBA2 Alpha-2Globulin;A2-GlobulinC92255 GLOBA2PT Alpha-2Globulin/TotalProteinAn estimation of the glomerular filtration rate adjustedfor body size based on cystatin C.A measurement of the gamma glutamyl transferase in abiological specimen.A relative measurement (ratio or percentage) of thegamma glutamyl transferase to creatinine in abiological specimen.A measurement of the glutamate dehydrogenase in abiological specimen.A measurement of the proteins contributing to thealpha 1 fraction in a biological specimen.A relative measurement (ratio or percentage) ofalpha-1-fraction proteins to total proteins in abiological specimen.A measurement of the proteins contributing to thealpha 2 fraction in a biological specimen.A relative measurement (ratio or percentage) ofalpha-2-fraction proteins to total proteins in abiological specimen.Glomerular Filtration Rate fromCystatin C Adjusted for BSAGamma Glutamyl TranspeptidaseMeasurementGamma Glutamyl Transferase toCreatinine Ratio MeasurementGlutamate DehydrogenaseMeasurementAlpha-1 Globulin MeasurementAlpha-1 Globulin to Total ProteinRatio MeasurementAlpha-2 Globulin MeasurementAlpha-2 Globulin to Total ProteinRatio MeasurementC92256 GLOBB Beta Globulin A measurement of the proteins contributing to the betafraction in a biological specimen.Beta Globulin MeasurementC92294 GLOBBPT BetaGlobulin/TotalProteinA relative measurement (ratio or percentage) of betafraction proteins to total proteins in a biologicalspecimen.Beta Globulin to Total Protein RatioMeasurementC92257 GLOBG Gamma Globulin A measurement of the proteins contributing to thegamma fraction in a biological specimen.Gamma Globulin MeasurementC92295 GLOBGPT GammaGlobulin/TotalProteinA relative measurement (ratio or percentage) ofgamma fraction proteins to total proteins in abiological specimen.Gamma Globulin to Total ProteinRatio MeasurementC74738 GLOBUL Globulin A measurement of the globulin protein in a biologicalspecimen.Globulin Protein MeasurementC80183 GLP1 Glucagon-LikePeptide-1; TotalGlucagon-LikePeptide-1C80164 GLP1AC Glucagon-LikePeptide-1, ActiveFormA measurement of the total glucagon-like peptide-1 ina biological specimen.A measurement of the active form of glucagon-likepeptide-1 in a biological specimen.Glucagon-like Peptide-1MeasurementActive Glucagon-like Peptide-1MeasurementC41376 GLUC Glucose A measurement of the glucose in a biologicalspecimen.C74859 GLUCAGON Glucagon A measurement of the glucagon hormone in abiological specimen.Plasma Glucose MeasurementGlucagon MeasurementC96652 GLUCCLR GlucoseClearanceA measurement of the glucose clearance in abiological specimen.Glucose Clearance MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 173 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC79447 GLUCCRT Glucose/CreatinineC74739 GLUTAM Glutamate;Glutamic AcidC100448 GLYCRLFR Free Glycerol;Free GlycerinC82019 GMCSF GranulocyteMacrophageColony StmFactorC74860 GNRH GonadotropinReleasingHormone;LuteinisingHormoneReleasingHormoneA relative measurement (ratio or percentage) of theglucose to creatinine in a biological specimen.A measurement of the glutamate in a biologicalspecimen.A measurement of the amount of unbound glycerol in abiological specimen.A measurement of the granulocyte macrophage colonystimulating factor in a biological specimen.A measurement of the gonadotropin releasinghormone in a biological specimen.Glucose to Creatinine RatioMeasurementGlutamate MeasurementFree Glycerol MeasurementGranulocyte Macrophage ColonyStm Factor MeasurementGonadotropin Releasing HormoneMeasurementC80186 GOLD Gold A measurement of the gold in a biological specimen. Gold MeasurementC96654 GRAN Granulocytes A measurement of the granulocytes in a biologicalspecimen.Granulocyte CountC98866 GRANCE Granulocytes/Total CellsC96675 GRANIM ImmatureGranulocytesC100445 GRANIMLE ImmatureGranulocytes/LeukocytesC74861 GRWHIH GrowthHormoneInhibitingHormone;SomatostatinC74862 GRWHRH GrowthHormoneReleasingHormone;SomatocrininC80185 GST GlutathioneS-Transferase,TotalC79433 GSTAL AlphaGlutathione-S-TransferaseC80166 GSTALCRT GlutathioneS-Transferase,Alpha/CreatA relative measurement (ratio or percentage) of thegranulocytes to total cells in a biological specimen(for example a bone marrow specimen).A measurement of the total immature granulocytes in abiological specimen.A relative measurement (ratio or percentage) of theimmature granulocytes to leukocytes in a biologicalspecimen (for example a bone marrow specimen).A measurement of the growth hormone inhibitinghormone in a biological specimen.A measurement of the growth hormone releasinghormone in a biological specimen.A measurement of the total glutathione-s-transferasein a biological specimen.A measurement of the alpha form of glutathioneS-transferase in a biological specimen.A relative measurement (ratio or percentage) of thealpha glutathione-S-transferase to creatinine in abiological specimen.Granulocyte to Total CellRatioMeasurementImmature Granulocyte CountImmature Granulocytes toLeukocytes Ratio MeasurementGrowth Hormone InhibitingHormone MeasurementGrowth Hormone ReleasingHormone MeasurementGlutathione-S-TransferaseMeasurementAlpha Glutathione-S-TransferaseMeasurementAlpha Glutathione-S-Transferase toCreatinine Ratio MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 174 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonymCDISC DefinitionNCI Preferred TermC79435GSTCREATGlutathione-S-Transferase/CreatinineA relative measurement (ratio or percentage) of theglutathione S-transferase to creatinine in a biologicalspecimen.Glutathione-S-Transferase toCreatinine Ratio MeasurementC79457GSTMUMuGlutathione-S-TransferaseA measurement of the mu form of glutathioneS-transferase in a biological specimen.Mu Glutathione-S-TransferaseMeasurementC79458GSTMUCRTMuGlutathione-S-Transferase/CreatinineA relative measurement (ratio or percentage) of themu gamma glutamyl transpeptidase to creatinine in abiological specimen.Mu Glutathione-S-Transferase toCreatinine Ratio MeasurementC80203GSTPIGlutathioneS-Transferase, PiA measurement of the Pi glutathione-s-transferase in abiological specimen.Pi Glutathione S-TransferaseMeasurementC80207GSTTHGlutathioneS-Transferase,ThetaA measurement of the theta glutathione-s-transferasein a biological specimen.Theta Glutathione S-TransferaseMeasurementC80165GUSAGlucuronidase,AlphaA measurement of the alpha glucuronidase in abiological specimen.Alpha Glucuronidase MeasurementC80170GUSBGlucuronidase,BetaA measurement of the beta glucuronidase in abiological specimen.Beta Glucuronidase MeasurementC92534HAABHepatitis A VirusAntibodyA measurement of the antibody reaction of abiological specimen to the Hepatitis A virus.Hepatitis A Antibody MeasurementC92271HAABIGMHepatitis A VirusAntibody IgMA measurement of hepatitis A virus antibody IgM in abiological specimen.Hepatitis A Virus Antibody IgMMeasurementC74604HAIRYCEHairy CellsA measurement of the hairy cells (b-cell lymphocyteswith hairy projections from the cytoplasm) in abiological specimen.Hairy Cell CountC103405HALBABHuman AlbuminAntibodyA measurement of the human albumin antibody in abiological specimen.Human Albumin AntibodyMeasurementC103406HAMABHAMAA measurement of the human anti-mouse antibody in abiological specimen.C74740HAPTOGHaptoglobinA measurement of the haptoglobin protein in abiological specimen.Human Anti-Mouse AntibodyMeasurementHaptoglobin Protein MeasurementC98740HASIGEABHumanAnti-Sheep IgEAntibodyA measurement of the human anti-sheep IgE antibodiesin a biological specimen.Human Anti-Sheep IgE AntibodyMeasurementC98741HASIGGABHumanAnti-Sheep IgGAntibodyA measurement of the human anti-sheep IgGantibodies in a biological specimen.Human Anti-Sheep IgG AntibodyMeasurementC98742HASIGMABHumanAnti-Sheep IgMAntibodyA measurement of the human anti-sheep IgMantibodies in a biological specimen.Human Anti-Sheep IgM AntibodyMeasurementC92541HAVVLDHAV Viral LoadA measurement of the hepatitis A viral load in abiological specimen.Hepatitis A Viral Load MeasurementC64849HBA1CHemoglobinA1CA measurement of the glycosylated hemoglobin in abiological specimen.Glycosylated HemoglobinMeasurementC96660HBCABHepatitis B VirusCore AntibodyA measurement of the hepatitis B virus core antibodyin a biological specimen.Hepatitis B Virus Core AntibodyMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresourceSource Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 175 of 423 -A>
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonymCDISC DefinitionNCI Preferred TermC96661HBCIGMABHepatitis B VirusCore IgMAntibodyA measurement of the hepatitis B virus core IgMantibody in a biological specimen.Hepatitis B Virus Core IgM AntibodyMeasurementC103404HBDNAHepatitis B DNAA measurement of hepatitis B virus DNA in abiological specimen.Hepatitis B DNA MeasurementC96662HBEABHepatitis B Viruse AntibodyA measurement of the hepatitis B e antibody in abiological specimen.Hepatitis B Virus e AntibodyMeasurementC96663HBEAGHepatitis B Viruse AntigenA measurement of the hepatitis B e antigen in abiological specimen.Hepatitis B Virus e AntigenMeasurementC74711HBSABAnti-HBs;HBsAb; HepatitisB Virus SurfaceAntibodyA measurement of the surface antibody reaction of abiological specimen to the Hepatitis B virus.Hepatitis B Surface AntibodyMeasurementC64850HBSAGHBsAg; HepatitisB Virus SurfaceAntigenA measurement of the surface antigen reaction of abiological specimen to the Hepatitis B virus.Hepatitis B Virus Surface AntigenMeasurementC92542HBVVLDHBV Viral LoadA measurement of the hepatitis B viral load in abiological specimen.Hepatitis B Viral Load MeasurementC92535HCABHepatitis C VirusAntibodyA measurement of the antibody reaction of abiological specimen to the Hepatitis C virus.Hepatitis C Antibody MeasurementC64851HCGChoriogonadotropin Beta;Pregnancy TestA measurement of the Choriogonadotropin Beta in abiological specimen.Choriogonadotropin BetaMeasurementC64796C92543HCTHematocritThe percentage of a whole blood specimen that iscomposed of red blood cells (erythrocytes).HCVVLDHCV Viral LoadA measurement of the hepatitis C viral load in abiological specimen.HematocritHepatitis C Viral Load MeasurementC96664HDABHepatitis D VirusAntibody;Hepatitis DeltaAntibodyA measurement of the hepatitis D virus antibody in abiological specimen.Hepatitis D Virus AntibodyMeasurementC61041HDLHDL CholesterolA measurement of the high density lipoproteincholesterol in a biological specimen.Serum HDL CholesterolMeasurementC80187HDL2HDL-Cholesterol Subclass 2A measurement of the high-density lipoprotein (HDL)cholesterol subclass 2 in a biological specimen.HDL-Cholesterol Subclass 2MeasurementC80188HDL3HDL-Cholesterol Subclass 3A measurement of the high-density lipoprotein (HDL)cholesterol subclass 3 in a biological specimen.HDL-Cholesterol Subclass 3MeasurementC100425HDLCLDLCHDLCholesterol/LDLCholesterolRatioA relative measurement (ratio or percentage) of theamount of HDL cholesterol compared to LDLcholesterol in a biological specimen.HDL Cholesterol to LDLCholesterol Ratio MeasurementC103402HDLPSZHDL ParticleSizeA measurement of the average particle size ofhigh-density lipoprotein in a biological specimen.HDL Particle Size MeasurementC96665HEIGMABHepatitis E VirusIgM AntibodyA measurement of the hepatitis E virus IgM antibody ina biological specimen.Hepatitis E Virus IgM AntibodyMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresourceSource Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 176 of 423 -A>
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74709 HEINZ Heinz Bodies;Heinz-ErhlichBodiesA measurement of the Heinz bodies (small roundinclusions within the body of a red blood cell) in abiological specimen.Heinz-Ehrlich Body MeasurementC74658 HELMETCE Helmet Cells A measurement of the Helmet cells (specializedKeratocytes with two projections on either end that aretapered and hornlike) in a biological specimen.C96668 HEXK Hexokinase A measurement of the hexokinase in a biologicalspecimen.C64848 HGB Hemoglobin A measurement of the hemoglobin in a biologicalspecimen.C92258 HGBA Hemoglobin A A measurement of the hemoglobin A in a biologicalspecimen.C92259 HGBA2 Hemoglobin A2 A measurement of the hemoglobin A2 in a biologicalspecimen.C92260 HGBB Hemoglobin B A measurement of the hemoglobin B in a biologicalspecimen.C92261 HGBC Hemoglobin C A measurement of the hemoglobin C in a biologicalspecimen.Helmet Cell CountHexokinase MeasurementHemoglobin MeasurementHemoglobin A MeasurementHemoglobin A2 MeasurementHemoglobin B MeasurementHemoglobin C MeasurementC92262 HGBF Hemoglobin F;FetalHemoglobinA measurement of the hemoglobin F in a biologicalspecimen.Hemoglobin F MeasurementC96689 HGBMET Methemoglobin A measurement of the methemoglobin in a biologicalspecimen.C96616 HGBOXY Oxyhemoglobin A measurement of the oxyhemoglobin in a biologicalspecimen.C92272 HGRNA Hepatitis G RNA A measurement of the Hepatitis G RNA in a biologicalspecimen.C80189 HISTAMIN Histamine A measurement of the histamine in a biologicalspecimen.Methemoglobin MeasurementOxyhemoglobin MeasurementHepatitis G RNA MeasurementHistamine MeasurementC74714 HIV<strong>12</strong>AB HIV-1/2AntibodyA measurement of the antibody reaction of abiological specimen to the either the HIV-1 or HIV-2virus.HIV-1 HIV-2 Antibody MeasurementC74713 HIV1AB HIV-1 Antibody A measurement of the antibody reaction of abiological specimen to the HIV-1 virus.C74715 HIV2AB HIV-2 Antibody A measurement of the antibody reaction of abiological specimen to the HIV-2 virus.HIV-1 Antibody MeasurementHIV-2 Antibody MeasurementC92266 HIV2NUAC HIV-2 NucleicAcidC92265 HIVI24AG HIV-1 p24AntigenC92263 HIVIMONA HIV-1 Group Mand O NucleicAcidC92264 HIVIOAB HIV-1 Group OAntibodyA measurement of the HIV-2 nucleic acids in abiological specimen.A measurement of the HIV-1 p24 antigen in abiological specimen.A measurement of the HIV-1 group M and O nucleicacids in a biological specimen.A measurement of the HIV-1 group O antibody in abiological specimen.HIV-2 Nucleic Acid MeasurementHIV-1 p24 Antigen MeasurementHIV-1 Group M and O Nucleic AcidMeasurementHIV-1 Group O AntibodyMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 177 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92544 HIVVLD HIV Viral Load A measurement of the HIV viral load in a biologicalspecimen.HIV Viral Load MeasurementC100460 HLAB27AG HLA-B27Antigen; HumanLeukocyteAntigen B27A measurement of the human leukocyte antigen B27(HLA-B27) in a biological specimen.HLA-B27 Antigen MeasurementC96659 HMOSIDRN Hemosiderin A measurement of the hemosiderin complex in abiological specimen.Hemosiderin MeasurementC100447 HOMAIR Homostat ModelAssess of InsulinRstn;HomeostaticModelAssessment ofInsulinResistanceAn assessment of beta-cell function and insulinresistance based on fasting blood glucose and insulinconcentrations.Homeostatic Model Assessment ofInsulin ResistanceC74741 HOMOCY Homocysteine A measurement of the homocysteine amino acid in abiological specimen.Homocysteine Acid MeasurementC74704 HOWJOL Howell-JollyBodiesC103403 HPLIGGAB Helicobacterpylori IgGAntibodyA measurement of the Howell-Jolly bodies (spherical,blue-black condensed DNA inclusions within the bodyof a red blood cell that appear under Wright-stain) in abiological specimen.A measurement of the Helicobacter pylori IgGantibody in a biological specimen.Howell-Jolly Body MeasurementHelicobacter pylori IgG AntibodyMeasurementC64802 HPOCROM Hypochromia An observation which indicates that the hemoglobinconcentration in a red blood cell specimen has fallenbelow a specified level.HypochromiaC74640 HRYCELY HairyCells/LymphocytesC96698 HS<strong>12</strong>GGAB Herpes SimplexVirus 1/2 IgGAntibody;HSV-1/2 IgG AbC96666 HS<strong>12</strong>GMAB Herpes SimplexVirus 1/2 IgMAntibody;HSV-1/2 IgM AbC96697 HS1IGGAB Herpes SimplexVirus 1 IgGAntibody; HSV-1IgG AntibodyC98738 HS1IGMAB Herpes SimplexVirus 1 IgMAntibodyC96667 HS2IGGAB Herpes SimplexVirus 2 IgGAntibody; HSV-2IgG AntibodyA relative measurement (ratio or percentage) of thehairy cells (b-cell lymphocytes with hairy projectionsfrom the cytoplasm) to all lymphocytes in a biologicalspecimen .A measurement of the herpes simplex virus 1 and 2IgG antibody in a biological specimen.A measurement of the herpes simplex virus 1 and 2IgM antibody in a biological specimen.A measurement of the herpes simplex virus 1 IgGantibody in a biological specimen.A measurement of the Herpes Simplex virus 1 IgMantibodies in a biological specimen.A measurement of the herpes simplex virus 2 IgGantibody in a biological specimen.Hairy Cell to Lymphocyte RatioMeasurementHerpes Simplex Virus 1/2 IgGAntibody MeasurementHerpes Simplex Virus 1/2 IgMAntibody MeasurementHerpes Simplex Virus 1 IgGAntibody MeasurementHerpes Simplex Virus 1 IgMAntibody MeasurementHerpes Simplex Virus 2 IgGAntibody MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 178 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98739 HS2IGMAB Herpes SimplexVirus 2 IgMAntibodyC81984 HTPHAB HeterophileAntibodiesC74863 HVA HomovanillicAcidA measurement of the Herpes Simplex virus 2 IgMantibodies in a biological specimen.A measurement of the heterophile antibodies in abiological specimen.A measurement of the homovanillic acid metabolite ina biological specimen.Herpes Simplex Virus 2 IgMAntibody MeasurementHeterophile Antibody MeasurementHomovanillic Acid MeasurementC74879 HYDCDN Hydrocodone A measurement of the hydrocodone present in abiological specimen.C74880 HYDMRPHN Hydromorphone A measurement of the hydromorphone present in abiological specimen.C102275 HYDROGEN Hydrogen A measurement of the hydrogen in a biologicalspecimen.C96669 HYPERCHR Hyperchromia A measurement of the prevalence of the erthrocyteswith an elevated hemoglobin concentration.C80190 HYPRLN Hydroxyproline A measurement of the total hydroxyproline in abiological specimen.Hydrocodone MeasurementHydromorphone MeasurementHydrogen MeasurementHyperchromia MeasurementHydroxyproline MeasurementC746<strong>12</strong> HYPSEGCE HypersegmentedCellsC92273 IAH1N1VL Influenza AH1N1 Viral LoadC103408 IAIGGAB Influenza A IgGAntibodyC74718 IBCT Total IronBinding CapacityC74719 IBCU Unsaturated IronBinding CapacityC103409 IBIGGAB Influenza B IgGAntibodyC81985 IC5<strong>12</strong>AB Islet Cell 5<strong>12</strong>AntibodyC81986 IC5<strong>12</strong>AG Islet Cell 5<strong>12</strong>AntigenA measurement of the hypersegmented (more than fivelobes) neutrophils in a biological specimen.A measurement of the Influenza A H1N1 viral load in abiological specimen.A measurement of the Influenza A IgG antibody in abiological specimen.A measurement of the amount of iron needed to fullysaturate the transferrin in a biological specimen.A measurement of the binding capacity of unsaturatediron in a biological specimen.A measurement of the Influenza B IgG antibody in abiological specimen.A measurement of the islet cell 5<strong>12</strong> antibody in abiological specimen.A measurement of the islet cell 5<strong>12</strong> antigen in abiological specimen.Hypersegmented NeutrophilMeasurementInfluenza A H1N1 Viral LoadMeasurementInfluenza A IgG AntibodyMeasurementTotal Iron Binding CapacityUnsaturated Iron Binding CapacityMeasurementInfluenza B IgG AntibodyMeasurementIslet Cell 5<strong>12</strong> AntibodyMeasurementIslet Cell 5<strong>12</strong> Antigen MeasurementC81994 IFNA Interferon Alpha A measurement of the interferon alpha in a biologicalspecimen.C81995 IFNB Interferon Beta A measurement of the interferon beta in a biologicalspecimen.Interferon Alpha MeasurementInterferon Beta MeasurementC81996 IFNG InterferonGammaC81969 IGA ImmunoglobulinAC98745 IGD ImmunoglobulinDC81970 IGE ImmunoglobulinEA measurement of the interferon gamma in abiological specimen.A measurement of the Immunoglobulin A in abiological specimen.A measurement of the Immunoglobulin D in abiological specimen.A measurement of the Immunoglobulin E in abiological specimen.Interferon Gamma MeasurementImmunoglobulin A MeasurementImmunoglobulin D MeasurementImmunoglobulin E MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 179 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74864 IGF1 Insulin-likeGrowth Factor-1C74865 IGF2 Insulin-likeGrowth Factor-2C81971 IGG ImmunoglobulinGC81972 IGM ImmunoglobulinMC102280 IL2SRA SolubleInterleukin 2ReceptorActivityA measurement of the insulin-like growth factor-1 in abiological specimen.A measurement of the insulin-like growth factor-2 in abiological specimen.A measurement of the Immunoglobulin G in abiological specimen.A measurement of the Immunoglobulin M in abiological specimen.A measurement of the soluble interleukin 2 receptoractivity in a biological specimen.Insulin Like Growth Factor-1MeasurementInsulin Like Growth Factor-2MeasurementImmunoglobulin G MeasurementImmunoglobulin M MeasurementSoluble Interleukin 2 ReceptorActivity MeasurementC103410 ILE Isoleucine A measurement of the isoleucine in a biologicalspecimen.C82044 INDICAN Indican A measurement of the indican present in a biologicalspecimen.Isoleucine MeasurementIndican MeasurementC92274 INFAVLD Influenza A ViralLoadC81987 INGAPAB Islet NeogenesisAssoc ProteinAntibodyA measurement of the Influenza A viral load in abiological specimen.A measurement of the islet neogenesis associatedprotein antibody in a biological specimen.Influenza A Viral Load MeasurementIslet Neogenesis Associated ProteinAntibody MeasurementC82020 INHIBINA Inhibin A A measurement of the inhibin A in a biologicalspecimen.C96681 INHIBINB Inhibin B A measurement of the inhibin B in a biologicalspecimen.C98748 INLCLR Inulin Clearance A measurement of the volume of serum or plasma thatwould be cleared of inulin by excretion of urine for aspecified unit of time (e.g. one minute).Inhibin A MeasurementInhibin B MeasurementInulin ClearanceC64805 INR Prothrombin Intl.NormalizedRatioA ratio that represents the prothrombin time for aplasma specimen, divided by the result for a controlplasma specimen, further standardized for theInternational Sensitivity Index of the tissue factor(thromboplastin) used in the test.International Normalized Ratio ofProthrombin TimeC74788 INSULIN Insulin A measurement of the insulin in a biological specimen. Insulin MeasurementC74805 INTLK1 Interleukin 1 A measurement of the interleukin 1 in a biologicalspecimen.C74806 INTLK10 Interleukin 10 A measurement of the interleukin 10 in a biologicalspecimen.C74807 INTLK11 Interleukin 11 A measurement of the interleukin 11 in a biologicalspecimen.C74808 INTLK<strong>12</strong> Interleukin <strong>12</strong> A measurement of the interleukin <strong>12</strong> in a biologicalspecimen.C74809 INTLK13 Interleukin 13 A measurement of the interleukin 13 in a biologicalspecimen.C74810 INTLK14 Interleukin 14 A measurement of the interleukin 14 in a biologicalspecimen.Interleukin 1 MeasurementInterleukin 10 MeasurementInterleukin 11 MeasurementInterleukin <strong>12</strong> MeasurementInterleukin 13 MeasurementInterleukin 14 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 180 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74811 INTLK15 Interleukin 15 A measurement of the interleukin 15 in a biologicalspecimen.C748<strong>12</strong> INTLK16 Interleukin 16 A measurement of the interleukin 16 in a biologicalspecimen.C74813 INTLK17 Interleukin 17 A measurement of the interleukin 17 in a biologicalspecimen.C74814 INTLK18 Interleukin 18 A measurement of the interleukin 18 in a biologicalspecimen.C74815 INTLK19 Interleukin 19 A measurement of the interleukin 19 in a biologicalspecimen.C74816 INTLK2 Interleukin 2 A measurement of the interleukin 2 in a biologicalspecimen.C74817 INTLK20 Interleukin 20 A measurement of the interleukin 20 in a biologicalspecimen.C74818 INTLK21 Interleukin 21 A measurement of the interleukin 21 in a biologicalspecimen.C74819 INTLK22 Interleukin 22 A measurement of the interleukin 22 in a biologicalspecimen.C74820 INTLK23 Interleukin 23 A measurement of the interleukin 23 in a biologicalspecimen.C74821 INTLK24 Interleukin 24 A measurement of the interleukin 24 in a biologicalspecimen.C74822 INTLK25 Interleukin 25 A measurement of the interleukin 25 in a biologicalspecimen.C74823 INTLK26 Interleukin 26 A measurement of the interleukin 26 in a biologicalspecimen.C74824 INTLK27 Interleukin 27 A measurement of the interleukin 27 in a biologicalspecimen.C74825 INTLK28 Interleukin 28 A measurement of the interleukin 28 in a biologicalspecimen.C74826 INTLK29 Interleukin 29 A measurement of the interleukin 29 in a biologicalspecimen.C74827 INTLK3 Interleukin 3 A measurement of the interleukin 3 in a biologicalspecimen.C74828 INTLK30 Interleukin 30 A measurement of the interleukin 30 in a biologicalspecimen.C74829 INTLK31 Interleukin 31 A measurement of the interleukin 31 in a biologicalspecimen.C74830 INTLK32 Interleukin 32 A measurement of the interleukin 32 in a biologicalspecimen.C74831 INTLK33 Interleukin 33 A measurement of the interleukin 33 in a biologicalspecimen.C74832 INTLK4 Interleukin 4 A measurement of the interleukin 4 in a biologicalspecimen.Interleukin 15 MeasurementInterleukin 16 MeasurementInterleukin 17 MeasurementInterleukin 18 MeasurementInterleukin 19 MeasurementInterleukin 2 MeasurementInterleukin 20 MeasurementInterleukin 21 MeasurementInterleukin 22 MeasurementInterleukin 23 MeasurementInterleukin 24 MeasurementInterleukin 25 MeasurementInterleukin 26 MeasurementInterleukin 27 MeasurementInterleukin 28 MeasurementInterleukin 29 MeasurementInterleukin 3 MeasurementInterleukin 30 MeasurementInterleukin 31 MeasurementInterleukin 32 MeasurementInterleukin 33 MeasurementInterleukin 4 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 181 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74833 INTLK5 Interleukin 5 A measurement of the interleukin 5 in a biologicalspecimen.C74834 INTLK6 Interleukin 6 A measurement of the interleukin 6 in a biologicalspecimen.C74835 INTLK7 Interleukin 7 A measurement of the interleukin 7 in a biologicalspecimen.C74836 INTLK8 Interleukin 8 A measurement of the interleukin 8 in a biologicalspecimen.C74837 INTLK9 Interleukin 9 A measurement of the interleukin 9 in a biologicalspecimen.Interleukin 5 MeasurementInterleukin 6 MeasurementInterleukin 7 MeasurementInterleukin 8 MeasurementInterleukin 9 MeasurementC100439 IOHEXCLR IohexolClearanceC98749 IOTCLR IothalamateClearanceC98750 IOTCLRBS IothalamateClearanceAdjusted forBSAC102276 IRF ImmatureReticulocyteFractionA measurement of the volume of serum or plasma thatwould be cleared of Iohexol through excretion for aspecified unit of time.A measurement of the volume of serum or plasma thatwould be cleared of iothalamate by excretion of urinefor a specified unit of time (e.g. one minute).A measurement of the volume of serum or plasma thatwould be cleared of iothalamate by excretion of urinefor a specified unit of time (e.g. one minute), adjustedfor body size.A measurement of the immature reticulocyte fractionpresent in a biological specimen.Iohexol ClearanceIothalamate ClearanceIothalamate Clearance Adjusted forBSAImmature Reticulocyte FractionMeasurementC74679 IRON Iron; FE A measurement of the iron in a biological specimen. Iron MeasurementC80180 ISOPRF2 F2-Isoprostane A measurement of the F2-isoprostane in a biologicalspecimen.C100459 JO1AB Jo-1 Antibody A measurement of the Jo-1 antibody in a biologicalspecimen.C64853 K Potassium A measurement of the potassium in a biologicalspecimen.F2 Isoprostane MeasurementJo-1 Antibody MeasurementPotassium MeasurementC79462 KCREAT Potassium/CreatinineA relative measurement (ratio or percentage) of thepotassium to creatinine in a biological specimen.Potassium to Creatinine RatioMeasurementC64854 KETONES Ketones A measurement of the ketones in a biologicalspecimen.Ketone MeasurementC100433 KIM1 Kidney InjuryMolecule-1;KIM-1; HepatitisA Virus CellularReceptor 1C98730 KLCFR Kappa LightChain, FreeC98731 KLCLLCFR Kappa LtChain,Free/Lambda LtChain,FreeA measurement of the kidney injury molecule-1(Kim-1) in a biological specimen.A measurement of the free kappa light chain in abiological specimen.A relative measurement (ratio or percentage) of thefree kappa light chain to the free lambda light chain ina biological specimen.Kidney Injury Molecule-1MeasurementFree Kappa Light ChainMeasurementFree Kappa Light Chain to FreeLambda Light Chain RatioMeasurementC96688 KRCYMG Megakaryocytes A measurement of the megakaryocytes per unit of abiological specimen.Megakaryocyte CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 182 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98867 KRCYMGCE Megakaryocytes/Total CellsA relative measurement (ratio or percentage) of themegakaryocytes to total cells in a biological specimen(for example a bone marrow specimen).Megakaryocyte to Total CellRatioMeasurementC96682 KURLOFCE Kurloff Cells A measurement of the large secretorygranule-containing immune cells in a biologicalspecimen taken from members of certain genera of theCaviidae family.Kurloff Cells MeasurementC79450 LACTICAC Lactic Acid;2-hydroxypropanoic acid; LactateC64855 LDH LactateDehydrogenaseC74887 LDH1 LDH Isoenzyme1C79451 LDH1LDH LDH Isoenzyme1/LDHC74888 LDH2 LDH Isoenzyme2C79452 LDH2LDH LDH Isoenzyme2/LDHC74889 LDH3 LDH Isoenzyme3C79453 LDH3LDH LDH Isoenzyme3/LDHC74890 LDH4 LDH Isoenzyme4C79454 LDH4LDH LDH Isoenzyme4/LDHC74891 LDH5 LDH Isoenzyme5C79455 LDH5LDH LDH Isoenzyme5/LDHC79449 LDHCREAT LactateDehydrogenase/CreatinineA measurement of the lactic acid in a biologicalspecimen.A measurement of the lactate dehydrogenase in abiological specimen.A measurement of the lactate dehydrogenaseisoenzyme 1 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 1 to total lactatedehydrogenase in a biological specimen.A measurement of the lactate dehydrogenaseisoenzyme 2 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 2 to total lactatedehydrogenase in a biological specimen.A measurement of the lactate dehydrogenaseisoenzyme 3 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 3 to total lactatedehydrogenase in a biological specimen.A measurement of the lactate dehydrogenaseisoenzyme 4 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 4 to total lactatedehydrogenase in a biological specimen.A measurement of the lactate dehydrogenaseisoenzyme 5 in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase isoenzyme 5 to total lactatedehydrogenase in a biological specimen.A relative measurement (ratio or percentage) of thelactate dehydrogenase to creatinine in a biologicalspecimen.Lactic Acid MeasurementLactate DehydrogenaseMeasurementLactate Dehydrogenase Isoenzyme 1MeasurementLDH Isoenzyme 1 to LDH RatioMeasurementLactate Dehydrogenase Isoenzyme 2MeasurementLDH Isoenzyme 2 to LDH RatioMeasurementLactate Dehydrogenase Isoenzyme 3MeasurementLDH Isoenzyme 3 to LDH RatioMeasurementLactate Dehydrogenase Isoenzyme 4MeasurementLDH Isoenzyme 4 to LDH RatioMeasurementLactate Dehydrogenase Isoenzyme 5MeasurementLDH Isoenzyme 5 to LDH RatioMeasurementLactate Dehydrogenase to CreatinineRatio MeasurementC61042 LDL LDL Cholesterol A measurement of the low density lipoproteincholesterol in a biological specimen.Serum LDL CholesterolMeasurementC1034<strong>12</strong> LDLPSZ LDL ParticleSizeA measurement of the average particle size oflow-density lipoprotein in a biological specimen.LDL Particle Size MeasurementC74866 LEPTIN Leptin A measurement of the leptin hormone in a biologicalspecimen.Leptin MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 183 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64856 LEUKASE LeukocyteEsteraseC79467 LGLUCLE Large UnstainedCells/LeukocytesC74659 LGUNSCE Large UnstainedCellsC74790 LH LuteinizingHormoneC64807 LIPASE TriacylglycerolLipaseC96683 LKM1AB Liver KidneyMicrosomalType 1 Antibody;LKM-1C100456 LKM1IAAB Liver KidneyMicrosomalType 1 IgA AbC100454 LKM1IGAB Liver KidneyMicrosomalType 1 IgG AbC100455 LKM1IMAB Liver KidneyMicrosomalType 1 IgM AbC98732 LLCFR Lambda LightChain, FreeA measurement of the enzyme which indicates thepresence of white blood cells in a biologicalspecimen.A relative measure (ratio or percentage) of the largeunstained cells to leukocytes in a biological specimen.A measurement of the large, peroxidase-negative cellswhich cannot be further characterized (i.e. as largelymphocytes, virocytes, or stem cells) present in abiological specimen.A measurement of the luteinizing hormone in abiological specimen.A measurement of the pancreatic lipase in a biologicalspecimen.A measurement of the liver kidney microsomal type 1antibody in a biological specimen.A measurement of the liver kidney microsomal type 1IgA antibodies in a biological specimen.A measurement of the liver kidney microsomal type 1IgG antibodies in a biological specimen.A measurement of the liver kidney microsomal type 1IgM antibodies in a biological specimen.A measurement of the free lambda light chain in abiological specimen.Leukocyte Esterase MeasurementLarge Unstained Cells to LeukocytesRatio MeasurementLarge Unstained Cell CountLuteinizing Hormone MeasurementTriacylglycerol Lipase MeasurementLiver Kidney Microsomal Type 1Antibody MeasurementLiver Kidney Microsomal Type 1IgA Antibody MeasurementLiver Kidney Microsomal Type 1IgG Antibody MeasurementLiver Kidney Microsomal Type 1IgM Antibody MeasurementFree Lambda Light ChainMeasurementC82022 LPA Lipoprotein-a A measurement of the lipoprotein-a in a biologicalspecimen.Lipoprotein a MeasurementC92275 LPNAG LegionellapneumophilaAntigenC92277 LPNGMAB Legionellapneumophila IgGIgM AntibodyC92276 LPNIGGAB Legionellapneumophila IgGAntibodyA measurement of the Legionella pneumophila antigenin a biological specimen.A measurement of the Legionella pneumophila IgG andIgM in a biological specimen.A measurement of the Legionella pneumophila IgGantibody in a biological specimen.Legionella pneumophila AntigenMeasurementLegionella pneumophila IgG IgMAntibody MeasurementLegionella pneumophila IgGAntibody MeasurementC92278 LPNIGMAB Legionellapneumophila IgMAntibodyA measurement of the Legionella pneumophila IgMantibody in a biological specimen.Legionella pneumophila IgMAntibody MeasurementC75354 LSD Lysergic AcidDiethylamide;AcidA measurement of the lysergic acid diethylamine(LSD) in a biological specimen.Lysergide MeasurementC103413 LTB4 Leukotriene B4 A measurement of the leukotriene B4 in a biologicalspecimen.Leukotriene B4 MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 184 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103414 LTD4 Leukotriene D4 A measurement of the leukotriene D4 in a biologicalspecimen.C103415 LTE4 Leukotriene E4 A measurement of the leukotriene E4 in a biologicalspecimen.Leukotriene D4 MeasurementLeukotriene E4 MeasurementC82021 LTF Lactoferrin;LactotransferrinA measurement of the lactoferrin in a biologicalspecimen.Lactoferrin MeasurementC51949 LYM Lymphocytes A measurement of the lymphocytes in a biologicalspecimen.Lymphocyte CountC64818 LYMAT LymphocytesAtypicalC64819 LYMATLE LymphocytesAtypical/LeukocytesC98751 LYMCE Lymphocytes/Total CellsC100444 LYMIM ImmatureLymphocytesC100443 LYMIMLE ImmatureLymphocytes/LeukocytesC64820 LYMLE Lymphocytes/LeukocytesA measurement of the atypical lymphocytes in abiological specimen.A relative measurement (ratio or percentage) of theatypical lymphocytes to leukocytes in a biologicalspecimen.A relative measurement (ratio or percentage) of thelymphocytes to total cells in a biological specimen(for example a bone marrow specimen).A measurement of the immature lymphocytes in abiological specimen.A relative measurement (ratio or percentage) of theimmature lymphocytes to leukocytes in a biologicalspecimen.A relative measurement (ratio or percentage) of thelymphocytes to leukocytes in a biological specimen.Atypical Lymphocyte CountAtypical Lymphocyte To LeukocyteRatioLymphocyte to Total Cell RatioMeasurementImmature LymphocytesMeasurementImmature Lymphocytes toLeukocytes Ratio MeasurementLymphocyte To Leukocyte RatioC74613 LYMMCE Lymphoma Cells A measurement of the malignant lymphocytes in abiological specimen.Lymphoma Cell CountC74910 LYMMCELY LymphomaCells/LymphocytesC81955 LYMPHOTC Lymphotactin;ChemokineLigand 1C74618 LYMPL PlasmacytoidLymphocytes;PlymphocytesC74648 LYMPLLY PlasmacytoidLymphocytes/LymphocytesC74629 LYMRCT ReactiveLymphocytesC74654 LYMRCTLY ReactiveLymphocytes/LymphocytesA relative measurement (ratio or percentage) of themalignant lymphocytes to all lymphocytes in abiological specimen.A measurement of the lymphotactin in a biologicalspecimen.A measurement of the plasmacytoid lymphocytes(lymphocytes with peripherally clumped chromatin andoften deep blue cytoplasm, and that appear similar toplasma cells) in a biological specimen.A relative measurement (ratio or percentage) of theplasmacytoid lymphocytes (lymphocytes withperipherally clumped chromatin and often deep bluecytoplasm, and that appear similar to plasma cells) toall lymphocytes in a biological specimen.A measurement of the reactive lymphocytes(lymphocytes which have become large due to anantigen reaction) in a biological specimen.A relative measurement (ratio or percentage) of thereactive lymphocytes (lymphocytes which havebecome large due to an antigen reaction) to alllymphocytes in a biological specimen.Lymphoma Cell to LymphocyteRatio MeasurementLymphotactin MeasurementPlasmacytoid Lymphocyte CountPlasmacytoid Lymphocyte toLymphocyte Ratio MeasurementReactive Lymphocyte CountMeasurementReactive Lymphocyte toLymphocyte Ratio MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 185 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64821 MACROCY Macrocytes A measurement of the macrocytes in a biologicalspecimen.Macrocyte CountC74614 MAYHEG May-HegglinAnomalyC64797 MCH Ery. MeanCorpuscularHemoglobinC64798 MCHC Ery. MeanCorpuscularHGBConcentrationC82025 MCP1 MonocyteChemotacticProtein 1C92291 MCPROT MonoclonalImmunoglobulinProtein;ParaproteinC80191 MCSF MacrophageColonyStimulatingFactorC64799 MCV Ery. MeanCorpuscularVolumeC81956 MDC Macrophage-Derived Chemokine;ChemokineLigand 22C75359 MDMA 3,4-methylenedioxymethamphetamine; EcstasyA measurement of the May-Hegglin anomaly in ablood sample. This anomaly is characterized by large,misshapen platelets and the presence of Dohle bodiesin leukocytes.A quantitative measurement of the mean amount ofhemoglobin per erythrocyte in a biological specimen.A quantitative measurement of the mean amount ofhemoglobin per erythrocytes in a specified volume ofa biological specimen.A measurement of the monocyte chemotactic protein1 in a biological specimen.A measurement of homogenous immunoglobulinresulting from the proliferation of a single clone ofplasma cells in a biological specimen.A measurement of the macrophage colony stimulatingfactor in a biological specimen.A quantitative measurement of the mean volume oferythrocytes in a biological specimen.A measurement of the macrophage-derived chemokinein a biological specimen.A measurement of the3,4-methylenedioxymethamphetamine (MDMA) in abiological specimen.May-Hegglin Anomaly MeasurementErythrocyte Mean CorpuscularHemoglobinErythrocyte Mean CorpuscularHemoglobin ConcentrationMonocyte Chemotactic Protein 1MeasurementMonoclonal Protein MeasurementMacrophage Colony StimulatingFactor MeasurementErythrocyte Mean CorpuscularVolumeMacrophage-Derived ChemokineMeasurement3,4-Methylenedioxymethamphetamine MeasurementC74615 METAMY Metamyelocytes A measurement of the metamyelocytes (small,myelocytic neutrophils with an indented nucleus) in abiological specimen.Metamyelocyte CountC98754 METAMYCE Metamyelocytes/Total CellsC74645 METAMYLE Metamyelocytes/LeukocytesC75348 METHAMPH MethamphetamineA relative measurement (ratio or percentage ) of themetamyelocytes (small, myelocytic neutrophils withan indented nucleus) to total cells in a biologicalspecimen (for example a bone marrow specimen).A relative measurement (ratio or percentage) of themetamyelocytes (small, myelocytic neutrophils withan indented nucleus) to all leukocytes in a biologicalspecimen.A measurement of the methamphetamine drug presentin a biological specimen.Metamyelocyte to Total Cell RatioMeasurementMetamyelocyte to Leukocyte RatioMeasurementMethamphetamine MeasurementC74881 METHDN Methadone A measurement of the methadone present in abiological specimen.Methadone MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 186 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74882 METHQLDN Methaqualone A measurement of the methaqualone present in abiological specimen.C64840 MG Magnesium A measurement of the magnesium in a biologicalspecimen.C79436 MGB Myoglobin A measurement of myoglobin in a biologicalspecimen.Methaqualone MeasurementMagnesium MeasurementMyoglobin MeasurementC79456 MGCREAT Magnesium/CreatinineA relative measurement (ratio or percentage) of themagnesium to creatinine in a biological specimen.Magnesium to Creatinine RatioMeasurementC64822 MICROCY Microcytes A measurement of the microcytes in a biologicalspecimen.Microcyte CountC82023 MIP1A MacrophageInflammatoryProtein 1 Alpha;ChemokineLigand 3C82024 MIP1B MacrophageInflammatoryProtein 1 Beta;ChemokineLigand 4A measurement of the macrophage inflammatoryprotein 1 alpha in a biological specimen.A measurement of the macrophage inflammatoryprotein 1 beta in a biological specimen.Macrophage Inflammatory Protein 1Alpha MeasurementMacrophage Inflammatory Protein 1Beta MeasurementC74867 MLATONIN Melatonin A measurement of the melatonin hormone in abiological specimen.Melatonin MeasurementC74660 MLIGCE Malignant Cells,NOSC74643 MLIGCEBC Malignant Cells,NOS/BloodCellsC96690 MMA MethylmalonicAcidC80192 MMP1 MatrixMetalloproteinase 1; InterstitialCollagenaseC80193 MMP2 MatrixMetalloproteinase 2; Gelatinase AC80194 MMP3 MatrixMetalloproteinase 3; Stromelysin1C80195 MMP7 MatrixMetalloproteinase 7; MatrilysinC80196 MMP8 MatrixMetalloproteinase 8; NeutrophilCollagenaseA measurement of the malignant cells of all types in abiological specimen.A relative measurement (ratio or percentage) of themalignant cells of all types to all blood cells in abiological specimen.A measurement of the methylmalonic acid in abiological specimen.A measurement of the matrix metalloproteinase 1 in abiological specimen.A measurement of the matrix metalloproteinase 2 in abiological specimen.A measurement of the matrix metalloproteinase 3 in abiological specimen.A measurement of the matrix metalloproteinase 7 in abiological specimen.A measurement of the matrix metalloproteinase 8 in abiological specimen.Malignant Cell CountMalignant Cell to Blood Cell RatioMeasurementMethylmalonic Acid MeasurementMatrix Metalloproteinase 1MeasurementMatrix Metalloproteinase 2MeasurementMatrix Metalloproteinase 3MeasurementMatrix Metalloproteinase 7MeasurementMatrix Metalloproteinase 8MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 187 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC80197 MMP9 MatrixMetalloproteinase 9; Gelatinase BA measurement of the matrix metalloproteinase 9 in abiological specimen.Matrix Metalloproteinase 9MeasurementC64823 MONO Monocytes A measurement of the monocytes in a biologicalspecimen.C74631 MONOBL Monoblasts A measurement of the monoblast cells in a biologicalspecimen.Monocyte CountMonoblast CountC74646 MONOBLLE Monoblasts/LeukocytesC98872 MONOCE Monocytes/TotalCellsC96676 MONOIM ImmatureMonocytesC96677 MONOIMLE ImmatureMonocytes/LeukocytesC64824 MONOLE Monocytes/LeukocytesA relative measurement (ratio or percentage) of themonoblasts to leukocytes in a biological specimen.A relative measurement (ratio or percentage) of themonocytes to total cells in a biological specimen (forexample a bone marrow specimen).A measurement of the immature monocytes in abiological specimen.A relative measurement (ratio or percentage) ofimmature monocytes to total leukocytes in abiological specimen.A relative measurement (ratio or percentage) of themonocytes to leukocytes in a biological specimen.Monoblast to Leukocyte RatioMeasurementMonocytes to TotalCellRatioMeasurementImmature Monocyte CountImmature Monocyte to LeukocyteRatio MeasurementMonocyte To Leukocyte RatioC74883 MORPHINE Morphine A measurement of the morphine present in a biologicalspecimen.Morphine MeasurementC96686 MPC Mean PlateletComponentA measurement of the mean platelet component(platelet activity) in a blood specimen.Mean Platelet ComponentMeasurementC80198 MPO Myeloperoxidase A measurement of the myeloperoxidase in a biologicalspecimen.Myeloperoxidase MeasurementC92280 MPOAB MyeloperoxidaseAntibodyC74730 MPV Mean PlateletVolumeA measurement of the myeloperoxidase antibody in abiological specimen.A measurement of the average size of the plateletspresent in a blood sample.Myeloperoxidase AntibodyMeasurementMean Platelet Volume MeasurementC74721 MUCTHR Mucous Threads A measurement of the mucous threads present in abiological specimen.C74632 MYBLA Myeloblasts A measurement of the myeloblast cells in a biologicalspecimen.Mucous Thread MeasurementMyeloblast CountC64825 MYBLALE Myeloblasts/LeukocytesC92283 MYBLAT1 Type IMyeloblastsC92284 MYBLAT2 Type IIMyeloblastsC92285 MYBLAT3 Type IIIMyeloblastsA relative measure (ratio or percentage) of themyeloblasts to leukocytes in a biological specimen.A measurement of type I myeloblast cells per unit of abiological specimen.A measurement of type II myeloblast cells per unit of abiological specimen.A measurement of type III myeloblast cells per unit ofa biological specimen.Myeloblast To Leukocyte RatioType I Myeloblasts MeasurementType II Myeloblasts MeasurementType III Myeloblasts MeasurementC74662 MYCY Myelocytes A measurement of the myelocytes in a biologicalspecimen.Myelocyte CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 188 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98868 MYCYCE Myelocytes/Total CellsC64826 MYCYLE Myelocytes/LeukocytesC103418 MYELINAB MyelinAntibodiesC92242 MYPCERPC Myeloid/Erythroid RatioC92241 MYTBGIR Mycobacteriumtuberculosis IFNGammaResponseC92279 MYTBNUAC MycobacteriumtuberculosisNucleic AcidC79464 NACREAT Sodium/CreatinineC79459 NAG N-AcetylGlucosamide;N-AcetylGlucosamineC103419 NAGASE NAG;Beta-N-acetyl-D-glucosaminidaseC79460 NAGCREAT N-AcetylGlucosamide/CreatinineC79437 NCTD5P 5 PrimeNucleotidase;5'-RibonucleotidePhosphohydrolaseA relative measurement (ratio or percentage) of themyelocytes to total cells in a biological specimen (forexample a bone marrow specimen).A relative measurement (ratio or percentage) of themyelocytes to leukocytes in a biological specimen.A measurement of the myelin antibodies in abiological specimen.A relative measurement (ratio or percentage) ofmyeloid progenitor cells to erythrocyte precursorcells in a biological specimen.A measurement of the amount of interferon gammathat is released when Mycobacterium tuberculosisantigen is incubated with a biological specimen,usually blood. Increased amounts of interferon gammasuggests a prior or existing M. tuberculosis infection.A measurement of the Mycobacterium tuberculosisnucleic acid in a biological specimen.A relative measurement (ratio or percentage) of thesodium to creatinine in a biological specimen.A measurement of N-acetyl glucosamide in abiological specimen.A measurement of theN-acetyl-beta-D-glucosaminidase in a biologicalspecimen.A relative measurement (ratio or percentage) of theN-acetyl glucosamide to creatinine in a biologicalspecimen.A measurement of the 5'-nucleotidase in a biologicalspecimen.Myelocyte to Total CellRatioMeasurementMyelocyte To Leukocyte RatioMyelin Antibodies MeasurementMyeloid to Erythroid RatioMeasurementMycobacterium tuberculosisInterferon Gamma ResponseMycobacterium tuberculosis NucleicAcid MeasurementSodium to Creatinine RatioMeasurementN-Acetyl Glucosamide MeasurementN-acetyl-beta-D-glucosaminidaseMeasurementN-Acetyl Glucosamide to CreatinineRatio Measurement5 Prime Nucleotidase MeasurementC80199 NEOPTERN Neopterin A measurement of the neopterin in a biologicalspecimen.C63321 NEUT Neutrophils A measurement of the neutrophils in a biologicalspecimen.Neopterin MeasurementAbsolute Neutrophil CountC64830 NEUTB Neutrophils BandFormC64831 NEUTBLE Neutrophils BandForm/LeukocytesC98763 NEUTCE Neutrophils/Total CellsA measurement of the banded neutrophils in abiological specimen.A relative measurement (ratio or percentage) of thebanded neutrophils to leukocytes in a biologicalspecimen.A relative measurement (ratio or percentage) of theneutrophils to total cells in a biological specimen (forexample a bone marrow specimen).Neutrophil Band Form CountNeutrophil Band Form To LeukocyteRatioNeutrophil to Total Cell RatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 189 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96651 NEUTGT GiantNeutrophilsC96678 NEUTIM ImmatureNeutrophilsC100442 NEUTIMLE ImmatureNeutrophils/LeukocytesC64827 NEUTLE Neutrophils/LeukocytesC84822 NEUTMM NeutrophilicMetamyelocytesC84823 NEUTMY NeutrophilicMyelocytesC81997 NEUTSG Neutrophils,SegmentedC82045 NEUTSGLE Neutrophils,Segmented/LeukocytesC74628 NEUTVAC VacuolatedNeutrophilsC92298 NGON NeisseriagonorrhoeaeScreeningA measurement of the giant neutrophils in a biologicalspecimen.A measurement of the total immature neutrophils in abiological specimen.A relative measurement (ratio or percentage) of theimmature neutrophils to leukocytes in a biologicalspecimen.A relative measurement (ratio or percentage) of theneutrophils to leukocytes in a biological specimen.A measurement of the neutrophilic metamyelocytes ina biological specimen.A measurement of the neutrophilic myelocytes in abiological specimen.A measurement of the segmented neutrophils in abiological specimen.A relative measurement (ratio or percentage) ofsegmented neutrophils to leukocytes in a biologicalspecimen.A measurement of the neutrophils containing smallvacuoles in a biological specimen.Examination of a biological specimen to detect thepresence of Neisseria gonorrhoeae.Giant Neutrophil CountImmature Neutrophil CountImmature Neutrophils to LeukocytesRatio MeasurementNeutrophil To Leukocyte RatioNeutrophilic Metamyelocyte CountNeutrophilic Myelocyte CountSegmented Neutrophil CountSegmented Neutrophil to LeukocyteRatio MeasurementVacuolated Neutrophil CountNeisseria gonorrhoeae ScreeningC64810 NITRITE Nitrite A measurement of the nitrite in a urine specimen. Nitrite MeasurementC98762 NKCE Natural KillerCellsC74868 NOREPIN Noradrenaline;NorepinephrineC100434 NPAP Non-ProstaticAcidPhosphataseA measurement of the total natural killer cells in abiological specimen.A measurement of the norepinephrine hormone in abiological specimen.A measurement of the non-prostatic acid phosphatasein a biological specimen.Natural Killer Cell CountNoradrenaline MeasurementNon-Prostatic Acid PhosphataseMeasurementC74892 NPY Neuropeptide Y A measurement of the neuropeptide Y in a biologicalspecimen.C74743 NTELOP N-telopeptide A measurement of the N-telopeptide in a biologicalspecimen.Neuropeptide Y MeasurementN-telopeptide MeasurementC82039 NTXI Type I CollagenN-TelopeptidesC82041 NTXII Type II CollagenN-TelopeptidesA measurement of the type I collagen N-telopeptidesin a biological specimen.A measurement of the type II collagen N-telopeptidesin a biological specimen.Type I Collagen N-TelopeptideMeasurementType II Collagen N-TelopeptideMeasurementC74686 OCCBLD Occult Blood A measurement of the blood in body products such as astool sample, not detectable on gross examination.C74796 OPIATE Opiate A measurement of any opiate class drug present in abiological specimen.C74801 OSMLTY Osmolality A measurement of the osmoles of solute per unit ofbiological specimen.Occult Blood MeasurementOpiate MeasurementOsmolality MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 190 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74802 OSMRTY Osmolarity A measurement of the osmoles of solute per liter ofsolution.C74744 OSTEOC Osteocalcin A measurement of the osteocalcin in a biologicalspecimen.C100452 OVAPARS Ova and Parasite A measurement of the parasites and ova in a biologicalspecimen.Osmolarity MeasurementOsteocalcin MeasurementOva and Parasite MeasurementC92250 OXALATE Oxalate;EthanedioateA measurement of the oxalate in a biologicalspecimen.Oxalate MeasurementC96614 OXYCAP Oxygen Capacity A measurement of the maximum amount of oxygenthat can be combined chemically with hemoglobin in avolume of blood.Oxygen Capacity MeasurementC74884 OXYCDN Oxycodone;OxycontinC68032 OXYSAT OxygenSaturationC74869 OXYTOCIN Oxytocin;OxytoxinC96625 P1NP Procollagen 1N-TerminalPropeptide;Amino-terminalpropeptide oftype 1procollagen;P1NPAminoterm Type1A measurement of the oxycodone present in abiological specimen.A measurement of the oxygen-hemoglobin saturationof a volume of blood.A measurement of the oxytocin hormone in abiological specimen.A measurement of the procollagen 1 N-terminalpropeptide in a biological specimen.Oxycodone MeasurementOxygen Saturation MeasurementOxytocin MeasurementProcollagen 1 N-TerminalPropeptide MeasurementC102279 P50OXYGN P50 Oxygen A measurement of the partial pressure of oxygen whenhemoglobin is half saturated in a biological specimen.P50 Oxygen MeasurementC82030 PAI1 PlasminogenActivatorInhibitor-1C81989 PAI1AG PlasminogenActivatorInhibitor-1 AGC80204 PAP Prostatic AcidPhosphataseC82031 PAPPA Pregnancy-Associated PlasmaProtein-AC74616 PAPPEN PappenheimerBodiesC96617 PB19GGAB Parvovirus B19IgG AntibodyC96618 PB19GMAB Parvovirus B19IgM AntibodyA measurement of the plasminogen activatorinhibitor-1 in a biological specimen.A measurement of the plasminogen activatorinhibitor-1 antigen in a biological specimen.A measurement of the prostatic acid phosphatase in abiological specimen.A measurement of the pregnancy-associated plasmaprotein-A in a biological specimen.A measurement of the cells containing PappenheimerBodies (violet or blue staining ferritin granules usuallyfound along the periphery of the red blood cells) in abiological specimen.A measurement of the Parvovirus B19 IgG antibody ina biological specimen.A measurement of the Parvovirus B19 IgM antibody ina biological specimen.Plasminogen Activator Inhibitor-1MeasurementPlasminogen Activator Inhibitor-1Antigen MeasurementProstatic Acid PhosphataseMeasurementPregnancy-Associated PlasmaProtein-A MeasurementPappenheimer Body CountParvovirus B19 IgG AntibodyMeasurementParvovirus B19 IgM AntibodyMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 191 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC82625 PCO2 Partial PressureCarbon DioxideC74694 PCP Phencyclidine;PhenylcyclohexylpiperidineA measurement of the pressure of carbon dioxide in abiological specimen.A measurement of the phencyclidine present in abiological specimen.Partial Pressure of Carbon DioxideMeasurementPhencyclidine MeasurementC103430 PCT Procalcitonin A measurement of the procalcitonin in a biologicalspecimen.Procalcitonin MeasurementC81962 PDW PlateletDistributionWidthC74617 PELGERH Pelger HuetAnomalyC81988 PEMAB PemphigoidAntibodiesA measurement of the range of platelet sizes in abiological specimen.A measurement of the Pelger Huet Anomaly (nuclei ofneutrophils and eosinophils appear rod-like, sphericalor dumbbell shaped) in a biological specimen.A measurement of the pemphigoid antibodies in abiological specimen.Platelet Distribution WidthPelger Huet Anomaly MeasurementPemphigoid Antibody MeasurementC100<strong>12</strong>2 PEPSNG Pepsinogen A measurement of the pepsinogen in a biologicalspecimen.Pepsinogen MeasurementC100469 PEPSNGA Pepsinogen A;PGAC100470 PEPSNGC Pepsinogen C;PGCC100467 PEPSNGI Pepsinogen I;PGIC100468 PEPSNGII Pepsinogen II;PGIIA measurement of the pepsinogen A in a biologicalspecimen.A measurement of the pepsinogen C in a biologicalspecimen.A measurement of the pepsinogen I in a biologicalspecimen.A measurement of the pepsinogen II in a biologicalspecimen.Pepsinogen A MeasurementPepsinogen C MeasurementPepsinogen I MeasurementPepsinogen II MeasurementC100446 PERTHRBL Proerythroblast A measurement of the proerythroblasts in a biologicalspecimen.Proerythroblast MeasurementC103343 PG TotalProstaglandinA measurement of the total prostaglandin in abiological specimen.Prostaglandin MeasurementC103431 PGD2 Prostaglandin D2 A measurement of the prostaglandin D2 in a biologicalspecimen.Prostaglandin D2 MeasurementC103432 PGD2S Prostaglandin D2SynthaseA measurement of the prostaglandin D2 synthase in abiological specimen.Prostaglandin D2 SynthaseMeasurementC103434 PGE1 Prostaglandin E1 A measurement of the prostaglandin E1 in a biologicalspecimen.C103435 PGE2 Prostaglandin E2 A measurement of the prostaglandin E2 in a biologicalspecimen.Prostaglandin E1 MeasurementProstaglandin E2 MeasurementC103433 PGES Prostaglandin ESynthaseC103436 PGF1A Prostaglandin F1AlphaC103437 PGF2A Prostaglandin F2AlphaA measurement of the prostaglandin E synthase in abiological specimen.A measurement of the prostaglandin F1 alpha in abiological specimen.A measurement of the prostaglandin F2 alpha in abiological specimen.Prostaglandin E SynthaseMeasurementProstaglandin F1 AlphaMeasurementProstaglandin F2 AlphaMeasurementC45997 PH pH A measure of the acidity or alkalinity of a fluid on ascale of 0 to 14.pHSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 192 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74695 PHENTHZ Phenothiazine;DibenzothiazineC64857 PHOS Phosphate;Phosphorus;InorganicPhosphateC79461 PHOSCRT Phosphate/CreatinineA measurement of the phenothiazine present in abiological specimen.A measurement of the phosphate in a biologicalspecimen.A relative measurement (ratio or percentage) of thephosphate to creatinine in a biological specimen.Phenothiazine MeasurementPhosphate MeasurementPhosphate to Creatinine RatioMeasurementC96623 PHOSLPD Phospholipid A measurement of the phospholipids in a biologicalspecimen.Phospholipid MeasurementC82033 PICP ProcollagenType I CarboxyTerm PeptideA measurement of the procollagen-1 carboxy-terminalpeptide in a biological specimen.Procollagen Type I CarboxyTerminal Peptide MeasurementC51951 PLAT Platelets A measurement of the platelets in a biologicalspecimen.C103427 PLATAGGR Platelet Function A measurement of the association of platelets to oneanother via adhesion molecules in a biological sample.Platelet CountPlatelet Aggregation MeasurementC96624 PLATCLMP Platelet Clumps;PLT ClumpsA measurement of the platelet clumps in a biologicalspecimen.Platelet Clumps CountC74728 PLATGNT Giant Platelets A measurement of the giant (larger than 7um indiameter) platelets in a biological specimen.Giant Platelet CountC100424 PLATHCT PlateletHematocrit;ThrombocytocritA relative measurement (ratio or percentage) of theproportion of the volume of blood taken up byplatelets.Platelet Hematocrit MeasurementC74729 PLATLRG Large Platelets A measurement of the large (between 4 um and 7um indiameter) platelets in a biological specimen.Large Platelet CountC96679 PLSIMCE Immature PlasmaCellsC96680 PLSIMCLY Immature PlasmaCells/LymphocytesC74661 PLSMCE Mature PlasmaCells;PlasmacytesC98869 PLSMCECE PlasmaCells/Total CellsC74911 PLSMCELY Mature PlasmaCells/LymphocytesA measurement of the immature plasma cells in abiological specimen.A relative measurement (ratio or percentage) ofimmature plasma cells to total lymphocytes in abiological specimen.A measurement of the mature plasma cells(plasmacytes) in a biological specimen.A relative measurement (ratio or percentage) of themature plasma cells (plasmacytes) to total cells in abiological specimen (for example a bone marrowspecimen).A relative measurement (ratio or percentage) of themature plasma cells (plasmacytes) to all lymphocytesin a biological specimen.Immature Plasma Cell CountImmature Plasma Cell toLymphocyte Ratio MeasurementMature Plasma Cell CountPlasma Cell to Total CellRatioMeasurementMature Plasma Cell to LymphocyteRatio MeasurementC100453 PLSMDM Plasmodium Examination of a biological specimen to detect thepresence of any protozoan belonging to thePlasmodium genus.Plasmodium MeasurementC74619 PLSPCE PrecursorPlasma Cells;PlasmablastA measurement of the precursor (blast stage) plasmacells (antibody secreting cells derived from B cells viaantigen stimulation) in a biological specimen.Precursor Plasma Cell CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 193 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74650 PLSPCELY PrecursorPlasmaCells/LymphocytesC80201 PNCTPP PancreaticPolypeptideC7<strong>12</strong>51 PO2 Partial PressureOxygenA relative measurement (ratio or percentage) of theprecursor (blast stage) plasma cells (antibodysecreting cells derived from B cells via antigenstimulation) to all lymphocytes in a biologicalspecimen.A measurement of the pancreatic polypeptide in abiological specimen.A measurement of the pressure of oxygen in abiological specimen.Precursor Plasma Cell toLymphocyte Ratio MeasurementPancreatic PolypeptideMeasurementPartial Pressure of OxygenMeasurementC79602 POIKILO Poikilocytes A measurement of the odd-shaped erythrocytes in awhole blood specimen.Poikilocyte MeasurementC74649 POIKRBC Poikilocytes/ErythrocytesA relative measurement (ratio or percentage) of thepoikilocytes, or irregularly shaped erythrocytes, to allerythrocytes in a biological specimen.Poikilocyte to Erythrocyte RatioMeasurementC64803 POLYCHR Polychromasia A measurement of the blue-staining characteristic ofnewly generated erythrocytes.C100435 PREALB Prealbumin A measurement of the prealbumin in a biologicalspecimen.PolychromasiaPrealbumin MeasurementC64829 PRLYMLE Prolymphocytes/LeukocytesA relative measurement (ratio or percentage) ofprolymphocytes to leukocytes in a biologicalspecimen.Prolymphocyte To Leukocyte RatioC74791 PROGEST Progesterone A measurement of the progesterone hormone in abiological specimen.C81967 PROINSUL Proinsulin A measurement of the proinsulin in a biologicalspecimen.C74870 PROLCTN Prolactin A measurement of the prolactin hormone in abiological specimen.C74620 PROLYM Prolymphocytes A measurement of the prolymphocytes in a biologicalspecimen.Progesterone MeasurementProinsulin MeasurementProlactin MeasurementProlymphocyte CountC74651 PROLYMLY Prolymphocytes/LymphocytesC74652 PROMONLE Promonocytes/LeukocytesA relative measurement (ratio or percentage) of theprolymphocytes to all lymphocytes in a biologicalspecimen.A relative measurement (ratio or percentage) of thepromonocytes to all leukocytes in a biologicalspecimen.Prolymphocyte to Lymphocyte RatioMeasurementPromonocyte to Lymphocyte RatioMeasurementC74621 PROMONO Promonocytes A measurement of the promonocytes in a biologicalspecimen.C74622 PROMY Promyelocytes A measurement of the promyelocytes (immaturemyelocytes) in a biological specimen.Promonocyte CountPromyelocyte CountC98773 PROMYCE Promyelocytes/Total CellsC74653 PROMYLE Promyelocytes/LeukocytesA relative measurement (ratio or percentage) of thepromyelocytes (immature myelocytes) to total cells ina biological specimen (for example a bone marrowspecimen).A relative measurement (ratio or percentage) of thepromyelocytes (immature myelocytes) to allleukocytes in a biological specimen.Promyelocyte to Total Cell RatioMeasurementPromyelocyte to Lymphocyte RatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 194 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74885 PROPOX Propoxyphene A measurement of the propoxyphene present in abiological specimen.C64858 PROT Protein A measurement of a group of complex organicmacromolecules composed of one or morealpha-L-amino acid chains in a biological specimen.Propoxyphene MeasurementTotal Protein MeasurementC79463 PROTCRT Protein/CreatinineC92240 PROTOSML Protein/Osmolality;Protein/Osmolality RatioA relative measurement (ratio or percentage) of theprotein to creatinine in a biological specimen.A relative measurement (ratio or percentage) of totalproteins to the osmolality of a biological specimen.Protein to Creatinine RatioMeasurementProtein to Osmolality RatioMeasurementC100436 PROTS Protein S A measurement of the protein S in a biologicalspecimen.Protein S MeasurementC17634 PSA Prostate SpecificAntigenA measurement of the prostate specific antigen in abiological specimen.Prostate Specific AntigenMeasurementC74696 PSDEPHD Pseudoephedrine A measurement of the pseudoephedrine present in abiological specimen.Pseudoephedrine MeasurementC62656 PT ProthrombinTimeC98774 PTA ProthrombinActivity; FactorII ActivityC82034 PTF1_2 ProthrombinFragments 1 + 2C81964 PTHCT ParathyroidHormone,C-TerminalC74784 PTHFG ParathyroidHormone,FragmentedC74789 PTHI ParathyroidHormone, IntactC81965 PTHMM ParathyroidHormone,Mid-MoleculeC81966 PTHNT ParathyroidHormone,N-TerminalC103451 PTHW ParathyroidHormone, WholeA blood clotting measurement that evaluates theextrinsic pathway of coagulation.A measurement of the biological activity ofprothrombin in a biological specimen.A measurement of the prothrombin fragments 1 and 2in a biological specimen.A measurement of the C-terminal fragment ofparathyroid hormone in a biological specimen.A measurement of the fragmented parathyroidhormone in a biological specimen.A measurement of the intact parathyroid hormone(consisting of amino acids 1-84 or 7-84) in abiological specimen.A measurement of the mid-molecule fragment ofparathyroid hormone in a biological specimen.A measurement of the N-terminal fragment ofparathyroid hormone in a biological specimen.A measurement of the whole parathyroid hormone(consisting of amino acids 1-84) in a biologicalspecimen.Prothrombin TimeProthrombin Activity MeasurementProthrombin Fragments 1 and 2MeasurementC-Terminal Parathyroid HormoneMeasurementFragmented Parathyroid HormoneMeasurementIntact Parathyroid HormoneMeasurementMid-Molecule Parathyroid HormoneMeasurementN-Terminal Parathyroid HormoneMeasurementWhole Parathyroid HormoneMeasurementC80211 PYRIDNLN Pyridinoline A measurement of the pyridinoline in a biologicalspecimen.Pyridinoline MeasurementC80202 PYY Peptide YY;Peptide TyrosineTyrosineA measurement of the peptide YY in a biologicalspecimen.Peptide YY MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 195 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC81957 RANTES Reg upon ActNormal T-cellExprd Secrtd;ChemokineLigand 5C51946 RBC Erythrocytes;Red Blood CellsC92245 RBCCLMP Erythrocyte CellClumps; RedBlood CellClumps; RBCClumpsC96605 RBCGHOST ErythrocyteGhosts; RBCGhostsC92296 RBCMORPH Erythrocyte CellMorphology;Red Blood CellMorphology;RBCMorphologyC74705 RBCNUC NucleatedErythrocytes;Nucleated RedBlood CellsC82046 RBCNUCLE NucleatedErythrocytes/LeukocytesC74647 RBCNURBC NucleatedErythrocytes/Erythrocytes;Nucleated RedBloodCells/ErythrocytesC100437 RBP Retinol BindingProteinC64800 RDW ErythrocytesDistributionWidthC74893 RENIN Renin;AngiotensinogenaseA measurement of the RANTES (regulated onactivation, normally, T-cell expressed, and secreted)chemokine in a biological specimen.A measurement of the total erythrocytes in abiological specimen.A measurement of red blood cell clumps in abiological specimen.A measurement of the erythrocyte ghosts(erythrocytes in which hemoglobin has been removedthrough hemolysis) in a biological specimen.An examination or assessment of the form andstructure of red blood cells.A measurement of the nucleated erythrocytes (large,immature nucleated erythrocytes) in a biologicalspecimen.A relative measurement (ratio or percentage) ofnucleated erythrocytes to leukocytes in a biologicalspecimen.A relative measurement (ratio or percentage) of thenucleated erythrocytes (large, immature nucleatederythrocytes) to all erythrocytes in a biologicalspecimen.A measurement of the total retinol binding protein in abiological specimen.A value derived from mean corpuscular volume and thestandard deviation of the red blood cell volume in awhole blood specimen.A measurement of the renin in a biological specimen.Reg upon Act Normal T-cell ExprdSecrtd MeasurementErythrocyte CountErythrocyte Cell ClumpsMeasurementErythrocyte Ghost CountErythrocyte Cell MorphologyNucleated Red Blood Cell CountNucleated Erythrocyte to LeukocyteRatio MeasurementNucleated Red Blood Cell toErythrocyte Ratio MeasurementRetinol Binding ProteinMeasurementErythrocyte Distribution WidthMeasurementRenin MeasurementC80205 RESISTIN Resistin A measurement of the resistin in a biologicalspecimen.Resistin MeasurementC102274 RETCRRBC HCT CorrectedReticulocytes/ErythrocytesA relative measurement (ratio or percentage) of thehematocrit corrected reticulocytes to erythrocytes in abiological specimen.Hematocrit Corrected Reticulocytesto Erythrocytes Ratio MeasurementC51947 RETI Reticulocytes A measurement of the reticulocytes in a biologicalspecimen.Reticulocyte CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 196 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102273 RETIHCR HematocritCorrectedReticulocytesC98776 RETIHGB ReticulocyteHemoglobinC64828 RETIRBC Reticulocytes/ErythrocytesC74717 RF RheumatoidFactorC100457 RNPAB Ribonucleoprotein Antibody;RNP AntibodyC74624 ROULEAUX RouleauxFormationC74716 RPR Rapid PlasmaReaginA measurement of the hematocrit correctedreticulocytes in a biological specimen.A measurement of the amount of hemoglobin inreticulocytes.A relative measurement (ratio or percentage) ofreticulocytes to erythrocytes in a biological specimen.A measurement of the rheumatoid factor antibody in abiological specimen.A measurement of the ribonucleoprotein antibody in abiological specimen.A measurement of the number of stacking red bloodcells within a biological specimen.A measurement of the antibodies produced by cellulardamage caused by Treponema pallidum (syphilis) in abiological specimen.Hematocrit Corrected ReticulocyteCountReticulocyte HemoglobinMeasurementReticulocyte To Erythrocyte RatioRheumatoid Factor MeasurementRibonucleoprotein AntibodyMeasurementRouleaux Formation CountRapid Plasma Reagin MeasurementC96628 RPTLTIME Reptilase Time A measurement of the time it takes a plasma sample toclot after adding the active enzyme reptilase.Reptilase Time MeasurementC81968 RT3 Triiodothyronine, ReverseC103440 RUBIGGAB Rubella IgGAntibodyC82035 SCF Stem CellFactor; KITLigandA measurement of the reverse triiodothyronine in abiological specimen.A measurement of the Rubella IgG antibody in abiological specimen.A measurement of the stem cell factor in a biologicalspecimen.Reverse TriiodothyronineMeasurementRubella IgG Antibody MeasurementStem Cell Factor MeasurementC74706 SCHISTO Schistocytes A measurement of the schistocytes (fragmented redblood cells) in a biological specimen.Schistocyte CountC74656 SCKCERBC SickleCells/ErythrocytesA relative measurement (ratio or percentage) of thesickle cells (sickle shaped red blood cells) to allerythrocytes in a biological specimen.Sickle Cell to Erythrocyte RatioMeasurementC74626 SCKLCE Sickle Cells A measurement of the sickle cells (sickle shaped redblood cells) in a biological specimen.Sickle Cell CountC100458 SCL70AB Scl-70 Antibody;Scleroderma-70AntibodyC79465 SDH SorbitolDehydrogenaseA measurement of the Scl-70 antibody in a biologicalspecimen.A measurement of the sorbitol dehydrogenase in abiological specimen.Scl-70 Antibody MeasurementSorbitol DehydrogenaseMeasurementC74871 SECRETIN Secretin A measurement of the secretin hormone in abiological specimen.C74625 SEZCE Sezary Cells A measurement of the Sezary cells (atypicallymphocytes with cerebriform nuclei) in a biologicalspecimen.Secretin MeasurementSezary Cell CountC74655 SEZCELY SezaryCells/LymphocytesA relative measurement (ratio or percentage of theSezary cells (atypical lymphocytes with cerebriformnuclei) to all lymphocytes in a biological specimen.Sezary Cell to Lymphocyte RatioMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 197 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74745 SHBG Sex HormoneBindingGlobulin; SexHormoneBinding ProteinC74876 SIXMAM 6-MonoacetylmorphineC92236 SJSSAAB Ro Antibody;Sjogrens SS-AAntibodyC92237 SJSSBAB La Antibody;Sjogrens SS-BAntibodyC100438 SLTFRNRC SolubleTransferrinReceptorC74627 SMDGCE Smudge Cells;Basket CellsA measurement of the sex hormone binding (globulin)protein in a biological specimen.A measurement of the 6-monoacetylmorphine presentin a biological specimen.A measurement of the Sjogrens SS-A antibody in abiological specimen.A measurement of the Sjogrens SS-B antibody in abiological specimen.A measurement of the soluble transferrin receptor in abiological specimen.A measurement of the smudge cells (the nuclearremnant of a ruptured white blood cell) in a biologicalspecimen.Sex Hormone Binding ProteinMeasurement6-MonoacetylmorphineMeasurementSjogren's SS-A AntibodyMeasurementSjogren's SS-B AntibodyMeasurementSoluble Transferrin ReceptorMeasurementSmudge Cell CountC92281 SMTHAB Smith Antibody A measurement of the Smith antibody in a biologicalspecimen.C64809 SODIUM Sodium A measurement of the sodium in a biologicalspecimen.Smith Antibody MeasurementSodium MeasurementC80360 SOMATRO Somatotrophin;GrowthHormoneA measurement of the somatotrophin (growth)hormone in a biological specimen.Somatotropin MeasurementC74663 SPERM Spermatozoa A measurement of the spermatozoa cells present in abiological specimen.C102281 SPERMMTL Sperm Motility A measurement of the sperm capable of forward,progressive movement in a semen specimen.Spermatozoa Cell CountSperm Motility MeasurementC64832 SPGRAV Specific Gravity A ratio of the density of a fluid to the density of water. Specific GravityC74707 SPHERO Spherocytes A measurement of the spherocytes (small,sphere-shaped red blood cells) in a biologicalspecimen.C74872 SRTONIN Serotonin A measurement of the serotonin hormone in abiological specimen.Spherocyte CountSerotonin MeasurementC96567 STIPBASO BasophilicStipplingA measurement of the basophilic stippling in abiological specimen.Basophilic Stippling MeasurementC74708 STOMCY Stomatocytes A measurement of the stomatocytes (red blood cellswith an oval or rectangular area of central pallor,producing the appearance of a cell mouth) in abiological specimen.Stomatocyte CountC92282 STRPLOAB Streptolysin OAntibody;AntistreptolysinOA measurement of Streptolysin O Antibody in abiological specimen.Streptolysin O AntibodyMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 198 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92533 SVCAM1 Soluble VascularCell AdhesionMolecule 1C74747 T3 Triiodothyronine; Total T3C74787 T3FR Triiodothyronine, Free; Free T3C74748 T3UP TriiodothyronineUptakeC74794 T4 Thyroxine; TotalT4C74786 T4FR Thyroxine, Free;Free T4C74746 TBG ThyroxineBinding GlobulinC64801 TEARDCY Dacryocytes;Tear ShapedErythrocytes;Teardrop CellsC74793 TESTOS Testosterone;TotalTestosteroneC74785 TESTOSFR Testosterone,FreeA measurement of the soluble vascular cell adhesionmolecule 1 in a biological specimen.A measurement of the total (free and bound)triiodothyronine in a biological specimen.A measurement of the free triiodothyronine in abiological specimen.A measurement of the binding of triiodothyonine tothyroxine binding globulin protein in a biologicalspecimen.A measurement of the total (free and bound) thyroxinein a biological specimen.A measurement of the free thyroxine in a biologicalspecimen.A measurement of the thyroxine binding globulinprotein in a biological specimen.A measurement of dacryocytes in a biologicalspecimen.A measurement of the total (free and bound)testosterone in a biological specimen.A measurement of the free testosterone in a biologicalspecimen.Soluble Vascular Cell AdhesionMolecule 1Triiodothyronine MeasurementFree Triiodothyronine MeasurementTriiodothyronine UptakeMeasurementTotal Thyroxine MeasurementFree Thyroxine MeasurementThyroxine Binding Globulin ProteinMeasurementDacryocyte AnalysisTotal Testosterone MeasurementFree Testosterone MeasurementC82037 TFERRIN Transferrin A measurement of the transferrin in a biologicalspecimen.Transferrin MeasurementC98792 TFRRNSAT TransferrinSaturationA measurement of the iron bound to transferrin in abiological specimen.Transferrin Saturation MeasurementC103446 TGLOB TG A measurement of the thyroglobulin in a biologicalspecimen.C74873 THRMPTN Thrombopoietin A measurement of the thrombopoietin hormone in abiological specimen.Thyroglobulin MeasurementThrombopoietin MeasurementC81990 THYAB ThyroidAntibodiesC81991 THYAMAB ThyroidAntimicrosomalAntibodiesC81992 THYATAB ThyroidAntithyroglobulin AntibodiesC96639 THYPXD Thyroperoxidase;ThyroidPeroxidaseC96638 THYPXDAB ThyroperoxidaseAntibodyA measurement of the thyroid antibodies in abiological specimen.A measurement of the thyroid antimicrosomalantibodies in a biological specimen.A measurement of the thyroid antithyroglobulinantibodies in a biological specimen.A measurement of the thyroperoxidase in a biologicalspecimen.A measurement of the thyroperoxidase antibody in abiological specimen.Thyroid Antibody MeasurementThyroid Antimicrosomal AntibodyMeasurementThyroid Antithyroglobulin AntibodyMeasurementThyroperoxidase MeasurementThyroperoxidase AntibodyMeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 199 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC82036 TIMP1 Tissue InhibitorofMetalloproteinase 1C74751 TNF Tumor NecrosisFactor; TumorNecrosis FactoralphaC96641 TOXGRAN ToxicGranulationC81993 TPAAG TissuePlasminogenActivatorAntigenC84811 TPRONP Non-Phosphorylated Tau ProteinC84810 TPROT Tau Protein;Total Tau ProteinC848<strong>12</strong> TPROTP PhosphorylatedTau ProteinC80208 TRAP TotalRadical-TrapAntioxidantPotentialC100420 TRCYANDP TricyclicAntidepressantsC96636 TRGTCE Leptocytes;Target Cells;Codocytes;Mexican HatCellsC74874 TRH ThyrotropinReleasingHormone;ThyrotropinReleasing FactorA measurement of the tissue inhibitor ofmetalloproteinase 1 in a biological specimen.A measurement of the total tumor necrosis factor(cachexin) cytokine in a biological specimen.A measurement of the toxic granulation in neutrophilicblood cells.A measurement of the tissue plasminogen activatorantigen in a biological specimen.A measurement of the non-phosphorylated Tau proteinin a biological specimen.A measurement of the total Tau protein in a biologicalspecimen.A measurement of the phosphorylated Tau protein in abiological specimen.A measurement of the ability of the antioxidants in abiological specimen to buffer free radicals in asuspension.A measurement of tricyclic antidepressants in abiological specimen.A measurement of the target cells in a biologicalspecimen.A measurement of the thyrotropin releasing hormonein a biological specimen.Tissue Inhibitor ofMetalloproteinase 1 MeasurementTumor Necrosis FactorMeasurementToxic Granulation MeasurementTissue Plasminogen ActivatorAntigen MeasurementNonphosphorylated Tau ProteinMeasurementTau Protein MeasurementPhosphorylated Tau ProteinMeasurementTotal Radical-Trap AntioxidantPotential MeasurementTricyclic AntidepressantMeasurementTarget Cell CountThyrotropin Releasing HormoneMeasurementC92238 TRICH Trichomonas Examination of a biological specimen to detect thepresence of any protozoan belonging to theTrichomonas genus.C648<strong>12</strong> TRIG Triglycerides A measurement of the triglycerides in a biologicalspecimen.C74749 TROPONI Troponin I A measurement of the actin binding troponin in abiological specimen.C74750 TROPONT Troponin T A measurement of the tropomyosin binding troponin ina biological specimen.C92292 TRYPTASE Tryptase A measurement of the tryptase in a biologicalspecimen.Trichomonas ScreeningTriglyceride MeasurementTroponin I MeasurementTroponin T MeasurementTryptase MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 200 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64813 TSH Thyrotropin A measurement of the thyrotropin in a biologicalspecimen.C80365 TT Thrombin Time A measurement of the time it takes a plasma sample toclot after adding the active enzyme thrombin. (NCI)C74723 TURB Turbidity A measurement of the opacity of a biologicalspecimen.C103445 TXB2 Thromboxane B2 A measurement of the thromboxane B2 in a biologicalspecimen.Thyrotropin MeasurementThrombin TimeTurbidity MeasurementThromboxane B2 MeasurementC103344 TXB2_D11 11-Dehydro-Thromboxane B2A measurement of the 11-dehydro-thromboxane B2 ina biological specimen.11-Dehydro-Thromboxane B2MeasurementC64814 URATE Urate; Uric Acid A measurement of the urate in a biological specimen. Urate MeasurementC64815 UREA Urea A measurement of the urea in a biological specimen. Urea MeasurementC96645 UREACRT Urea/Creatinine A relative measurement (ratio or percentage) of theurea to creatinine in a biological specimen.C64816 UROBIL Urobilinogen A measurement of the urobilinogen in a biologicalspecimen.Urea to Creatinine RatioMeasurementUrobilinogen MeasurementC82042 VCAM1 Vascular CellAdhesionMolecule 1C92514 VEGF VascularEndothelialGrowth FactorA measurement of the vascular cell adhesion molecule1 in a biological specimen.A measurement of the vascular endothelial growthfactor in a biological specimen.Vascular Cell Adhesion Molecule 1MeasurementVascular Endohelial Growth FactorMeasurementC759<strong>12</strong> VISC Viscosity The resistance of a liquid to sheer forces and flow.(NCI)ViscosityC74895 VITA Vitamin A;RetinolC74896 VITB1 Thiamine;Vitamin B1A measurement of the Vitamin A in a biologicalspecimen.A measurement of the thiamine in a biologicalspecimen.Vitamin A MeasurementVitamin B1 MeasurementC64817 VITB<strong>12</strong> Vitamin B<strong>12</strong> A measurement of the Vitamin B<strong>12</strong> in a serumspecimen.Vitamin B<strong>12</strong> MeasurementC74897 VITB17 Vitamin B17;AmygdalinC74898 VITB2 Riboflavin;Vitamin B2C74899 VITB3 Niacin; VitaminB3C74900 VITB5 Vitamin B5;Pantothenic AcidC74901 VITB6 Vitamin B6;PyridoxineC74902 VITB7 Vitamin B7;BiotinC74676 VITB9 Folic Acid;Vitamin B9A measurement of the Vitamin B17 in a biologicalspecimen.A measurement of the riboflavin in a biologicalspecimen.A measurement of the niacin in a biological specimen.A measurement of the Vitamin B5 in a biologicalspecimen.A measurement of the Vitamin B6 in a biologicalspecimen.A measurement of the Vitamin B7 in a biologicalspecimen.A measurement of the folic acid in a biologicalspecimen.Vitamin B17 MeasurementVitamin B2 MeasurementVitamin B3 MeasurementVitamin B5 MeasurementVitamin B6 MeasurementVitamin B7 MeasurementFolic Acid MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 201 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC74903 VITC Vitamin C;Ascorbic AcidC84818 VITD Vitamin D; TotalVitamin DC74904 VITD2 Vitamin D2;ErgocalciferolC74905 VITD3 Vitamin D3;CholecalciferolC92267 VITDAT 1,25-Dihydroxyvitamin D; ActiveVitamin DC92268 VITDIT 25-Hydroxyvitamin D; InactiveVitamin DA measurement of the Vitamin C in a biologicalspecimen.A measurement of the total Vitamin D in a biologicalspecimen.A measurement of the Vitamin D2 in a biologicalspecimen.A measurement of the Vitamin D3 in a biologicalspecimen.A measurement of the total active Vitamin D in abiological specimen.A measurement of the total inactive Vitamin D in abiological specimen.Vitamin C MeasurementVitamin D MeasurementVitamin D2 MeasurementVitamin D3 Measurement1, 25-Dihydroxyvitamin DMeasurement25-Hydroxyvitamin D MeasurementC74906 VITE Vitamin E A measurement of the Vitamin E in a biologicalspecimen.Vitamin E MeasurementC103448 VITECHOL VitaminE/CholesterolC74907 VITK Vitamin K;NaphthoquinoneC103449 VITK1 Phylloquinone;PhytomenadioneC64806 VLDL VLDLCholesterolC103450 VLDLPSZ VLDL ParticleSizeC74875 VMA VanillylMandelic Acid;VanilmandelicAcidA relative measurement (ratio or percentage) ofvitamin E to total cholesterol in a biological specimen.A measurement of the total Vitamin K in a biologicalspecimen.A measurement of the Vitamin K1 in a biologicalspecimen.A measurement of the very low density lipoproteincholesterol in a biological specimen.A measurement of the average particle size ofvery-low-density lipoprotein in a biological specimen.A measurement of the vanillyl mandelic acidmetabolite in a biological specimen.Vitamin E to Cholesterol RatioMeasurementVitamin K MeasurementVitamin K1 MeasurementSerum VLDL CholesterolMeasurementVLDL Particle Size MeasurementVanillyl Mandelic AcidMeasurementC74720 VOLUME Volume A measurement of the volume of a biologicalspecimen.Volume MeasurementC98795 VZVIGAAB Varicella ZosterVirus IgAAntibodyC98796 VZVIGGAB Varicella ZosterVirus IgGAntibodyC98797 VZVIGMAB Varicella ZosterVirus IgMAntibodyC51948 WBC Leukocytes;White BloodCellsA measurement of the Varicella Zoster virus IgAantibodies in a biological specimen.A measurement of the Varicella Zoster virus IgGantibodies in a biological specimen.A measurement of the Varicella Zoster virus IgMantibodies in a biological specimen.A measurement of the leukocytes in a biologicalspecimen.Varicella Zoster Virus IgA AntibodyMeasurementVaricella Zoster Virus IgG AntibodyMeasurementVaricella Zoster Virus IgM AntibodyMeasurementLeukocyte CountSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 202 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C65047 - LBTESTCD - Laboratory Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92246 WBCCLMP Leukocyte CellClumps; WhiteBlood CellClumps; WBCClumpsC92297 WBCMORPH Leukocyte CellMorphology;White BloodCellMorphology;WBCMorphologyA measurement of white blood cell clumps in abiological specimen.An examination or assessment of the form andstructure of white blood cells.Leukocyte Cell ClumpsMeasurementLeukocyte Cell MorphologyC74664 YEAST Yeast Cells A measurement of the yeast cells present in abiological specimen.C92239 YEASTHYP Yeast Hyphae Examination of a biological specimen to detect thepresence of any yeast cells that are in the long,filamentous and branching phase of growth.Yeast Cell MeasurementYeast Hyphae ScreeningC80210 ZINC Zinc A measurement of the zinc in a biological specimen. Zinc MeasurementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 203 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89971 - MATEST - Macroscopic Findings Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16033 Clinical Signs Follow-up Clinical SignsFollow-upThe process by which information about the healthstatus of a subject is obtained after the subject is nolonger receiving study medication.Follow-upC90390Gross PathologicalExaminationGrossPathologicalExaminationAn assessment of macroscopic pathological findings.Gross Pathological ExaminationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 204 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89972 - MATESTCD - Macroscopic Findings Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16033 CLSFUP Clinical SignsFollow-upC90390 GROSPATH GrossPathologicalExaminationThe process by which information about the healthstatus of a subject is obtained after the subject is nolonger receiving study medication.An assessment of macroscopic pathological findings.Follow-upGross Pathological ExaminationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 205 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90017 - MIRESCAT - Microscopic Histopathology Result CategoryCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC14172 BENIGN Benign For neoplasms, a non-infiltrating andnon-metastasizing neoplastic process that ischaracterized by the absence of morphologic featuresassociated with malignancy (e.g., severe atypia, nuclearpleomorphism, tumor cell necrosis, and abnormalmitoses). For other conditions, a process that is mildin nature and not dangerous to health. (NCI)C14143 MALIGNANT Malignant Refers to abnormal cell activity manifested bydecreased control over growth and function, causingtumor growth or spread into surrounding tissue andadverse effects to the host. (NCI)C14174 METASTATIC Metastatic A term referring to the pathologic observation of atumor extension or migration from its original site ofgrowth to another non-adjacent site.BenignMalignantMetastaticC53529 NON-NEOPLASTIC Non-neoplasticDisorderAny disorder other than abnormal tissue growthresulting from uncontrolled cell proliferation. (NCI)Non-Neoplastic DisorderC89084 UNDETERMINED Undetermined A term referring to the lack of definitive clinical orpathologic criteria for a tumor to predict its clinicalcourse or classify it as benign or malignant.UndeterminedSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 206 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89973 - MITEST - Microscopic Findings Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90424 Microscopic Examination MicroscopicExaminationAn assessment by microscope (e.g., light, electron,confocal, etc.).Microscopic ExaminationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 207 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89974 - MITESTCD - Microscopic Findings Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90424 MIEXAM MicroscopicExaminationAn assessment by microscope (e.g., light, electron,confocal, etc.).Microscopic ExaminationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 208 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89975 - MTHTRM - Method of TerminationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90355ANESTHETIZED CERVICALDISLOCATIONA method of sacrifice whereby a subject isanesthetized and the spinal column is dislocated fromthe skull and brain.Anesthesia and Cervical DislocationEuthanasiaC90356ANESTHETIZEDDECAPITATIONA method of sacrifice whereby a subject isanesthetized and the head is removed from the body.Anesthesia and DecapitationEuthanasiaC90357ANESTHETIZEDEXSANGUINATIONA method of sacrifice whereby a subject isanesthetized and the body is drained of blood.Anesthesia and ExsanguinationEuthanasiaC90358ANESTHETIZEDINTRAVENOUS KCLA method of sacrifice whereby a subject isanesthetized and potassium chloride is administeredintravenously to induce cardiac arrest.Anesthesia and IntravenousPotassium Chloride EuthanasiaC90400BARBITURATE OVERDOSEINTRAPERITONEALA method of sacrifice whereby a subject isanesthetized and barbiturate(s) are administeredintraperitoneally to induce respiratory arrest.Intraperitoneal Barbiturate OverdoseEuthanasiaC90401BARBITURATE OVERDOSEINTRAVENOUSA method of sacrifice whereby a subject isanesthetized and barbiturate(s) are administeredintravenously to induce respiratory arrest.Intravenous Barbiturate OverdoseEuthanasiaC90371 CERVICAL DISLOCATION A method of sacrifice whereby the spinal column isdislocated from the skull and brain.C90369 CO2 A method of sacrifice whereby a subject inhalescarbon dioxide until death occurs.C90375 DECAPITATION A method of sacrifice whereby a subject's head isremoved from the body.Cervical DislocationCarbon Dioxide EuthanasiaDecapitationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 209 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66789 - ND - Not DoneCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49484 NOT DONE Indicates a task, process or examination that has eithernot been initiated or completed. (NCI)Not DoneSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 210 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC7644ADAMANTINOMA,MALIGNANTADAMANTINOMAA low-grade malignant neoplasm composed ofepithelial cells and a spindle cell osteo-fibrousproliferation.AdamantinomaC4200ADENOACANTHOMA,MALIGNANTADENOACANTHOMAA malignant neoplasm arising from glandular cells thatincludes focal or extensive areas of squamousmetaplasia.Adenocarcinoma with SquamousMetaplasiaC3766ADENOCARCINOMA,CLEAR CELL, MALIGNANTCLEAR CELLCARCINOMA;MESONEPHROID CLEAR CELLADENOCARCINOMA;MESONEPHROID CLEAR CELLCARCINOMAA malignant neoplasm comprising glandular epithelialclear cells.Clear Cell AdenocarcinomaC7359ADENOCARCINOMA,ENDOMETRIAL,MALIGNANTADENOCARCINOMA OFENDOMETRIUM;ADENOCARCINOMA OF THEENDOMETRIUMA malignant glandular neoplasm of the uterine lining.Endometrial AdenocarcinomaC2852ADENOCARCINOMA,MALIGNANTA malignant neoplasm arising from glandular cells.AdenocarcinomaC267<strong>12</strong>ADENOCARCINOMA,MUCINOUS, MALIGNANTGELATINOUSADENOCARCINOMA; MUCOIDADENOCARCINOMA;MUCINOUSCARCINOMA;MUCOUSADENOCARCINOMA; COLLOIDADENOCARCINOMA; COLLOIDCARCINOMA;MUCOIDCARCINOMA;MUCOUSCARCINOMA;GELATINOUSCARCINOMAAn adenocarcinoma comprising neoplastic glandularcells containing intracytoplasmic mucin.Mucinous AdenocarcinomaC2853ADENOCARCINOMA,PAPILLARY, MALIGNANTAn adenocarcinoma with papillary growth pattern.Papillary AdenocarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 211 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC40310ADENOCARCINOMA,SEBACEOUS, MALIGNANTCARCINOMAOFSEBACEOUSGLAND;CARCINOMAOF THESEBACEOUSGLAND;SEBACEOUSGLANDCARCINOMAAn adenocarcinoma with sebaceous differentiation.Sebaceous CarcinomaC8984 ADENOFIBROMA, BENIGN BENIGNMIXEDMUELLERIANTUMORBenign mixed neoplasm comprised ofepithelial/glandular and mesenchymal structures.AdenofibromaC4159 ADENOLIPOMA, BENIGN Benign mixed neoplasm comprised ofepithelial/glandular and lipomatous structures.LipoadenomaC4196ADENOMA, ACINAR CELL,BENIGNACINIC CELLADENOMA;ACINARADENOMAA benign glandular epithelial neoplasm comprisingsecretory cells forming acinar patterns.Acinar Cell AdenomaC7580ADENOMA, ADNEXAL,BENIGNADENOMA OFADNEXA;ADENOMA OFSKINAPPENDAGE;ADNEXALADENOMAA benign glandular epithelial neoplasm comprisingsecretory cells forming acinar patterns.Skin Appendage AdenomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 2<strong>12</strong> of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC9003ADENOMA,ADRENOCORTICAL ,BENIGNCORTICALCELLADENOMA;ADRENALCORTICALADENOMA;ADRENOCORTICALADENOMA;BENIGNADRENALADENOMA;BENIGNADENOMA OFTHE ADRENALGLAND;ADENOMA OFTHE ADRENALGLAND;ADRENALADENOMA;ADENOMA OFADRENALGLAND;ADENOMA OFTHE ADRENALCORTEX;BENIGNADRENALGLANDADENOMA;BENIGNADENOMA OFADRENALGLAND;ADENOMA OFADRENALCORTEX;ADRENALGLANDADENOMAA benign neoplasm arising from any of the adrenalcortical layers.Adrenal Cortex AdenomaC3329ADENOMA, ANTERIORLOBE PITUITARY GLAND,BENIGNADENOMA OFPITUITARYGLAND;ADENOMA OFPITUITARY;ADENOMA OFTHE PITUITARYGLAND;ADENOMA OFTHEPITUITARY;PITUITARYADENOMAA benign neoplasm of the anterior lobe of the pituitarygland.Pituitary Gland AdenomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 213 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC5950ADENOMA, BASAL CELL,BENIGNBASAL CELLADENOMA OFSALIVARYGLAND; BASALCELLADENOMA OFTHE SALIVARYGLAND; BASALCELLADENOMA;BENIGN BASALCELL TUMORA benign epithelial neoplasm characterized by thepresence of/arising from basal cells.Salivary Gland Basal Cell AdenomaC2855 ADENOMA, BENIGN A benign neoplasm arising from epithelium. AdenomaC3494ADENOMA, BRONCHIAL,BENIGNA benign neoplasia of the lung, arising from bronchialepithelium.Lung Papillary AdenomaC4140ADENOMA,BRONCHIOLOALVEOLAR,BENIGNADENOMA OFTHE ALVEOLI;ADENOMA OFALVEOLIA benign lung neoplasm arising from thealveolar/bronchiolar epithelium.Alveolar AdenomaC46101ADENOMA, C-CELL,BENIGNPARAFOLLICULAR CELLADENOMAA benign neoplasm arising from C-cells of the thyroidgland.Neoplastic C-Cell HyperplasiaC6088ADENOMA, CERUMINOUSGLAND, BENIGNCERUMINOUSADENOMA;CERUMINOUSADENOMA OFEXTERNALAUDITORYCANAL;CERUMINOUSADENOMA OFTHEEXTERNALAUDITORYCANAL;CERUMINOMAA benign epithelial neoplasm derived from ceruminousglands in the external auditory canal.External Auditory Canal CeruminousAdenomaC4151ADENOMA, CLEAR CELL,BENIGNA benign neoplasm comprising glands containingepithelial clear cells.Clear Cell AdenomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 214 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3502ADENOMA, FOLLICULARCELL, BENIGNADENOMA OFTHE THYROIDGLAND;THYROIDADENOMA;FOLLICULARADENOMA OFTHE THYROID;THYROIDFOLLICULARADENOMA;ADENOMA OFTHYROID;ADENOMA OFTHYROIDGLAND;FOLLICULARADENOMA OFTHE THYROIDGLAND;FOLLICULARADENOMA OFTHYROIDGLAND;ADENOMA OFTHE THYROID;FOLLICULARADENOMA OFTHYROID;THYROIDGLANDADENOMA;FOLLICULARADENOMAA benign neoplasm arising from follicular cells of thethyroid gland.Thyroid Gland Follicular AdenomaC3758ADENOMA,HEPATOCELLULAR,BENIGNADENOMA OFTHE LIVERCELLS; LIVERCELLADENOMA;HCA;ADENOMA OFLIVER CELLSA benign epithelial neoplasm arising from hepatocytes.Hepatocellular AdenomaC7<strong>12</strong>6ADENOMA,HEPATOCHOLANGIOCELLULAR, BENIGNA benign neoplasm arising from the intrahepatic bileduct.Intrahepatic Bile Duct AdenomaC65184ADENOMA, ISLET CELL,BENIGNISLET CELLADENOMAA benign neoplasm arising from the islet cells of thepancreas.Islet Cell AdenomaC46119ADENOMA, LIGHT CELL,BENIGNA benign epithelial neoplasm of the thyroid glandcomprising follicular cells with cytoplasmic clearing.Thyroid Gland Clear Cell FollicularAdenomaC2973ADENOMA, MUCINOUS,BENIGNPSEUDOMUCINOUSCYSTADENOMA; MUCINOUSCYSTOMA;MUCINOUSADENOMAA benign, cystic epithelial neoplasm comprising cellscontaining intracytoplasmic mucin.Mucinous CystadenomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 215 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC79951ADENOMA, PAPILLARY,BENIGNA benign epithelial neoplasm characterized by thepresence of papillary epithelial patterns.Papillary AdenomaC60490ADENOMA, PARSDISTALIS, BENIGNRat Pars DistalisAdenomaA benign epithelial neoplasm arising from the parsdistalis of the anterior pituitary gland.Rat Pars Distalis AdenomaC60493ADENOMA, PARSINTERMEDIA, BENIGNRat ParsIntermediaAdenomaA benign epithelial neoplasm arising from the parsintermedia of the anterior pituitary gland.Rat Pars Intermedia AdenomaC98723ADENOMA, PITUITARYGLAND, BENIGNA benign neoplasm of the pituitary gland.Experimental Organism PituitaryGland AdenomaC8383ADENOMA, RENAL CELL,BENIGNRENALTUBULEADENOMAA benign neoplasm arising from the renal cortex.Renal AdenomaC39956ADENOMA, RETE TESTIS,BENIGNA benign epithelial neoplasm arising from the retetestis.Rete Testis AdenomaC4174ADENOMA, SEBACEOUS,BENIGNSEBACEOUSGLANDADENOMA;ADENOMA OFTHESEBACEOUSGLAND;ADENOMA OFSEBACEOUSGLAND; SKINAPPENDAGESEBACEOUSADENOMAA benign neoplasm arising from sebaceous glands.Sebaceous AdenomaC92180ADENOMA, SERTOLIFORMTUBULAR, BENIGNRat SertoliformTubular AdenomaA benign tubular adenoma with Sertoli celldifferentiation.Rat Sertoliform Tubular AdenomaC7560ADENOMA, SWEATGLAND, BENIGNADENOMA OFTHE SWEATGLAND;ADENOMA OFSWEAT GLANDA benign epithelias neoplasm arising from sweatglands.Sweat Gland AdenomaC4133ADENOMA, TUBULARCELL, BENIGNA benign neoplasm arising from glandular epithelium,characterized by a tubular architectural pattern.Tubular AdenomaC79953ADENOMA,TUBULOSTROMAL,BENIGNA benign ovarian epithelial neoplasm characterized bythe presence of tubular structures and interstitialstroma.Tubulostromal AdenomaC84357ADENOMA, YOLK SAC,BENIGNA benign epithelial neoplasm arising from the yolk sac.Yolk Sac AdenomaC98800ADENOMA, ZYMBAL'SGLANDA benign neoplasm of the rodent Zymbal's gland withsebaceous and/or squamous differentiation.Zymbal's Gland AdenomaC3726 ADENOMYOMA, BENIGN A benign neoplasm characterized by the presence of aglandular and a mesenchymal component.AdenomyomaC83488ADRENAL TUMOR,SUBCAPSULAR, BENIGNSUBCAPSULARSINGLE CELLADENOMA,ADRENALA benign neoplasm located beneath the adrenalcapsule.Benign Subcapsular Adrenal TumorSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 216 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC83489ADRENAL TUMOR,SUBCAPSULAR,MALIGNANTSUBCAPSULARSINGLE CELLCARCINOMA,ADRENALA malignant neoplasm located beneath the adrenalcapsule.Malignant Subcapsular AdrenalTumorC82973ADRENAL TUMOR,SUBCAPSULAR,UNDETERMINEDSUBCAPSULARCELL TUMOR,ADRENALA neoplasm located beneath the adrenal capsule inwhich the malignancy status has not been established.Subcapsular Adrenal TumorC2858ADRENOCORTICALNEOPLASM,UNDETERMINEDADRENALCORTEXTUMOR;ADRENOCORTICALNEOPLASM;ADRENOCORTICAL TUMORA neoplasm of the adrenal cortex in which themalignancy status has not been established.Adrenal Cortex NeoplasmC7111AMELOBLASTOMA,BENIGNA benign odontogenic neoplasm arising from theepithelial component of the embryonic tooth.Benign AmeloblastomaC54297AMELOBLASTOMA,MALIGNANTMALIGNANTAMELOBLASTOMAA malignant odontogenic neoplasm arising from theepithelial component of the embryonic tooth.Metastasizing AmeloblastomaC4313AMELOBLASTOMA,UNDETERMINEDA odontogenic neoplasm arising from the epithelialcomponent of the embryonic tooth of undeterminedmalignancy status.AmeloblastomaC3799 ANGIOFIBROMA, BENIGN FIBROANGIOMA, BENIGN;FIBROUSPAPULE;TELANGIECTATIC FIBROMA;ANGIOFIBROMATOUSHYPERPLASIAA benign, morphologic variant of fibromacharacterized by the presence of numerous dilatedvascular channels.AngiofibromaC3733 ANGIOLIPOMA, BENIGN ANGIOLIPOMA A lipoma characterized by prominent vascularization. AngiolipomaC4209ARRHENOBLASTOMA,BENIGNARRHENOBLASTOMA,BENIGNA benign Sertoli-Leydig neoplasm of the ovarycharacterized by the presence of Sertoli cells intubules without evidence of significant nuclear atypiaor mitotic activity.Well Differentiated OvarianSertoli-Leydig Cell TumorC4210ARRHENOBLASTOMA,MALIGNANTARRHENOBLASTOMA,MALIGNANTA malignant Sertoli-Leydig neoplasm of the ovarycharacterized by the presence of a sarcomatoid stromawhich contains primitive gonadal stromal cells.Poorly Differentiated OvarianSertoli-Leydig Cell TumorC9477ASTROCYTOMA,GRADE IIIANAPLASTIC , MALIGNANT ASTROCYTICTUMOR;GRADE IIIASTROCYTOMA; GRADE IIIASTROCYTICNEOPLASM;MALIGNANTASTROCYTOMAA malignant neoplasm of the brain or spinal cordoriginating from astrocytes, exhibiting poordifferentiation (anaplasia).Anaplastic AstrocytomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 217 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC7173ASTROCYTOMA, DIFFUSE,MALIGNANTASTROCYTOMA, DIFFUSEA malignant astrocytic neoplasm characterized by ahigh degree of cellular differentiation, slow growth,and diffuse infiltration of neighboring brain structures.Diffuse AstrocytomaC4047ASTROCYTOMA,PILOCYTIC, BENIGNGRADE IASTROCYTICNEOPLASM;GRADE IASTROCYTOMA; GRADE IASTROCYTICTUMORA benign neoplasm of the brain or spinal cord arisingfrom astrocytes associated with single or multiplecysts.Pilocytic AstrocytomaC60781ASTROCYTOMA,UNDETERMINEDA neoplasm of the brain or spinal cord exhibitingastrocytic differentiation, but in which the malignancystatus has not been established.AstrocytomaC103391 BASALIOMA, BENIGN BASALIOMA A benign epithelial neoplasm arising from primaryepithelial germ cells of the piliary complex.Experimental Organism BenignBasaliomaC4614BASOSQUAMOUS TUMOR,BENIGNCUTANEOUSPAPILLOMA;PAPILLOMA OFSKIN;PAPILLOMA OFTHE SKINA benign papillary neoplasm of the skin.Skin PapillomaC2922BASOSQUAMOUS TUMOR,MALIGNANTBASOSQUAMOUSCARCINOMA;SKIN MIXEDBASAL ANDSQUAMOUSCELLCARCINOMA;BASOSQUAMOUS CELLCARCINOMAA basal cell carcinoma (skin neoplasm) which displayssquamous differentiation.Skin Basosquamous Cell CarcinomaC72074CARCINOID TUMOR,ATYPICAL, MALIGNANTMALIGNANTCARCINOIDTUMORA malignant neuroendocrine neoplasm arising fromenterochromaffin cells in the gastrointestinal tract and(less common) in the bronchi exhibiting atypia.Atypical Carcinoid TumorC2915CARCINOID TUMOR,UNDETERMINEDCARCINOID;WELL-DIFFERENTIATEDENDOCRINENEOPLASM;CARCINOIDNEOPLASMA neuroendocrine neoplasm arising fromenterochromaffin cells in the gastrointestinal tract and(less common) the bronchi with undeterminedmalignancy status.Carcinoid TumorSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 218 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4147CARCINOMA, ACIDOPHIL,MALIGNANTEOSINOPHILCARCINOMA;ACIDOPHILADENOCARCINOMA;ACIDOPHILCARCINOMA;EOSINOPHILADENOMCARCINOMA;EOSINOPHILADENOMCARCINOMAADENOCARCINOMAA malignant epithelial neoplasm of the anteriorpituitary gland in which the neoplastic cells stainpositive with acidic dyes.Pituitary Gland Acidophil CarcinomaC3768CARCINOMA, ACINARCELL, MALIGNANTACINARADENOCARCINOMA; ACINICCELLADENOCARCINOMA; ACINARCARCINOMA;ACINAR CELLADENOCARCINOMA; ACINICCELLCARCINOMAA malignant glandular epithelial neoplasm comprisingsecretory cells forming acinar patterns.Acinar Cell CarcinomaC3727CARCINOMA,ADENOSQUAMOUS,MALIGNANTMIXEDADENOCARCINOMA ANDSQUAMOUSCARCINOMA;MIXEDADENOCARCINOMA ANDEPIDERMOIDCELLCARCINOMA;MIXEDADENOCARCINOMA ANDSQUAMOUSCELLCARCINOMA;MIXEDADENOCARCINOMA ANDEPIDERMOIDCARCINOMAAn epithelial neoplasm composed of malignantglandular and malignant squamous cells.Adenosquamous CarcinomaC3775CARCINOMA, ADNEXAL,MALIGNANTSKINAPPENDAGECARCINOMA;CARCINOMAOF ADNEXA;CARCINOMAOF SKINAPPENDAGEA malignant epithelial neoplasm arising fromsebaceous or sweat glands or from hair follicles.Adnexal CarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 219 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC9325CARCINOMA,ADRENOCORTICAL,MALIGNANTCORTICALCELLCARCINOMA;ADENOCARCINOMA,ADRENOCORTICAL,MALIGNANT;ADRENALCORTEXADENOCARCINOMA;ADRENALCORTEXCANCER;ADRENALCORTICALADENOCARCINOMA;ADRENALCORTICALCARCINOMA;ADRENOCORTICALCARCINOMAA malignant epithelial neoplasm arising from adrenalcortical cells.Adrenal Cortex CarcinomaC2921CARCINOMA, BASAL CELL,MALIGNANTBASAL CELLEPITHELIOMA;BASAL CELLCANCER;BASAL CELLCARCINOMAOF SKIN;BASAL CELLCARCINOMAOF THE SKIN;BASAL CELLCARCINOMA;BASAL CELLEPITHELIOMA;BASAL CELLSKINCARCINOMA;BCCA malignant epithelial neoplasm arising from basalcells of the epidermis and pilosebaceous unit of skin[the dermis].Skin Basal Cell CarcinomaC35875CARCINOMA, BRONCHIAL,MALIGNANTA malignant neoplasia of the lung, arising frombronchial epithelium.Bronchogenic CarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 220 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC2923CARCINOMA,BRONCHIOLOALVEOLAR,MALIGNANTBRONCHIOALVEOLARADENOCARCINOMA OF LUNG;BRONCHIOLO-ALVEOLARLUNGCARCINOMA;BRONCHIOLOALVEOLARADENOCARCINOMA OF LUNG;BRONCHIOLOALVEOLARLUNGADENOCARCINOMA; BAC;BRONCHIOLO-ALVEOLARCARCINOMAOF LUNG;BRONCHIOLO-ALVEOLARCARCINOMAOF THE LUNG;BRONCHIOALVEOLAR LUNGCARCINOMA;BRONCHIOLOALVEOLARADENOCARCINOMA OF THELUNG;BRONCHIOALVEOLARADENOCARCINOMA OF THELUNGA malignant lung neoplasm originating from thealveolar/bronchiolar epithelium.Bronchioloalveolar CarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 221 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3879CARCINOMA, C-CELL,MALIGNANTPARAFOLLICULAR CELLCARCINOMA; CCELLCARCINOMA;MEDULLARYCARCINOMAOF THYROIDGLAND;THYROIDGLANDNEUROENDOCRINECARCINOMA;MEDULLARYCARCINOMAOF THETHYROIDGLAND;MEDULLARYTHYROIDCANCER;PARAFOLLICULAR CELLCARCINOMA;MEDULLARYTHYROIDCARCINOMA;MEDULLARYCARCINOMAOF THYROID;MEDULLARYCARCINOMA;MTC;MEDULLARYCARCINOMAOF THETHYROID;MEDULLARYTHYROIDGLANDCARCINOMA;THYROIDMEDULLARYCARCINOMAA neuroendocrine malignant epithelial neoplasmarising from C-cells of the thyroid gland.Thyroid Gland Medullary CarcinomaC4176CARCINOMA,CERUMINOUS GLAND,MALIGNANTA malignant neoplasm derived from ceruminous glandsin the external auditory canal.Ceruminous AdenocarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 222 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4715CARCINOMA, CHOROIDPLEXUS, MALIGNANTCHOROIDPLEXUSCANCER;CARCINOMAOF THECHOROIDPLEXUS;CARCINOMAOF CHOROIDPLEXUS;CANCER OFTHE CHOROIDPLEXUS;CANCER OFCHOROIDPLEXUS;ANAPLASTICCHOROIDPLEXUSPAPILLOMAA malignant neoplasm arising from the choroid plexusof the brain.Choroid Plexus CarcinomaC3752CARCINOMA,EMBRYONAL, MALIGNANTCARCINOMA,EMBRYONAL,MALIGNANTA non-seminomatous malignant germ cell neoplasm ofthe testis or ovary.Embryonal CarcinomaC7558CARCINOMA,ENDOMETRIAL,MALIGNANTCARCINOMAOFENDOMETRIUM;CARCINOMAOF THEENDOMETRIUMA malignant epithelial neoplasm arising from the liningof the uterine body cavity.Endometrial CarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 223 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC8054CARCINOMA, FOLLICULARCELL, MALIGNANTFOLLICULARTHYROIDCANCER;WELL-DIFFERENTIATEDFOLLICULARCARCINOMA;FOLLICULARADENOCARCINOMA;FOLLICULARCANCER OFTHE THYROID;FOLLICULARCARCINOMAOF THETHYROIDGLAND;WELL-DIFFERENTIATEDFOLLICULARADENOCARCINOMA;FOLLICULARCARCINOMA;FOLLICULARCARCINOMAOF THYROIDGLAND;FOLLICULARCANCER OFTHYROID;FOLLICULARTHYROIDCARCINOMA;THYROIDFOLLICULARCARCINOMA;FOLLICULARCARCINOMAOF THETHYROID;FOLLICULARCANCER OFTHYROIDGLAND;FOLLICULARCANCER OFTHE THYROIDGLAND;FOLLICULARCARCINOMAOF THYROID;FOLLICULARTHYROIDGLANDCARCINOMAA malignant neoplasia arising from follicular cells ofthe thyroid gland.Thyroid Gland Follicular CarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 224 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3099CARCINOMA,HEPATOCELLULAR,MALIGNANTCARCINOMAOF LIVERCELLS;PRIMARYCARCINOMAOF THE LIVERCELLS; LIVERCELLCARCINOMA;HEPATOMA;CARCINOMAOF THE LIVERCELLS;PRIMARYCARCINOMAOF LIVERCELLS; HCCA malignant neoplasm arising from hepatocytes.Hepatocellular CarcinomaC103393CARCINOMA,HEPATOCHOLANGIOCELLULAR, MALIGNANTHEPATOCHOLANGIOCELLULARCARCINOMAA malignant mixed neoplasm of the liver comprisingneoplastic hepatocytes and bile duct epithelial cells;both elements displaying evidence of malignancy.Experimental Organism MalignantHepatocholangiocellular CarcinomaC2917CARCINOMA, IN SITU,MALIGNANTEPITHELIALTUMOR, INSITU,MALIGNANT;INTRAEPITHELIALCARCINOMA;NON-INVASIVECARCINOMA;CISA malignant epithelial neoplasm confined to theepithelial layer and without evidence of further tissueinvasion.Carcinoma In SituC3770CARCINOMA, ISLET CELL,MALIGNANTPANCREATICNEUROENDOCRINECARCINOMA;MALIGNANTPANCREATICENDOCRINETUMOR;MALIGNANTISLET CELLTUMOR; ISLETCELL CANCER;ISLET CELLCARCINOMAA malignant endocrine neoplasm arising from islets ofLangerhans of the pancreas.Pancreatic NeuroendocrineCarcinomaC2916 CARCINOMA, MALIGNANT EPITHELIOMAMALIGNANT;EPITHELIALCARCINOMA;MALIGNANTEPITHELIALNEOPLASM;MALIGNANTEPITHELIALTUMOR;MALIGNANTEPITHELIOMAA malignant epithelial neoplasm.CarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 225 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC6878CARCINOMA, MIXED,ACINAR-ISLET CELL,MALIGNANTA malignant pancreatic neoplasm characterized by thepresence of a mixture of acinar and islet cell elements.Mixed Acinar-NeuroendocrineCarcinoma of the PancreasC3773CARCINOMA,NEUROENDOCRINE CELL,MALIGNANTNEUROENDOCRINE TUMOR,MALIGNANT;NEUROENDOCRINE CELLCARCINOMA;NEUROENDOCRINECARCINOMAA malignant neuroendocrine neoplasm comprisingcells containing secretory granules.Neuroendocrine CarcinomaC60491CARCINOMA, PARSDISTALIS, MALIGNANTRat Pars DistalisCarcinomaA malignant epithelial neoplasm arising from the parsdistalis of the pituitary gland.Rat Pars Distalis CarcinomaC92183CARCINOMA, PARSINTERMEDIA, MALIGNANTRat ParsIntermediaCarcinomaA malignant epithelial neoplasm arising from the parsintermedia of the pituitary gland.Rat Pars Intermedia CarcinomaC9385CARCINOMA, RENALCELL, MALIGNANTADENOCARCINOMA OF THEKIDNEY;RENAL CELLCANCER;KIDNEYADENOCARCINOMA; RCC;RENAL CELLCARCINOMA,STAGEUNSPECIFIED;RENAL CELLADENOCARCINOMA;ADENOCARCINOMA OFKIDNEYA malignant neoplasm arising from renal parenchyma.Renal Cell CarcinomaC39807CARCINOMA, RENAL,TUBULAR, MALIGNANTMUCINOUSTUBULAR ANDSPINDLE CELLCARCINOMAOF THEKIDNEYA malignant neoplasm of the kidney, characterized bythe presence of tubules.Mucinous Tubular and Spindle CellCarcinoma of the KidneyC27004CARCINOMA, SPINDLECELL, MALIGNANTPSEUDOSARCOMATOUSCARCINOMA;SPINDLE CELLCARCINOMAA malignant epithelial neoplasm characterized by thepresence of spindle cells.Sarcomatoid CarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 226 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC27093CARCINOMA, SQUAMOUSCELL, IN SITU,MALIGNANTEPIDERMOIDCELLCARCINOMAIN SITU;EPIDERMOIDCARCINOMAIN SITU;GRADE IIISQUAMOUSINTRAEPITHELIAL NEOPLASIA;INTRAEPITHELIAL SQUAMOUSCELLCARCINOMA;SQUAMOUSCELLCARCINOMAIN SITU;GRADE 3SQUAMOUSINTRAEPITHELIAL NEOPLASIA;SQUAMOUSCARCINOMAIN SITUA malignant epithelial neoplasm confined to thesquamous epithelium, without invasion of underlyingtissues.Stage 0 Squamous Cell CarcinomaC2929CARCINOMA, SQUAMOUSCELL, MALIGNANTMALIGNANTEPIDERMOIDCELLNEOPLASM;SQUAMOUSCARCINOMA;EPIDERMOIDCARCINOMA;MALIGNANTSQUAMOUSCELL TUMOR;SQUAMOUSCELLEPITHELIOMA;EPIDERMOIDCELL CANCER;MALIGNANTSQUAMOUSCELLNEOPLASM;MALIGNANTEPIDERMOIDCELL TUMOR;SQUAMOUSCELL CANCERA malignant neoplasm arising from squamousepithelial cells.Squamous Cell CarcinomaC6938CARCINOMA, SWEATGLAND, MALIGNANTCARCINOMAOF SWEATGLAND;CARCINOMAOF THE SWEATGLANDA malignant neoplasm arising from sweat glands.Sweat Gland CarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 227 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC2930CARCINOMA,TRANSITIONAL CELL,MALIGNANTTRANSITIONALCARCINOMAA malignant neoplasm arising from transitionalepithelium, usually affecting the urinary bladder,ureter, or renal pelvis.Transitional Cell CarcinomaC65192CARCINOMA, TUBULARCELL, MALIGNANTA malignant glandular neoplasm exhibiting tubularstructures.Tubular AdenocarcinomaC80356CARCINOMA,TUBULOSTROMAL,MALIGNANTA malignant epithelial neoplasm of the ovary withtubular and stromal neoplastic components.Tubulostromal AdenocarcinomaC3692CARCINOMA,UNDIFFERENTIATED,MALIGNANTANAPLASTICCARCINOMA;CARCINOMA,UNDIFFERENTIATEDA malignant epithelial neoplasm exhibiting poordifferentiation (anaplasia).Undifferentiated CarcinomaC98801CARCINOMA, ZYMBAL'SGLANDA malignant neoplasm of the rodent Zymbal's glandwith sebaceous and/or squamous differentiation.Zymbal's Gland CarcinomaC34448CARCINOSARCOMA,MALIGNANTA malignant neoplasm comprising a mixture ofcarcinomatous and sarcomatous elements.CarcinosarcomaC5358CARDIAC SCHWANNOMA,BENIGNSCHWANNOMA,ENDOCARDIAL, BENIGNA benign peripheral nervous system neoplasm that iscomposed of well-differentiated Schwann cells andaffects the heart.Benign Cardiac SchwannomaC79950CHEMODECTOMA,BENIGNBENIGNCHEMODECTOMAA benign neoplasm of the chemoreceptor system (e.g.carotid body, glomus jugulare, glomus vagale).Benign Carotid Body ParagangliomaC3574CHEMODECTOMA,MALIGNANTMALIGNANTTUMOR OF THECAROTIDBODY;MALIGNANTCAROTIDBODY TUMOR;MALIGNANTNEOPLASM OFTHE CAROTIDBODY;MALIGNANTNEOPLASM OFCAROTIDBODY;MALIGNANTCAROTIDBODYNEOPLASM;MALIGNANTTUMOR OFCAROTIDBODYA malignant neoplasm of the chemoreceptor system(e.g. carotid body, glomus jugulare, glomus vagale).Malignant Carotid BodyParagangliomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 228 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC2932CHEMODECTOMA,UNDETERMINEDPARAGANGLIOMA OFCAROTIDBODY;CHEMODECTOMA;PARAGANGLIOMA OF THECAROTIDBODY; TUMOROF THECAROTIDBODY;CAROTIDBODY TUMOR;CAROTIDBODYCHEMODECTOMA; TUMOR OFCAROTIDBODYA neoplasm of the chemoreceptor system (e.g. carotidbody, glomus jugulare, glomus vagale) for which themalignancy status has not be established.Carotid Body ParagangliomaC35417CHOLANGIOCARCINOMA,INTRAHEPATIC,MALIGNANTINTRAHEPATICCARCINOMAOF BILE DUCT;INTRAHEPATICCARCINOMAOF THE BILEDUCT;INTRAHEPATICBILE DUCTCARCINOMAA malignant neoplasm of the liver arisingfrom/comprising cells resembling those of bile ducts.Intrahepatic CholangiocarcinomaC4436CHOLANGIOCARCINOMA,MALIGNANTCHOLANGIOCELLULARCARCINOMAA malignant neoplasm arising from/comprising cellsresembling those of bile ducts.CholangiocarcinomaC2942 CHOLANGIOMA, BENIGN HEPATOCHOLANGIOMA;CHOLANGIOMA; ADENOMAOF BILE DUCT;ADENOMA OFTHE BILEDUCT;CHOLANGIOADENOMA;HEPATOCHOLANGIOCELLULAR ADENOMAA benign neoplasm arising from/comprising cellsresembling those of bile ducts.Bile Duct AdenomaC53459 CHONDROMA, BENIGN A benign, well circumscribed neoplasm arising fromthe hyaline cartilage in soft tissue or bone. It ischaracterized by the presence of chondrocytes.ChondromaC2946CHONDROSARCOMA,MALIGNANTA malignant mesenchymal neoplasm arising fromcartilage-forming tissues.ChondrosarcomaC2947 CHORDOMA, MALIGNANT A malignant bone neoplasm arising from the remnantsof the fetal notochord.ChordomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 229 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC2948CHORIOCARCINOMA,MALIGNANTCHORIOEPITHELIOMAA malignant neoplasm arising from placentaltrophoblast cells. They generally arise in the uterus.ChoriocarcinomaC53684CONNECTIVE AND SOFTTISSUE NEOPLASM,BENIGNBENIGNCONNECTIVEAND SOFTTISSUENEOPLASM;BENIGNCONNECTIVEAND SOFTTISSUETUMOR;BENIGNMESENCHYMAL CELLNEOPLASM;BENIGNNEOPLASM OFTHE SOFTTISSUE ANDBONE; BENIGNTUMOR OF THESOFT TISSUEAND BONEA benign neoplasm arising from connective and softtissues that does not invade adjacent tissues ormetastasize to other anatomic sites.Benign Connective and Soft TissueNeoplasmC2964CRANIOPHARYNGIOMA,BENIGNNEOPLASM OFRATHKE'SPOUCH;TUMOR OFRATHKE'SPOUCH;RATHKEPOUCHTUMOR;RATHKEPOUCHNEOPLASM;RATHKE'SPOUCHTUMOR;RATHKE'SPOUCHNEOPLASM;CYSTOMAA benign epithelial neoplasm of the sellar region,presumably derived from Rathke pouch epithelium.CraniopharyngiomaC79949CRANIOPHARYNGIOMA,MALIGNANTCARCINOMAARISING FROMCRANIOPHARYNGIOMAA malignant epithelial neoplasm of the sellar region,presumably derived from Rathke pouch epithelium.Carcinoma Arising fromCraniopharyngiomaC2971CYSTADENOCARCINOMA,MALIGNANTA malignant cystic epithelial neoplasm arising fromglandular epithelium.CystadenocarcinomaC3777CYSTADENOCARCINOMA,PAPILLARY, MALIGNANTA malignant cystic epithelial neoplasm arising fromglandular epithelium exhibiting papillary structures.Papillary CystadenocarcinomaC2972 CYSTADENOMA, BENIGN CYSTOMA A benign cystic epithelial neoplasm arising fromglandular epithelium.CystadenomaC2974CYSTADENOMA,PAPILLARY, BENIGNA benign cystic epithelial neoplasm arising fromglandular epithelium exhibiting papillary structures.Papillary CystadenomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 230 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3555 DECIDUOMA, MALIGNANT MALIGNANTPLACENTALTUMOR;MALIGNANTPLACENTALNEOPLASM;MALIGNANTNEOPLASM OFPLACENTA;MALIGNANTTUMOR OF THEPLACENTA;MALIGNANTNEOPLASM OFTHEPLACENTA;MALIGNANTTUMOR OFPLACENTA;MALIGNANTPLACENTALTUMORA malignant neoplasm arising from decidua (placental)cells.Malignant Placental NeoplasmC4858DECIDUOMA,UNDETERMINEDPLACENTANEOPLASMS;PLACENTATUMORS;PLACENTATUMOR;TUMOR OFPLACENTA;TUMOR OF THEPLACENTA;PLACENTALTUMORS;PLACENTALTUMOR;NEOPLASM OFTHEPLACENTA;PLACENTANEOPLASM;NEOPLASM OFPLACENTAA neoplasm arising from decidua (placental) cells inwhich the malignancy status has not been established.Placental NeoplasmC9011 DERMOID CYST, BENIGN BENIGNCYSTICTERATOMA;DERMOID;MATURECYSTICTERATOMAA benign neoplasm comprised of a cyst, lined bymature epidermis-like tissue with dermal appendages.Dermoid CystC2996DYSGERMINOMA,MALIGNANTA malignant germ cell neoplasm characterized by thepresence of a monotonous primitive germ cellpopulation, primarily in the ovary.DysgerminomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 231 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4049EPENDYMOMA,WHO GRADE IIIANAPLASTIC , MALIGNANT EPENDYMALTUMOR;UNDIFFERENTIATEDEPENDYMALNEOPLASM;MALIGNANTEPENDYMOMA;UNDIFFERENTIATEDEPENDYMALTUMOR;UNDIFFERENTIATEDEPENDYMOMA; ANAPLASTICEPENDYMALNEOPLASM;ANAPLASTICEPENDYMALTUMOR; WHOGRADE IIIEPENDYMALNEOPLASMA malignant neoplasm of ependymal origin withanaplastic cellular morphology.Anaplastic EpendymomaC3017EPENDYMOMA,UNDETERMINEDWHO GRADE IIEPENDYMALTUMOR; WHOGRADE IIEPENDYMALNEOPLASMA neoplasm of ependymal origin, for which themalignancy status has not been established.EpendymomaC4092 EPITHELIOMA, BENIGN BENIGNEPITHELIALTUMOR;BENIGNEPITHELIOMA;BENIGNNEOPLASM OFEPITHELIUM;BENIGNTUMOR OF THEEPITHELIUM;BENIGNNEOPLASM OFTHEEPITHELIUM;BENIGNTUMOR OFEPITHELIUMA benign neoplasm arising from epithelial cells of theskin.Benign Epithelial NeoplasmC80349EPITHELIOMA, CYSTICKERATINIZING, BENIGNA benign cystic epithelial neoplasm featuring a centralkeratin mass surrounded by squamous epithelium.Cystic Keratinizing EpitheliomaC84356EPITHELIOMA,NON-KERATINIZING,BENIGNA benign cystic epithelial neoplasm characterized bythe absence of keratin production.Non-Keratinizing EpitheliomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 232 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC2928FIBROADENOCARCINOMA,MALIGNANTADENOCARCINOMA WITHPRODUCTIVEFIBROSIS;FIBROCARCINOMA;SCIRRHOUSCARCINOMAA malignant neoplasm originating from glandular cellswith a fibrous or fibroblastic component.Scirrhous AdenocarcinomaC3744 FIBROADENOMA, BENIGN FIBROADENOMA OFBREAST;BREASTFIBROADENOMA;FIBROADENOMA OF THEBREASTA benign neoplasm originating from glandular cellswith a fibrous or fibroblastic component.FibroadenomaC4249 FIBROLIPOMA, BENIGN A benign neoplasm comprising mature adipocytes,characterized by areas of abundant fibrous tissue.FibrolipomaC3041 FIBROMA, BENIGN A benign neoplasm arising from fibrous tissue. FibromaC4314FIBROMA, ODONTOGENIC,BENIGNCENTRALODONTOGENICFIBROMAA benign intraosseous neoplasm arising fromtooth-forming tissues in the mandible and maxilla,characterized by the presence of islands ofodontogenic epithelium.Odontogenic FibromaC8422FIBROMA, OSSIFYING,BENIGNOSSIFYINGFIBROMA;CEMENTIFYING FIBROMA;CEMENTO-OSSIFYINGFIBROMAA benign fibrous neoplasm characterized by amineralized component (woven bone, lamellar bone, orcementum-like material).Ossifying FibromaC66760 FIBROMYXOMA, BENIGN FIBROMYXOMAA benign soft-tissue neoplasm of uncertain lineage,characterized by the presence of neoplasticspindle-shaped to round cells and a fibromyxoidstroma.Fibromyxoid NeoplasmC3337FIBROPAPILLOMA,BENIGNA benign polypoid tumor comprising fibrous tissue andepithelium.Fibroepithelial PolypC3043FIBROSARCOMA,MALIGNANTA malignant mesenchymal neoplasm of the soft tissueand bone.FibrosarcomaC3788GANGLIOGLIOMA,UNDETERMINEDA neuroepithelial neoplasm comprised of neoplasticganglion cells and neoplastic glial cells, in which themalignancy status has not been established.GangliogliomaC3790GANGLIONEUROBLASTOMA, MALIGNANTA malignant neoplasm characterized by the presence ofneuroblastic and ganglion cells and a stroma withSchwannian differentiation.GanglioneuroblastomaC3049GANGLIONEUROMA,BENIGNNEURALCREST TUMOR,BENIGNA benign neoplasm characterized by the presence ofganglion cells and spindle cell proliferation, locatedprimarily in the brain, ganglia, or adrenal medulla.GanglioneuromaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 233 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC27477GIANT CELL TUMOR,BENIGNOSTEOCLASTOMA, BENIGN;BENIGN BONEGIANT CELLTUMORA benign neoplasm of bone comprised of giant cells(osteoclast-like) and mononuclear cells.Benign Giant Cell Tumor of BoneC4090GIANT CELL TUMOR,MALIGNANTMALIGNANTGIANT CELLTUMORA malignant neoplasm of bone comprised of giantcells (osteoclast-like) and mononuclear cells.Malignant Giant Cell NeoplasmC3055GIANT CELL TUMOR,UNDETERMINEDTUMOR OFGIANT CELL;TUMOR OF THEGIANT CELLA neoplasm of bone comprised of giant cells(osteoclast-like) and mononuclear cells, for which themalignancy status has not been established.Giant Cell TumorC4822 GLIOMA, MALIGNANT MALIGNANTNEUROGLIALNEOPLASM;MALIGNANTGLIALNEOPLASM;MALIGNANTNEUROGLIALTUMOR;MALIGNANTGLIAL TUMORA malignant neuroglial neoplasm. The term can applyto several primary neoplasm of the brain and spinalcord, including astrocytoma and oligodendroglioma inaddition to others.Malignant GliomaC3903GLIOMA, MIXED,MALIGNANTGLIOMA,MIXED; MIXEDNEUROGLIALTUMOR;MIXEDNEUROGLIALNEOPLASM;MIXED GLIALTUMOR;MIXED GLIALNEOPLASMA malignant neoplasm comprising two or more glialcell types (e.g., astrocytes, ependymal cells,oligodendrocytes).Mixed GliomaC3252GRANULAR CELL TUMOR,BENIGNBENIGNGRANULARCELL TUMOR;BENIGNGRANULARCELLNEOPLASM;BENIGNGRANULARCELLMYOBLASTOMA;MYOBLASTOMAA benign neoplasm, comprised of large cells withcytoplasmatic granules, occurring in variousorgans/tissues.Benign Granular Cell TumorSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 234 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4336GRANULAR CELL TUMOR,MALIGNANTMYOBLASTOMA,MALIGNANT;MALIGNANTGRANULARCELLNEOPLASM;MALIGNANTGRANULARCELLMYOBLASTOMAA malignant neoplasm, comprised of large cells withcytoplasmatic granules, occurring in variousorgans/tissues.Malignant Granular Cell TumorC4205GRANULOSA CELLTUMOR, MALIGNANTMALIGNANTGRANULOSACELL TUMORA malignant neoplasm of the ovary, originating fromgranulosa cells.Malignant Granulosa Cell TumorC3070GRANULOSA CELLTUMOR, UNDETERMINEDGRANULOSACELLNEOPLASMA neoplasm of the ovary (or testis), originating fromgranulosa cells for which the malignancy status has notbeen established.Granulosa Cell TumorC27520HAIR FOLLICLENEOPLASM, BENIGNBENIGNFOLLICULARNEOPLASM;BENIGNFOLLICULARTUMOR;BENIGN HAIRFOLLICLETUMORA benign neoplasm that arises from the hair follicle.Benign Hair Follicle NeoplasmC43310HAIR FOLLICLENEOPLASM, MALIGNANTMALIGNANTHAIRFOLLICLETUMORA malignant neoplasm that arises from the hairfollicle.Malignant Hair Follicle NeoplasmC7367HAIR FOLLICLENEOPLASM,UNDETERMINEDHAIRFOLLICLETUMOR; HAIRMATRIXNEOPLASM;HAIR MATRIXTUMOR;NEOPLASM OFHAIRFOLLICLE;NEOPLASM OFTHE HAIRFOLLICLEA neoplasm that arises from the hair follicle, for whichthe malignancy status has not been established.Hair Follicle NeoplasmC3075 HAMARTOMA, BENIGN A benign and excessive tumor-like growth of maturecells and normal tissues which grow in a disorganizedpattern.HamartomaC40426HAMARTOMA,LIPOMATOUS, BENIGNA benign hamartomatous tumor composedpredominantly of adipose tissue.Lipomatous HamartomaC3085 HEMANGIOMA, BENIGN ANGIOMA;BENIGNANGIOMA;BENIGNHEMANGIOMAA benign vascular neoplasm characterized by theformation of capillary-sized or cavernous vascularchannels.HemangiomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 235 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4300HEMANGIOPERICYTOMA,BENIGNA benign neoplasm originating from vascular pericytes(cells in the periphery of vessels).Benign HemangiopericytomaC4301HEMANGIOPERICYTOMA,MALIGNANTMALIGNANTHEMANGIOPERICYTOMANOSA malignant neoplasm originating from vascularpericytes (cells in the periphery of vessels).Malignant HemangiopericytomaC3087HEMANGIOPERICYTOMA,UNDETERMINEDA neoplasm originating from vascular pericytes (cellsin the periphery of vessels), for which the malignancystatus has not been established.HemangiopericytomaC3088HEMANGIOSARCOMA,MALIGNANTHEMANGIOSARCOMAA malignant vascular neoplasm arising fromendothelial cells.AngiosarcomaC3728HEPATOBLASTOMA,MALIGNANTHBL;PEDIATRICEMBRYONALHEPATOMA;PEDIATRICHEPATOBLASTOMAA malignant liver neoplasm composed of immaturehepatocytic elements.HepatoblastomaC3702 HIBERNOMA, BENIGN BROWN FATNEOPLASM;FETAL FATCELL LIPOMA;BROWN FATTUMORC103394 HIBERNOMA, MALIGNANT MALIGNANTHIBERNOMAA benign neoplasm of the brown adipose tissue.A malignant neoplasm arising from the brown adiposetissue in animals, usually in the subcutis and thethoracic cavity.HibernomaExperimental Organism MalignantHibernomaC98708 HISTIOCYTOMA, BENIGN A benign dermal neoplasm comprising of round cells,resembling histiocytes. The tumor commonly occursin young dogs and may regress spontaneously.Benign HistiocytomaC3739HISTIOCYTOMA, FIBROUS,BENIGNFIBROUSHISTIOCYTOMAA benign neoplasm composed of a fibroblastic and ahistiocytic component.Benign Fibrous HistiocytomaC4247HISTIOCYTOMA, FIBROUS,MALIGNANTMALIGNANTFIBROUSHISTIOCYTOMA OF THE SOFTTISSUE ANDBONE;FIBROXANTHOSARCOMA;MALIGNANTFIBROXANTHOMA; MFH;MALIGNANTFIBROUSHISTIOCYTOMA OF SOFTTISSUE ANDBONEA malignant neoplasm composed of a fibroblastic anda histiocytic component.Malignant Fibrous HistiocytomaC8402HISTIOCYTOMA, FIBROUS,UNDETERMINEDFIBROHISTIOCYTIC TUMORA neoplasm composed of a fibroblastic and ahistiocytic component, for which the malignancystatus has not been established.Fibrohistiocytic NeoplasmSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 236 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC35765HISTIOCYTOMA,UNDETERMINEDA neoplasm composed of histiocytic cells, for whichthe malignancy status has not been established.HistiocytomaC80351 ITO CELL TUMOR, BENIGN BENIGNSPONGIOTICPERICYTOMAA benign neoplasm of the liver composed of hepaticstellate cells (Ito cells).Benign Ito Cell TumorC80352ITO CELL TUMOR,MALIGNANTMALIGNANTSPONGIOTICPERICYTOMAA malignant neoplasm of the liver composed ofhepatic stellate cells (Ito cells).Malignant Ito Cell TumorC3146KERATOACANTHOMA,UNDETERMINEDAn epithelial skin neoplasm, composed of squamouscells, for which the malignancy status has not beenestablished.KeratoacanthomaC81854LEIOMYOBLASTOMA,BENIGNA benign neoplasm of the gastro-intestinal tract,originating from smooth muscle cells.Benign Epithelioid Cell TypeGastrointestinal Stromal TumorC81855LEIOMYOBLASTOMA,MALIGNANTA malignant neoplasm of the gastro-intestinal tract,originating from smooth muscle cells.Malignant Epithelioid Cell TypeGastrointestinal Stromal TumorC3486LEIOMYOBLASTOMA,UNDETERMINEDLEIOMYOBLASTOMA;EPITHELIOIDCELL TYPEGIST; STOUT'SLEIOMYOBLASTOMAA neoplasm of the gastro-intestinal tract, originatingfrom smooth muscle cells, for which the malignancystatus has not been established.Epithelioid Cell TypeGastrointestinal Stromal TumorC3157 LEIOMYOMA, BENIGN LEIOMYOMATOUSNEOPLASM;LEIOMYOMATOUS TUMOR;FIBROIDNEOPLASM;FIBROIDTUMOR;FIBROIDA benign neoplasm, originating from smooth musclecells.LeiomyomaC3158LEIOMYOSARCOMA,MALIGNANTLEIOMYOSARCOMASA malignant neoplasm, originating from smoothmuscle cells.LeiomyosarcomaC8923LEUKEMIA, ACUTEERYTHROID, MALIGNANTERYTHROBLASTIC LEUKEMIA;ACUTEERYTHROBLASTIC LEUKEMIA;M6 ACUTEMYELOIDLEUKEMIA;FAB M6A progressive, proliferative disease of blood cells,originating from immature erythroid cells.Acute Erythroid LeukemiaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 237 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3167LEUKEMIA, ACUTELYMPHOBLASTIC,MALIGNANTLYMPHOBLASTIC LEUKEMIA;ALL - ACUTELYMPHOCYTICLEUKEMIA;ACUTELYMPHOIDLEUKEMIA;PRECURSORCELLLYMPHOBLASTIC LEUKEMIA;ALL;PRECURSORLYMPHOBLASIC LEUKEMIA;ACUTELYMPHOGENOUS LEUKEMIA;ACUTELYMPHOCYTICLEUKEMIAS;ACUTELYMPHOCYTICLEUKAEMIAA progressive, proliferative disease of blood cells,originating from immature lymphoid cells.Acute Lymphoblastic LeukemiaC3170LEUKEMIA, ACUTEMEGAKARYOCYTIC,MALIGNANTACUTEMEGAKARYOCYTICLEUKEMIA;ACUTEMEGAKARYOBLASTICLEUKEMIA(FAB TYPE M7);FAB M7;ACUTE M7MYELOIDLEUKEMIAA progressive, proliferative disease of blood cells,originating from immature megakaryocytes.Acute Megakaryoblastic LeukemiaC4861LEUKEMIA, ACUTEMONOCYTIC, MALIGNANTACUTEMONOCYTICLEUKEMIA(FAB M5B);MONOCYTICLEUKEMIAA progressive, proliferative disease of blood cells,originating from immature monocytes.Acute Monocytic LeukemiaC3172LEUKEMIA,GRANULOCYTIC,MALIGNANTLEUKEMIAGRANULOCYTIC; LEUKEMIAMYELOID;NON-LYMPHOBLASTICLEUKEMIA;MYELOCYTICLEUKEMIA;NON-LYMPHOCYTICLEUKEMIA;MYELOGENOUS LEUKEMIAA progressive, proliferative disease of blood cells,originating from immature granulocytes.Myeloid LeukemiaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 238 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4664LEUKEMIA, LARGEGRANULARLYMPHOCYTIC,MALIGNANTT-GAMMALYMPHOPROLIFERATIVEDISORDER;LARGE CELLGRANULARLYMPHOIDLEUKEMIA;T-CELL LARGEGRANULARLYMPHOCYTICLEUKEMIA;LGLL;TGAMMALARGEGRANULARLYMPHOCYTELEUKEMIA; TGAMMALYMPHOPROLIFERATIVEDISORDER;LARGEGRANULARLYMPHOCYTOSIS; LARGEGRANULARLYMPHOCYTICLEUKEMIA;LARGE CELLGRANULARLYMPHOGENOUS LEUKEMIAA progressive, proliferative disease of blood cellswhich are large and granular, originating fromlymphoid cells.T-Cell Large Granular LymphocyteLeukemiaC7539LEUKEMIA,LYMPHOCYTIC,MALIGNANTLYMPHOGENOUS LEUKEMIA;LYMPHOCYTICLEUKEMIAA progressive, proliferative disease of blood cells,originating from lymphoid cells.Lymphoid LeukemiaC3161 LEUKEMIA, MALIGNANT LEUKEMIANOS;LEUKEMIAS,GENERAL;LEUKEMIAS;BLOOD(LEUKEMIA)A progressive, proliferative disease of blood cells,originating from myeloid or lymphoid stem cells.LeukemiaC42<strong>12</strong>LEYDIG CELL TUMOR,BENIGNADENOMA,INTERSTITIAL;BENIGNINTERSTITIALCELLNEOPLASM;BENIGNINTERSTITIALCELL TUMOR;BENIGNLEYDIG CELLNEOPLASMA benign neoplasm of the testis originating frominterstitial (Leydig) cells.Benign Leydig Cell TumorSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 239 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4213LEYDIG CELL TUMOR,MALIGNANTMALIGNANTINTERSTITIALCELL TUMOR;MALIGNANTLEYDIG CELLNEOPLASM;MALIGNANTINTERSTITIALCELLNEOPLASMA malignant neoplasm of the testis originating frominterstitial (Leydig) cells.Malignant Leydig Cell TumorC3188LEYDIG CELL TUMOR,UNDETERMINEDLEYDIG CELLTUMOR;LEYDIG CELLNEOPLASM;INTERSTITIALCELLNEOPLASM;INTERSTITIALCELL TUMORA neoplasm of the testis originating from interstitial(Leydig) cells, for which the malignancy status has notbeen established.Leydig Cell TumorC3192 LIPOMA, BENIGN A benign neoplasm composed of adipose tissue. LipomaC3194LIPOSARCOMA,MALIGNANTA malignant neoplasm composed of adipose tissue.LiposarcomaC3202 LUTEOMA, BENIGN LUTEOMA;LUTEINOMA;LUTEAL CELLTUMOR;LUTEAL CELLNEOPLASM;OVARIANSTROMALUTEOMAA benign neoplasm of the ovary, composed ofleuteinized granulosa-theca cells.Ovarian Stromal LuteomaC8965 LYMPHANGIOMA, BENIGN A benign neoplasm arising from the lymphatics. LymphangiomaC3205LYMPHANGIOSARCOMA,MALIGNANTMALIGNANTLYMPHANGIOENDOTHELIOMA;LYMPHANGIOENDOTHELIALSARCOMAA malignant neoplasm arising from the endothelialcells of the lymphatic vessels.LymphangiosarcomaC3209LYMPHOMA, FOLLICULAR,MALIGNANTLYMPHOMA,FOLLICULARCENTRE CELL;FOLLICULARCENTRE CELLLYMPHOMA;FOLLICLECENTERLYMPHOMA;FOLLICULARNON-HODGKINLYMPHOMA;FOLLICULARNON-HODGKIN'S LYMPHOMAA neoplasm of lymphoid cells which has at least apartial follicular pattern.Follicular LymphomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 240 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC35382LYMPHOMA, HISTIOCYTIC,MALIGNANTLYMPHOMA,LARGE CELL,MALIGNANTA malignant neoplasm of large lymphocytes, whichresemble histiocytes.True Histiocytic LymphomaC3461LYMPHOMA,IMMUNOBLASTIC,MALIGNANTA malignant neoplasm composed of immunoblasts(large B cells).Immunoblastic LymphomaC9360LYMPHOMA,LYMPHOBLASTIC,MALIGNANTPRECURSORCELLLYMPHOBLASTICLYMPHOMA;PRECURSORLYMPHOBLASTICLYMPHOMAA malignant neoplasm composed of lymphoblasts(lymphoid precursor cells).Lymphoblastic LymphomaC32<strong>12</strong>LYMPHOMA,LYMPHOPLASMACYTIC,MALIGNANTLYMPHOMA,PLASMACYTIC;IMMUNOCYTOMA,LYMPHOPLASMACYTICTYPE;LYMPHOPLASMACYTOIDLYMPHOMAA malignant neoplasm composed of lymphocytes(B-cells), lymphoplasmacytoid cells, and plasma cells.Lymphoplasmacytic LymphomaC3208 LYMPHOMA, MALIGNANT LYMPHOMA(HODGKINANDNON-HODGKIN); LYMPHOMA(HODGKIN'SANDNON-HODGKIN'S);MALIGNANTLYMPHOMAA malignant neoplasm composed of lymphocytes of B-or T/NK-cell phenotype.LymphomaC3463LYMPHOMA, MIXED,MALIGNANTDIFFUSEMIXED LARGEAND SMALLCELLLYMPHOMAA malignant neoplasm composed of a mixedlymphocyte population.Diffuse Mixed Cell LymphomaC7540LYMPHOMA, SMALLLYMPHOCYTIC,MALIGNANTSMALL B-CELLLYMPHOCYTICLYMPHOMA;SLL; B-CELLSMALLLYMPHOCYTICLYMPHOMA;LYMPHOMA,LYMPHOCYTIC, MALIGNANTA malignant neoplasm composed of smalllymphocytes.Small Lymphocytic LymphomaC26919LYMPHOSARCOMA,MALIGNANTAn antiquated term referring to a malignant lymphomathat is diffused and composed of small and largelymphocytes.LymphosarcomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 241 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3217MAST CELL TUMOR,BENIGNA benign neoplasm composed of mast cells.Benign MastocytomaC8991MAST CELL TUMOR,MALIGNANTA malignant neoplasm composed of mast cells.Malignant MastocytosisC9295MAST CELL TUMOR,UNDETERMINEDMAST CELLPROLIFERATIVE DISEASE;TUMOR OFMAST CELLS;NEOPLASM OFMAST CELLS;NEOPLASM OFTHE MASTCELLS; MASTCELL TUMOR;TUMOR OF THEMAST CELLSA neoplasm composed of mast cells, for which themalignancy status has not been determined.Mast Cell NeoplasmC3222MEDULLOBLASTOMA,MALIGNANTMEDULLOBLASTOMASA malignant, invasive embryonal neoplasm arisingfrom the cerebellum.MedulloblastomaC98709 MELANOCYTOMA, BENIGN A benign neoplasm or hamartoma composed ofmelanocytes.Benign MelanocytomaC3802MELANOMA,AMELANOTIC,MALIGNANTA malignant neoplasm composed of melanocytes,which lack melanin.Amelanotic MelanomaC3224 MELANOMA, MALIGNANT MALIGNANTMELANOMAA malignant neoplasm composed of melanocytes.MelanomaC77<strong>12</strong>MELANOMA, UVEAL,MALIGNANTMELANOMAOF UVEA;MELANOMAOF THE UVEA;INTRAOCULARMELANOMAA malignant neoplasm of the uvea composed ofmelanocytes.Uveal MelanomaC4051MENINGIOMA,ANAPLASTIC, MALIGNANTANAPLASTICMENINGIOMA;MALIGNANTMENINGIOMA;MENINGIOMA,MALIGNANTA malignant neoplasm of the meninges, exhibitingpoor differentiation (anaplasia).Anaplastic (Malignant) MeningiomaC4055 MENINGIOMA, BENIGN MENINGIOMA,BENIGNA benign neoplasm of the meninges.Benign MeningiomaC38938MENINGIOMA,MALIGNANTGRADE IIIMENINGIOMA;GRADE 3MENINGIOMA;WHO GRADE IIIMENINGIOMAA malignant neoplasm of the meninges.Grade III MeningiomaC3230MENINGIOMA,UNDETERMINEDA neoplasm of the meninges, for which the status ofmalignancy has not been determined.MeningiomaC4267MESENCHYMAL TUMOR,BENIGNA benign soft-tissue neoplasm comprising two ormore non-fibroblastic mesenchymal lines ofdifferentiation.Benign MesenchymomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 242 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3762 MESOTHELIOMA, BENIGN ADENOMATOID TUMOR,BENIGN;BENIGNMESOTHELIOMA;MESOTHELIOMA, BENIGN;BENIGNNEOPLASM OFMESOTHELIUM; BENIGNMESOTHELIALNEOPLASM;BENIGNTUMOR OFMESOTHELIUM; BENIGNMESOTHELIALTUMOR;BENIGNLOCALIZEDEPITHELIALMESOTHELIOMA; BENIGNTUMOR OF THEMESOTHELIUM; BENIGNNEOPLASM OFTHEMESOTHELIUMA benign neoplasm arising from mesothelial cells.Adenomatoid TumorC4456MESOTHELIOMA,MALIGNANTMALIGNANTTUMOR OFMESOTHELIUM; MALIGNANTNEOPLASM OFTHEMESOTHELIUM; MALIGNANTMESOTHELIALTUMOR;MALIGNANTNEOPLASM OFMESOTHELIUM; MALIGNANTMESOTHELIALNEOPLASM;MALIGNANTTUMOR OF THEMESOTHELIUMA malignant neoplasm originating from mesothelialcells of the pleura or peritoneum.Malignant MesotheliomaC3234MESOTHELIOMA,UNDETERMINEDA neoplasm originating from mesothelial cells of thepleura or peritoneum, for which the malignancy statushas not been established.MesotheliomaC4050 MIXED GLIOMA, BENIGN A benign neoplasm of the central nervous system withan astrocytic and oligodendrocytic component.C8602 MIXED TUMOR, BENIGN A benign neoplasm composed of epithelial and/ormyoepithelial cells and a mesenchymal component.OligoastrocytomaPleomorphic AdenomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 243 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3729MIXED TUMOR,MALIGNANTMALIGNANTMIXED TUMORA malignant neoplasm composed of epithelial and/ormyoepithelial cells and a mesenchymal component. Ageneral term for which the transformed cell types havenot been specified.Malignant Mixed NeoplasmC6930MIXED TUMOR,UNDETERMINEDTUMOR,MIXED; MIXEDTUMORA neoplasm composed of an epithelial componentand/or a mesenchymal component, for which themalignancy status has not been established. A generalterm for which the transformed cell types have notbeen specified.Mixed NeoplasmC8975MUELLERIAN TUMOR,MIXED, MALIGNANTMALIGNANTMIXEDMESODERMALTUMOR;MALIGNANTMIXEDMULLERIANTUMOR;MMMTA malignant neoplasm of the female reproductivesystem (mostly uterus and ovaries) originating fromthe Muellerian ducts and composed of carcinomatousand sarcomatous elements.Malignant Mixed Mesodermal(Mullerian) TumorC3736 MYELOLIPOMA, BENIGN MYELOLIPOMAA benign tumor of the adrenal gland composed ofadipocytes and hematopoietic/lymphoid cells.Adrenal Gland MyelolipomaC3242MYELOMA, PLASMA CELL,MALIGNANTMYELOMA;MULTIPLEMYELOMAA malignant neoplasm of the bone marrow composedof plasma cells.Plasma Cell MyelomaC7442MYOEPITHELIOMA,BENIGNA benign neoplasm composed of myoepithelial cells.Benign MyoepitheliomaC7596MYOEPITHELIOMA,MALIGNANTMYOEPITHELIALCARCINOMA;MALIGNANTMYOEPITHELIOMAA malignant neoplasm composed of myoepithelialcells.Malignant MyoepitheliomaC6577 MYXOMA, BENIGN A benign soft tissue neoplasm with a myxoid stromaformation.MyxomaC3255MYXOSARCOMA,MALIGNANTA malignant soft tissue neoplasm with a myxoidstroma formation.MyxosarcomaC4743NEOPLASM, BASAL CELL,BENIGNBENIGN BASALCELL TUMORA benign neoplasm composed of basal cells.Benign Basal Cell NeoplasmC7586NEOPLASM, BASAL CELL,MALIGNANTA malignant neoplasm composed of basal cells.Malignant Basal Cell NeoplasmC3677 NEOPLASM, BENIGN BENIGNTUMOR;BENIGNUNCLASSIFIABLE TUMORA general term used to describe autonomous growth oftissue where the originating cell type has not beencharacterized. The term benign indicates the absenceof morphologic features associated with malignancy(for instance severe atypia, nuclear pleomorphism,tumor cell necrosis, and abnormal mitoses).Benign NeoplasmC9305 NEOPLASM, MALIGNANT MALIGNANCY;CA; CANCER;MALIGNANTTUMORA general term for autonomous tissue growthexhibiting morphologic features of malignancy (e.g.severe atypia, nuclear pleomorphism, tumor cellnecrosis, abnormal mitoses, tissue invasiveness) andfor which the transformed cell type has not beenspecifically identified.Malignant NeoplasmSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 244 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3262NEOPLASM,UNDETERMINEDA general term for autonomous tissue growth in whichthe malignancy status has not been established and forwhich the transformed cell type has not beenspecifically identified.NeoplasmC40407NEPHROBLASTOMA,MALIGNANTEMBRYONALNEPHROMA;NEPHROBLASTOMA; WILMSTUMOR OF THEKIDNEY;RENAL WILMS'TUMOR;WILMS'TUMOR OF THEKIDNEYA malignant embryonal neoplasm of the kidney.Renal Wilms TumorC6963NEURAL CREST TUMOR,UNDETERMINEDA neoplasm composed of cells of neuroendocrineorigin for which the malignancy status has not beenestablished.Neuroendocrine NeoplasmC3270NEUROBLASTOMA,MALIGNANTNEUROBLASTOMA(SCHWANNIANSTROMA-POOR); NEURALCREST TUMOR,MALIGNANTA malignant neoplasm composed of neuroblastic cells.NeuroblastomaC3789NEUROBLASTOMA,OLFACTORY, MALIGNANTESTHESIONEUROBLASTOMA;OLFACTORYESTHESIONEUROBLASTOMA;ESTHESIONEUROBLASTOMA;ESTHESIONEUROEPITHELIOMA;OLFACTORYNEUROEPITHELIOMAA malignant neoplasm of the olfactory receptor cells,composed of neuroblastic cells.Olfactory NeuroblastomaC3272 NEUROFIBROMA, BENIGN An intraneural or extraneural neoplasm arising fromnerve tissues and neural sheaths, composed ofperineurial-like fibroblasts and Schwann cells.NeurofibromaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 245 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3059NEUROGLIAL TUMOR,UNDETERMINEDGLIOMA;NEOPLASM OFTHENEUROGLIA;NEUROGLIALNEOPLASMS;TUMOR OF THENEUROGLIA;TUMORS OFNEUROGLIA;TUMOR OFNEUROGLIA;NEOPLASMSOFNEUROGLIA;GLIALNEOPLASM;GLIAL TUMOR;GLIOMA;NEOPLASM OFNEUROGLIA;NEUROGLIALNEOPLASMA neoplasm of the central nervous system composedof glial cells (astrocytes, oligodendrocytes, ependymalcells), for which the malignancy status has not beendetermined.Neuroglial TumorC4306ODONTOGENIC TUMOR,BENIGNBENIGNODONTOGENICTUMORA benign neoplasm arising from tooth-forming tissues.Benign Odontogenic NeoplasmC3286ODONTOGENIC TUMOR,UNDETERMINEDODONTOGENICTUMORA neoplasm arising from tooth-forming tissues, forwhich the malignancy status has not been established.Odontogenic NeoplasmC3710ODONTOMA,AMELOBLASTIC, BENIGNAMELOBLASTICFIBROODONTOMA;FIBROAMELOBLASTICODONTOMAA benign neoplasm arising from tooth-forming tissueswith-enamel organ differentiation (but without enamelformation).Ameloblastic Fibro-OdontomaC7492ODONTOMA,AMELOBLASTIC,MALIGNANTA malignant neoplasm arising from tooth-formingtissues with-enamel organ differentiation (but withoutenamel formation).Ameloblastic CarcinomaC3287 ODONTOMA, BENIGN FIBROODONTOMA;FIBRO-ODONTOMAC48<strong>12</strong> ODONTOMA, MALIGNANT ODONTOGENICCARCINOMA;MALIGNANTODONTOGENICTUMOR;ODONTOGENICCARCINOSARCOMAA benign neoplasm of tooth origin.A malignant neoplasm of tooth origin.OdontomaMalignant Odontogenic NeoplasmSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 246 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4326OLIGODENDROGLIOMA,ANAPLASTIC, MALIGNANTWHO GRADE IIIOLIGODENDROGLIALNEOPLASM;OLIGODENDROGLIOMA;MALIGNANT;MALIGNANTOLIGODENDROGLIOMA;WHO GRADE IIIOLIGODENDROGLIALTUMOR;UNDIFFERENTIATEDOLIGODENDROGLIOMAA malignant neoplasm of the central nervous systemarising from oligodendrocytes, exhibiting poordifferentiation (anaplasia).Anaplastic OligodendrogliomaC3288OLIGODENDROGLIOMA,UNDETERMINEDWELLDIFFERENTIATEDOLIGODENDROGLIOMA;WELLDIFFERENTIATEDOLIGODENDROGLIALTUMOR; WHOGRADE IIOLIGODENDROGLIALTUMOR; WHOGRADE IIOLIGODENDROGLIALNEOPLASMA neoplasm of the central nervous system arising fromoligodendrocytes, for which the malignancy status hasnot been determined.OligodendrogliomaC7072 ONCOCYTOMA, BENIGN ONCOCYTOMA; ONCOCYTICTUMORA benign neoplasm composed of large cells withabundant eosinophilic granular cytoplasm (oncocytes).Oncocytic NeoplasmC3679ONCOCYTOMA,MALIGNANTHURTHLECELLCARCINOMA;HURTHLECELLADENOCARCINOMA;ONCOCYTICADENOCARCINOMA;ONCOCYTICCARCINOMAA malignant neoplasm composed of large epithelialcells with abundant granular eosinophilic cytoplasm(oncocytes).Oxyphilic AdenocarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 247 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3294OSTEOBLASTOMA,BENIGNGIANTOSTEOIDOSTEOMA;OSSIFYINGGIANT CELLTUMORA benign neoplasm of bone, characterized by theformation of osteoid tissue and large osteoblast-likecells.OsteoblastomaC3295OSTEOCHONDROMA,BENIGNA benign cartiliginous neoplasm arising from themetaphysis of bone.OsteochondromaC7155OSTEOCHONDROSARCOMA, MALIGNANTPRIMARYCHONDROSARCOMA;CONVENTIONALCHONDROSARCOMA OFBONE;PRIMARYCHONDROSARCOMA OF THEBONE;PRIMARYCHONDROSARCOMA OFBONE;PRIMARYBONECHONDROSARCOMAA malignant cartiliginous neoplasm of bone.Conventional ChondrosarcomaC4304OSTEOCLASTOMA,MALIGNANTGIANT CELLSARCOMA OFTHE BONE;GIANT CELLSARCOMA OFBONE; GIANTCELL BONESARCOMA;DEDIFFERENTIATED GIANTCELL TUMORA malignant neoplasm of bone comprised ofosteoclast-like giant cells and mononuclear cells.Malignant Giant Cell Tumor of BoneC3738OSTEOCLASTOMA,UNDETERMINEDGIANT CELLNEOPLASM OFBONE; BONEGIANT CELLTUMOR; BONEGIANT CELLNEOPLASMA neoplasm of bone comprised of osteoclast-like giantcells and mononuclear cells, for which the malignancystatus has not been established.Giant Cell Tumor of BoneSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 248 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3740 OSTEOFIBROMA, BENIGN DESMOIDTUMOR OFBONE;DESMOPLASTIC FIBROMA OFBONE;DESMOPLASTIC FIBROMA OFTHE BONE;DESMOPLASTIC FIBROMA;OSSEOUSDESMOPLASTIC FIBROMAA benign neoplasm characterized by osteolysis and thepresence of a rich collagenous stroma and spindlecells.Bone Desmoplastic FibromaC103392OSTEOFIBROMA, BONE,BENIGNOSTEOFIBROMA, BONEA benign neoplasm, morphologically a fibroma withosseous components.Experimental Organism Benign BoneOsteofibromaC3296 OSTEOMA, BENIGN A benign well-differentiated neoplasm of bone. OsteomaC8810OSTEOSARCOMA,EXTRASKELETAL,MALIGNANTEXTRAOSSEOUSOSTEOSARCOMA;EXTRASKELETALOSTEOGENICSARCOMA;SOFT TISSUEOSTEOSARCOMAA malignant bone-forming neoplasm, arising in tissueother than bone.Extraskeletal OsteosarcomaC9145OSTEOSARCOMA,MALIGNANTOSTEOGENICSARCOMAA malignant neoplasm usually arising from bone.OsteosarcomaC6803OVARIAN SEXCORD-STROMAL TUMOR,BENIGNSEX CORDSTROMALTUMOR,BENIGN;BENIGN SEXCORD-STROMAL NEOPLASMOF THEOVARY;BENIGN SEXCORD-STROMAL TUMOR OFTHE OVARY;BENIGNOVARIAN SEXCORD-STROMAL NEOPLASM;BENIGN SEXCORD-STROMAL TUMOR OFOVARY;BENIGN SEXCORD-STROMAL NEOPLASMOF OVARYA benign neoplasm of the ovary, originating from thesex-cord stroma.Benign Ovarian Sex Cord-StromalTumorSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 249 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC7440 PAPILLOMA, BENIGN A benign epithelial neoplasm that projects above thesurrounding epithelial surface.PapillomaC3698PAPILLOMA, CHOROIDPLEXUS, BENIGNPAPILLOMA OFCHOROIDPLEXUS;PAPILLOMA OFTHE CHOROIDPLEXUSA benign neoplasm of the choroid plexus of the centralnervous system.Choroid Plexus PapillomaC37<strong>12</strong>PAPILLOMA, SQUAMOUSCELL, BENIGNEPIDERMOIDPAPILLOMA;EPIDERMOIDCELLPAPILLOMA;KERATOTICPAPILLOMA;SQUAMOUSCELLPAPILLOMAA benign epithelial neoplasm characterized by apapillary growth pattern and a proliferation ofneoplastic squamous cells.Squamous PapillomaC4115PAPILLOMA,TRANSITIONAL CELL,BENIGNTRANSITIONALPAPILLOMAA benign papillary neoplasm composed of transitionalcells.Transitional Cell PapillomaC48314PARAGANGLIOMA,BENIGNBENIGNPARAGANGLIONICNEOPLASM;BENIGNNEUROENDOCRINE CELLTUMORA benign neoplasm arising from paraganglia locatedalong nerves composed of neoplastic neuroectodermalchromaffin cells.Benign ParagangliomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 250 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC8559PARAGANGLIOMA,MALIGNANTMALIGNANTTUMOR OF THEPARAGANGLION; MALIGNANTTUMOR OFPARAGANGLION; MALIGNANTPARAGANGLIONICNEOPLASM;MALIGNANTPARAGANGLIONIC TUMOR;MALIGNANTNEOPLASM OFPARAGANGLION; MALIGNANTPARAGANGLION NEOPLASM;MALIGNANTPARAGANGLION TUMOR;PARAGANGLION NEOPLASM;MALIGNANT;MALIGNANTNEOPLASM OFTHEPARAGANGLIONA malignant neoplasm arising from paraganglia locatedalong nerves composed of neoplastic neuroectodermalchromaffin cells.Malignant ParagangliomaC3308PARAGANGLIOMA,UNDETERMINEDPARAGANGLION NEOPLASM;NEOPLASM OFPARAGANGLION; TUMOR OFTHEPARAGANGLION;PARAGANGLION TUMOR;TUMOR OFPARAGANGLION;PARAGANGLIONICNEOPLASM;PARAGANGLIONIC TUMOR;NEOPLASM OFTHEPARAGANGLIONA neoplasm arising from paraganglia located alongnerves composed of neoplastic neuroectodermalchromaffin cells, for which the malignancy status hasnot been established.ParagangliomaC96805PERIPHERALCHOLANGIOCARCINOMA,MALIGNANTPERIPHERALCHOLANGIOCARCINOMAA malignant intrahepatic neoplasm arising from thesmall interlobular bile ducts.Peripheral IntrahepaticCholangiocarcinomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 251 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3798PERIPHERAL NERVESHEATH TUMOR,MALIGNANTMALIGNANTNEURILEMMOMA;MALIGNANTSCHWANNOMA;NEUROFIBROSARCOMA,MALIGNANT;MALIGNANTNEOPLASM OFPERIPHERALNERVESHEATH;MALIGNANTNEOPLASM OFTHEPERIPHERALNERVESHEATH;MALIGNANTNEURILEMMOMA;MALIGNANTNEURILEMOMA; MALIGNANTPERIPHERALNERVESHEATHNEOPLASM;MALIGNANTPERIPHERALNERVESHEATHTUMOUR;MALIGNANTTUMOR OFPERIPHERALNERVESHEATH;MALIGNANTTUMOR OF THEPERIPHERALNERVESHEATH;MPNSTA malignant neoplasm, originating from the sheaths ofthe peripheral nerve.Malignant Peripheral Nerve SheathTumorC48305PHEOCHROMOCYTOMA,BENIGNA benign neoplasm of the adrenal gland medulla.Benign Adrenal GlandPheochromocytomaC92181PHEOCHROMOCYTOMA,COMPLEX, BENIGNA benign neoplasm of the adrenal gland medulla,composed of medullary and neuroectodermalcomponents.Benign Adrenal Gland CompositePheochromocytomaC48306PHEOCHROMOCYTOMA,COMPLEX,UNDETERMINEDA neoplasm of the adrenal gland medulla, composed ofmedullary and neuroectodermal components, forwhich the malignancy status has not been established.Adrenal Gland CompositePheochromocytomaC92184PHEOCHROMOCYTOMA,COMPLEX,MALIGNANTA malignant neoplasm of the adrenal gland medulla,composed of medullary and neuroectodermalcomponents.Malignant Adrenal Gland CompositePheochromocytomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 252 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC4220PHEOCHROMOCYTOMA,MALIGNANTMALIGNANTPHEOCHROMOCYTOMA;MALIGNANTADRENALMEDULLARYPHEOCHROMOCYTOMA;MALIGNANTADRENALPHEOCHROMOCYTOMA;MALIGNANTADRENALGLANDPARAGANGLIOMA;MALIGNANTADRENALGLANDCHROMAFFINOMA;MALIGNANTADRENALGLANDCHROMAFFINNEOPLASM;MALIGNANTADRENALGLANDCHROMAFFINTUMOR;MALIGNANTADRENALGLANDCHROMAFFINPARAGANGLIOMA;PHEOCHROMOBLASTOMA;MALIGNANTADRENALMEDULLARYPARAGANGLIOMAA malignant neoplasm of the adrenal gland medulla.Malignant Adrenal GlandPheochromocytomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 253 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3326PHEOCHROMOCYTOMA,UNDETERMINEDADRENALPHEOCHROMOCYTOMA;CHROMAFFINPARAGANGLIOMA OF THEADRENALGLAND;INTRAADRENALPARAGANGLIOMA; ADRENALMEDULLARYPARAGANGLIOMA; ADRENALGLANDPARAGANGLIOMA; ADRENALMEDULLARYPHEOCHROMOCYTOMA; PCC;ADRENALGLANDCHROMAFFINPARAGANGLIOMA;PHAEOCHROMOCYTOMA;ADRENALGLANDCHROMAFFINOMA;PHEOCHROMOCYTOMAA neoplasm of the adrenal gland medulla, for which themalignancy status has not been established.Adrenal Gland PheochromocytomaC7368 PILOMATRIXOMA, BENIGN BENIGNPILOMATRICOMA;PILOMATRIXOMA;CALCIFYINGEPITHERLIOMA OFMALHERBE;BENIGNPILOMATRIXOMA; BENIGNHAIRFOLLICLENEOPLASMA benign adnexal neoplasm arising from hair-bearingskin surfaces.PilomatricomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 254 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC9344PINEOBLASTOMA,MALIGNANTPINEAL GLANDPNET; PINEALGLANDPRIMITIVENEUROECTODERMALNEOPLASM;PINEAL GLANDPRIMITIVENEUROECTODERMALTUMOR;PINEAL PNET;PINEALPRIMITIVENEUROECTODERMALNEOPLASM;PINEALPRIMITIVENEUROECTODERMALTUMOR; PNETOF PINEALGLAND; PNETOF THE PINEALGLAND;PRIMITIVENEUROECTODERMALNEOPLASM OFPINEALGLAND;PRIMITIVENEUROECTODERMALNEOPLASM OFTHE PINEALGLAND;PRIMITIVENEUROECTODERMALTUMOR OFPINEALGLAND;PRIMITIVENEUROECTODERMALTUMOR OF THEPINEAL GLANDA poorly differentiated malignant embryonal neoplasmarising from the pineal region of the brain.PineoblastomaC6966 PINEOCYTOMA, BENIGN PINEALOMA;BENIGNPINEALOMAA benign neoplasm of the brain arising from the pinealgland.PineocytomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 255 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC7157 PITUICYTOMA, BENIGN POSTERIORPITUITARYTUMOR;POSTERIORPITUITARYGLANDTUMOR;NEUROHYPOPHYSISNEOPLASM;NEUROHYPOPHYSIS TUMOR;POSTERIORPITUITARYNEOPLASMA neoplasm arising from the posterior lobe of thepituitary gland.Posterior Pituitary Gland NeoplasmC4665PLASMA CELL TUMOR,MALIGNANTPLASMACYTICTUMOR;PLASMA CELLDYSCRASIA;PLASMACYTICTUMOR;PLASMA CELLTUMOR;PLASMACYTICNEOPLASMA malignant neoplasm composed of plasma cells.Plasma Cell NeoplasmC3764POLYP, ADENOMATOUS,UNDETERMINEDPOLYPOIDADENOMAA neoplasm, originating from glandular epithelium,exhibiting polypoid growth, for which the malignancystatus has not been determined.Adenomatous PolypC6433POLYP, ENDOMETRIALSTROMAL, BENIGNPOLYP,STROMAL;POLYP OF THEENDOMETRIUM;ENDOMETRIALSTROMALPOLYP; POLYPOFENDOMETRIUM; ADENOMA,ENDOMETRIAL; GLANDULARENDOMETRIALPOLYP;ENDOMETRIALADENOMAA benign polypoid neoplasm of the endometriumprojecting into the endometrial cavity.Endometrial PolypC5680POLYP, INFLAMMATORY,BENIGNINFLAMMATORY POLYP OFTHE LARGEBOWEL;LARGE BOWELINFLAMMATORY POLYP;INFLAMMATORY POLY OFLARGE BOWELA non-neoplastic inflammatory polypoid lesion in thecolon and rectum.Colorectal Inflammatory PolypSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 256 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3340 POLYP, UNDETERMINED A protruding growth attached to the underlying tissueby a broad base or a thin stalk, which can be eitherinflammatory, hamartomatous, or neoplastic.PolypC3664 POLYP, VAGINAL, BENIGN POLYP OF THEVAGINA;POLYP OFVAGINAA benign polypoid growth arising from the vaginalwall.Vaginal PolypC4684RETICULOSIS, MALIGNANT ANGIOCENTRIC T-CELLLYMPHOMAA malignant lymphoid neoplasm composed ofEBV-positive NK/T cells arranged in an angiocentricpattern.Nasal Type Extranodal NK/T-CellLymphomaC7541RETINOBLASTOMA,MALIGNANTRBA malignant neoplasm originating in the nuclear layerof the retina.RetinoblastomaC3358 RHABDOMYOMA, BENIGN A benign neoplasm arising from skeletal muscle,characterized by the presence of rhabdomyoblasts.RhabdomyomaC3359RHABDOMYOSARCOMA,MALIGNANTA malignant mesenchymal neoplasm arising fromskeletal muscle.RhabdomyosarcomaC35815SARCOMA,GRANULOCYTIC,MALIGNANTA malignant neoplasm composed of myeloblasts,neutrophils and neutrophil precursors.Granulocytic SarcomaC27349SARCOMA, HISTIOCYTIC,MALIGNANTA malignant neoplasm composed of cells resemblinghistiocytes.Histiocytic SarcomaC83<strong>12</strong>SARCOMA,LEPTOMENINGEAL,MALIGNANTSARCOMA,MENINGEAL;SARCOMA OFLEPTOMENINGES; SARCOMAOF THELEPTOMENINGESA malignant mesenchymal neoplasm arising from theleptomeninges.Leptomeningeal SarcomaC9118 SARCOMA, MALIGNANT SARCOMA OFSOFT TISSUEAND BONE;SARCOMA OFTHE SOFTTISSUE ANDBONE;SARCOMA;MESENCHYMAL TUMOR,MALIGNANTA malignant mesenchymal neoplasm. A general termfor which the transformed cell type has not beenspecified.SarcomaC3520SARCOMA, MYELOID,MALIGNANTCHLOROMA;EXTRAMEDULLARYMYELOIDTUMORA malignant neoplasm composed of myeloblasts orimmature myeloid cells. It occurs in extramedullarysites or the bone.Myeloid SarcomaC3400SARCOMA, SYNOVIAL,MALIGNANTSSA malignant neoplasm that usually arises in thesynovial membranes of the joints and the synovial cellsof the tendons and bursae.Synovial SarcomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 257 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC27096SARCOMA,UNDIFFERENTIATED,MALIGNANTEMBRYONALSARCOMA,UNDIFFERENTIATEDA rare malignant neoplasm of the liver that is mostcommon in youth.Undifferentiated (Embryonal)SarcomaC3269 SCHWANNOMA, BENIGN NEURILEMMOMA;SCHWANNOMA;NEURINOMA;SCHWANNOMA (WHOGRADE I)C9309 SEMINOMA, MALIGNANT SEMINOMA,PURE;SEMINOMAA benign neoplasm of the peripheral nervous systemcomposed of well-differentiated Schwann cells.A malignant germ cell neoplasm of the testis.SchwannomaSeminomaC670<strong>12</strong>SERTOLI CELL TUMOR,BENIGNBENIGNANDROBLASTOMAA benign neoplasm of the testis or ovary, originatingfrom Sertoli cells.Benign Sertoli Cell TumorC67006SERTOLI CELL TUMOR,MALIGNANTMALIGNANTANDROBLASTOMAA malignant neoplasm of the testis or ovary,originating from Sertoli cells.Malignant Sertoli Cell TumorC98794SERTOLI CELL TUMOR,UNDETERMINEDAn ovarian neoplasm comprising Sertoli cells in whichthe malignancy status has not been established.Undetermined Sertoli Cell TumorC6569STROMAL NEPHROMA,MALIGNANTCMN;MESOBLASTICNEPHROMAA congenital malignant neoplasm of the kidneycharacterized by the presence of fibroblastic cells.Congenital Mesoblastic NephromaC8973STROMAL SARCOMA,ENDOMETRIAL,MALIGNANTESSA malignant, mesenchymal tumor of the uterinestroma.Endometrioid Stromal SarcomaC6926STROMAL SARCOMA,MALIGNANTSTROMALTUMOR,MALIGNANTA malignant neoplasm characterized by the presence ofatypical mesenchymal-stromal cells.Stromal SarcomaC66772STROMAL TUMOR,BENIGNA benign neoplasm composed of mesenchymalstromal cells.Benign Stromal TumorC67561STROMAL TUMOR,GONADAL, MALIGNANTSEX CORDSTROMALTUMOR,MALIGNANTA malignant neoplasm originating from the gonadal sexcord stroma.Malignant Sex Cord-Stromal TumorC3794STROMAL TUMOR,GONADAL,UNDETERMINEDSEXCORD-STROMAL TUMOR,UNDETERMINED; SEXCORD-STROMAL NEOPLASMA neoplasm originating from the gonadal sex cordstroma, for which the status of malignancy has notbeen determined.Sex Cord-Stromal TumorSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 258 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC3795SUBEPENDYMOMA,BENIGNWHO GRADE IEPENDYMALTUMOR; WHOGRADE IEPENDYMALNEOPLASM;SUBEPENDYMAL GLIOMAA benign neoplasm of the brain localized in the vicinityof a ventricular wall and is composed of glial tumorcell clusters embedded in an abundant fibrillary matrixwith frequent microcystic changes.SubependymomaC3829 SYNOVIOMA, BENIGN BENIGNNEOPLASM OFSYNOVIUM;BENIGNSYNOVIOMA;BENIGNNEOPLASM OFTHESYNOVIUM;BENIGNTUMOR OF THESYNOVIUM;BENIGNTUMOR OFSYNOVIUM;BENIGNSYNOVIALTUMORA benign neoplasm arising from the synovialmembrane.C67107 TERATOMA, BENIGN A benign germ-cell neoplasm derived from pluripotentcells and consisting of components from one or moreof the three germ-cell layers.Benign Synovial NeoplasmBenign TeratomaC4286 TERATOMA, MALIGNANT IMMATURETERATOMAA malignant germ-cell neoplasm derived frompluripotent cells and consisting of components fromone or more of the three germ-cell layers.Immature TeratomaC3403TERATOMA,UNDETERMINEDA germ-cell neoplasm derived from pluripotent cellsand consisting of components from one or more of thethree germ-cell layers, for which the malignancy statushas not been established.TeratomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 259 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC5219 THECOMA, BENIGN THECAL CELLTUMOR,BENIGN;BENIGNTHECAL CELLNEOPLASM OFTHE OVARY;BENIGNOVARIANTHECAL CELLTUMOR;BENIGNOVARIANTHECAL CELLNEOPLASM;BENIGNTHECAL CELLTUMOR OF THEOVARY;BENIGNTHECAL CELLTUMOR OFOVARY;BENIGNTHECOMA OFTHE OVARY;BENIGNTHECOMA OFOVARY;BENIGNTHECAL CELLNEOPLASM OFOVARYA benign sex-cord neoplasm of the ovary, originatingfrom theca cells.Benign Ovarian ThecomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 260 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC6929THECOMA, OVARIAN,MALIGNANTTHECOMA,MALIGNANT;MALIGNANTTHECOMA OFTHE OVARY;MALIGNANTTHECAL CELLNEOPLASM OFTHE OVARY;MALIGNANTTHECAL CELLNEOPLASM OFOVARY;MALIGNANTOVARIANTHECAL CELLNEOPLASM;MALIGNANTTHECAL CELLTUMOR OF THEOVARY;MALIGNANTTHECOMA OFOVARY;MALIGNANTOVARIANTHECAL CELLTUMOR;MALIGNANTTHECAL CELLTUMOR OFOVARYA malignant sex-cord neoplasm of the ovary,originating from theca cells.Malignant Ovarian ThecomaC66746 THYMOMA, BENIGN A benign neoplasm of the thymus, originating fromepithelial thymus cells.C76<strong>12</strong> THYMOMA, MALIGNANT A malignant neoplasm of the thymus, originating fromepithelial thymus cells.Benign ThymomaMalignant ThymomaC3411THYMOMA,UNDETERMINEDA neoplasm of the thymus, originating from epithelialthymus cells, for which the malignancy status has notbeen established.ThymomaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 261 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C88025 - NEOPLASM - Neoplasm TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC27132TRICHOEPITHELIOMA,BENIGNBROOKE'STUMOR;TRICHOGENICTRICHOBLASTOMA;TRICHOGENICADNEXALTUMOR;TRICHOEPITHELIOMAA benign hair follicle neoplasm with trichoblasticdifferentiation.TrichoblastomaC3011YOLK SAC TUMOR,MALIGNANTENDODERMALSINUS TUMOR;YOLK SACTUMOR SITEUNSPECIFIED;YOLK SACNEOPLASM;ENDODERMALSINUSNEOPLASMA non-seminomatous malignant germ cell tumorcomposed of primitive germ cells and which producean eosinophilic substance (alpha-fetoprotein).Yolk Sac TumorSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 262 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90004 - NEOSTAT - Neoplastic StatusCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC14172 BENIGN Benign For neoplasms, a non-infiltrating andnon-metastasizing neoplastic process that ischaracterized by the absence of morphologic featuresassociated with malignancy (e.g., severe atypia, nuclearpleomorphism, tumor cell necrosis, and abnormalmitoses). For other conditions, a process that is mildin nature and not dangerous to health. (NCI)C14143 MALIGNANT Malignant Refers to abnormal cell activity manifested bydecreased control over growth and function, causingtumor growth or spread into surrounding tissue andadverse effects to the host. (NCI)C14174 METASTATIC Metastatic A term referring to the pathologic observation of atumor extension or migration from its original site ofgrowth to another non-adjacent site.C89084 UNDETERMINED Undetermined A term referring to the lack of definitive clinical orpathologic criteria for a tumor to predict its clinicalcourse or classify it as benign or malignant.BenignMalignantMetastaticUndeterminedSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 263 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66742 - NY - No Yes ResponseCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49487 N No The non-affirmative response to a question. (NCI) NoC48660 NA NA Determination of a value is not relevant in the currentcontext. (NCI)C17998 U U; Unknown Not known, not observed, not recorded, or refused.(NCI)Not ApplicableUnknownC49488 Y Yes The affirmative response to a question. (NCI) YesSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 264 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89976 - OMTEST - Organ Measurement Test NameCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90426 Organ to Body Weight Ratio Organ to BodyWeight RatioC90427 Organ to Brain Weight Ratio Organ to BrainWeight RatioC90428 Organ to Heart Weight Ratio Organ to HeartWeight RatioA numeric comparison of the weight of an organ tobody weight.A numeric comparison of the weight of an organ to theweight of the brain.A numeric comparison of the weight of an organ to theweight of the heart.Organ to Body Weight RatioOrgan to Brain Weight RatioOrgan to Heart Weight RatioC25208 Weight Weight The vertical force exerted by a mass as a result ofgravity. (NCI)WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 265 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89977 - OMTESTCD - Organ Measurement Test CodeCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90427 OWBR Organ to BrainWeight RatioC90426 OWBW Organ to BodyWeight RatioC90428 OWHT Organ to HeartWeight RatioA numeric comparison of the weight of an organ to theweight of the brain.A numeric comparison of the weight of an organ tobody weight.A numeric comparison of the weight of an organ to theweight of the heart.Organ to Brain Weight RatioOrgan to Body Weight RatioOrgan to Heart Weight RatioC25208 WEIGHT Weight The vertical force exerted by a mass as a result ofgravity. (NCI)WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 266 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C95<strong>12</strong>0 - PHSPRP - Physical Properties Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC37927 Color Color The appearance of objects (or light sources) describedin terms of a person's perception of their hue andlightness (or brightness) and saturation. (NCI)C95110 Consistency Consistency A description about the firmness or make-up of anentity.C25333 Depth Depth The extent downward or inward; the perpendicularmeasurement from the surface downward to determinedeepness. (NCI)C25365 Description Description A written or verbal account, representation, statement,or explanation of something. (NCI)C25285 Diameter Diameter The length of a straight line passing through the centerof a circle or sphere and connecting two points on thecircumference. (NCI)C95109 Hair Cover Hair Cover A description of the quantity or quality of the hair orfur covering a biological entity. (NCI)C25334 Length Length The linear extent in space from one end of somethingto the other end, or the extent of something frombeginning to end. (NCI)C25677 Shape Shape The spatial arrangement of something as distinct fromits substance. (NCI)ColorConsistencyDepthDescriptionDiameterHair or Fur CoverLengthShapeC25757 Ulceration Ulceration The formation or development of an ulcer. (NCI) UlcerationC25345 Width Width The extent or measurement of something from side toside. (NCI)WidthSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 267 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C95<strong>12</strong>1 - PHSPRPCD - Physical Properties Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC37927 COLOR Color The appearance of objects (or light sources) describedin terms of a person's perception of their hue andlightness (or brightness) and saturation. (NCI)C95110 CONSIST Consistency A description about the firmness or make-up of anentity.C25333 DEPTH Depth The extent downward or inward; the perpendicularmeasurement from the surface downward to determinedeepness. (NCI)C25365 DESCR Description A written or verbal account, representation, statement,or explanation of something. (NCI)C25285 DIAMETER Diameter The length of a straight line passing through the centerof a circle or sphere and connecting two points on thecircumference. (NCI)C95109 HAIRCOV Hair Cover A description of the quantity or quality of the hair orfur covering a biological entity. (NCI)C25334 LENGTH Length The linear extent in space from one end of somethingto the other end, or the extent of something frombeginning to end. (NCI)C25677 SHAPE Shape The spatial arrangement of something as distinct fromits substance. (NCI)ColorConsistencyDepthDescriptionDiameterHair or Fur CoverLengthShapeC25757 ULCER Ulceration The formation or development of an ulcer. (NCI) UlcerationC25345 WIDTH Width The extent or measurement of something from side toside. (NCI)WidthSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 268 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85787AUC %Back ExtrapolationPredAUC %BackExtrapolationPredThe area under the curve (AUC) from the predictedconcentration value at time zero to the first measuredconcentration value as a percentage of the area underthe curve extrapolated to infinity. Applies only forintravascular bolus dosing.Predicted Area Under the CurvePercent Back ExtrapolationC85764 AUC %Extrapolation Obs AUC%ExtrapolationObsC85788 AUC %Extrapolation Pred AUC%ExtrapolationPredThe area under the curve (AUC) from the last observednon-zero concentration value to infinity as apercentage of the area under the curve extrapolated toinfinity.The area under the curve (AUC) from the last predictednon-zero concentration value to infinity as apercentage of the area under the curve extrapolated toinfinity.Observed Area Under the CurvePercent ExtrapolationPredicted Area Under the CurvePercent ExtrapolationC85564 AUC All AUC All The area under the curve (AUC) from the time ofdosing to the time of the last observation, regardlessof whether the last concentration is measurable or not.Area Under the Curve AllC92362 AUC All Norm by BMI AUC All Normby BMIC92306 AUC All Norm by Dose AUC All Normby DoseC92307 AUC All Norm by SA AUC All Normby SAC92308 AUC All Norm by WT AUC All Normby WTThe area under the curve (AUC) from the time ofdosing to the time of the last observation divided bythe body mass index, regardless of whether the lastconcentration is measurable or not.The area under the curve (AUC) from the time ofdosing to the time of the last observation divided bythe dose, regardless of whether the last concentrationis measurable or not.The area under the curve (AUC) from the time ofdosing to the time of the last observation divided bythe surface area, regardless of whether the lastconcentration is measurable or not.The area under the curve (AUC) from the time ofdosing to the time of the last observation divided bythe weight, regardless of whether the lastconcentration is measurable or not.AUC All Normalized by Body MassIndexAUC All Normalized by DoseAUC All Normalized by SurfaceAreaAUC All Normalized by WeightC85761 AUC Infinity Obs AUC Infinity Obs The area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration.Observed Area Under the CurveInfinityC92316AUC Infinity Obs Norm byBMIAUC Infinity ObsNorm by BMIThe area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration, divided by the body massindex.AUC Infinity Observed Normalizedby Body Mass IndexC96695AUC Infinity Obs Norm byDoseAUC Infinity ObsNorm by DoseThe area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration, divided by the dose.AUC Infinity Observed Normalizedby DoseC92317 AUC Infinity Obs Norm by SA AUC Infinity ObsNorm by SAThe area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration, divided by the surface area.AUC Infinity Observed Normalizedby Surface AreaC92318AUC Infinity Obs Norm byWTAUC Infinity ObsNorm by WTThe area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration, divided by the weight.AUC Infinity Observed Normalizedby WeightC85785 AUC Infinity Pred AUC InfinityPredThe area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration.Predicted Area Under the CurveInfinitySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 269 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92319AUC Infinity Pred Norm byBMIAUC InfinityPred Norm byBMIThe area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration, divided by the body massindex.AUC Infinity Predicted Normalizedby Body Mass IndexC85786AUC Infinity Pred Norm byDoseAUC InfinityPred Norm byDoseThe area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration, divided by the dose.Predicted Area Under the CurveInfinity by DoseC92320AUC Infinity Pred Norm bySAAUC InfinityPred Norm bySAThe area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration, divided by the surface area.AUC Infinity Predicted Normalizedby Surface AreaC92321AUC Infinity Pred Norm byWTAUC InfinityPred Norm byWTThe area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration, divided by the weight.AUC Infinity Predicted Normalizedby WeightC85567 AUC Over Dosing Interval AUC OverDosing IntervalThe area under the curve (AUC) for the definedinterval between doses (TAU).Area Under the Curve Over DosingIntervalC92322AUC Over Dosing IntervalNorm by BMIAUC OverDosing IntervalNorm by BMIThe area under the curve (AUC) for the definedinterval between doses (TAU) divided by the bodymass index.AUC Over Dosing IntervalNormalized by Body Mass IndexC92323AUC Over Dosing IntervalNorm by DoseAUC OverDosing IntervalNorm by DoseThe area under the curve (AUC) for the definedinterval between doses (TAU) divided by the dose.AUC Over Dosing IntervalNormalized by DoseC92324AUC Over Dosing IntervalNorm by SAAUC OverDosing IntervalNorm by SAThe area under the curve (AUC) for the definedinterval between doses (TAU) divided by the surfacearea.AUC Over Dosing IntervalNormalized by Surface AreaC92325AUC Over Dosing IntervalNorm by WTAUC OverDosing IntervalNorm by WTThe area under the curve (AUC) for the definedinterval between doses (TAU) divided by the weight.AUC Over Dosing IntervalNormalized by WeightC85566 AUC from T1 to T2 AUC from T1 toT2The area under the curve (AUC) over the interval fromT1 to T2.Area Under the Curve from T1 to T2C923<strong>12</strong>AUC from T1 to T2 Norm byBMIAUC from T1 toT2 Norm by BMIThe area under the curve (AUC) over the interval fromT1 to T2 divided by the body mass index.AUC from T1 to T2 Normalized byBody Mass IndexC92313AUC from T1 to T2 Norm byDoseAUC from T1 toT2 Norm byDoseThe area under the curve (AUC) over the interval fromT1 to T2 divided by the dose.AUC from T1 to T2 Normalized byDoseC92314AUC from T1 to T2 Norm bySAAUC from T1 toT2 Norm by SAThe area under the curve (AUC) over the interval fromT1 to T2 divided by the surface area.AUC from T1 to T2 Normalized bySurface AreaC92315AUC from T1 to T2 Norm byWTAUC from T1 toT2 Norm by WTThe area under the curve (AUC) over the interval fromT1 to T2 divided by the weight.AUC from T1 to T2 Normalized byWeightC85565 AUC to Last Nonzero Conc AUC to LastNonzero ConcThe area under the curve (AUC) from the time ofdosing to the last measurable concentration.Area Under the Curve From Dosingto Last ConcentrationC92309AUC to Last Nonzero ConcNorm by BMIAUC to LastNonzero ConcNorm by BMIThe area under the curve (AUC) from the time ofdosing to the last measurable concentration divided bythe body mass index.AUC Dosing to Last ConcentrationNormalized by Body Mass IndexC92310AUC to Last Nonzero ConcNorm by DoseAUC to LastNonzero ConcNorm by DoseThe area under the curve (AUC) from the time ofdosing to the last measurable concentration divided bythe dose.AUC Dosing to Last ConcentrationNormalized by DoseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 270 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92311AUC to Last Nonzero ConcNorm by SAAUC to LastNonzero ConcNorm by SAThe area under the curve (AUC) from the time ofdosing to the last measurable concentration divided bythe surface area.AUC Dosing to Last ConcentrationNormalized by Surface AreaC92305AUC to Last Nonzero ConcNorm by WTAUC to LastNonzero ConcNorm by WTThe area under the curve (AUC) from the time ofdosing to the last measurable concentration divided bythe weight.AUC Dosing to Last ConcentrationNormalized by WeightC85766 AUMC % Extrapolation Obs AUMC %ExtrapolationObsC85790 AUMC % Extrapolation Pred AUMC %ExtrapolationPredC85765 AUMC Infinity Obs AUMC InfinityObsThe area under the moment curve (AUMC) from thelast observed non-zero concentration value to infinityas a percentage of the area under the moment curveextrapolated to infinity.The area under the moment curve (AUMC) from thelast predicted non-zero concentration value to infinityas a percentage of the area under the moment curveextrapolated to infinity.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration.Observed Area Under the FirstMoment Curve PercentExtrapolationPredicted Area Under the FirstMoment Curve PercentExtrapolationObserved Area Under the FirstMoment Curve InfinityC92330AUMC Infinity Obs Norm byBMIAUMC InfinityObs Norm byBMIThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration, divided by thebody mass index.AUMC Infinity ObservedNormalized by Body Mass IndexC92331AUMC Infinity Obs Norm byDoseAUMC InfinityObs Norm byDoseThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration, divided by thedose.AUMC Infinity ObservedNormalized by DoseC92332AUMC Infinity Obs Norm bySAAUMC InfinityObs Norm by SAThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration, divided by thesurface area.AUMC Infinity ObservedNormalized by Surface AreaC92333AUMC Infinity Obs Norm byWTAUMC InfinityObs Norm byWTThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration, divided by theweight.AUMC Infinity ObservedNormalized by WeightC85789 AUMC Infinity Pred AUMC InfinityPredThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration.Predicted Area Under the FirstMoment Curve InfinityC92334AUMC Infinity Pred Norm byBMIAUMC InfinityPred Norm byBMIThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration, divided by thebody mass index.AUMC Infinity PredictedNormalized by Body Mass IndexC92335AUMC Infinity Pred Norm byDoseAUMC InfinityPred Norm byDoseThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration, divided by thedose.AUMC Infinity PredictedNormalized by DoseC92336AUMC Infinity Pred Norm bySAAUMC InfinityPred Norm bySAThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration, divided by thesurface area.AUMC Infinity PredictedNormalized by Surface AreaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 271 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92337AUMC Infinity Pred Norm byWTAUMC InfinityPred Norm byWTThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration, divided by theweight.AUMC Infinity PredictedNormalized by WeightC85570 AUMC Over Dosing Interval AUMC OverDosing IntervalThe area under the first moment curve (AUMC) for thedefined interval between doses (TAU).Area Under the First Moment CurveOver Dosing IntervalC92338AUMC Over Dosing IntervalNorm by BMIAUMC OverDosing IntervalNorm by BMIThe area under the first moment curve (AUMC) for thedefined interval between doses (TAU) divided by thebody mass index.AUMC Over Dosing IntervalNormalized by Body Mass IndexC92339AUMC Over Dosing IntervalNorm by DoseAUMC OverDosing IntervalNorm by DoseThe area under the first moment curve (AUMC) for thedefined interval between doses (TAU) divided by thedose.AUMC Over Dosing IntervalNormalized by DoseC92340AUMC Over Dosing IntervalNorm by SAAUMC OverDosing IntervalNorm by SAThe area under the first moment curve (AUMC) for thedefined interval between doses (TAU) divided by thesurface area.AUMC Over Dosing IntervalNormalized by Surface AreaC92341AUMC Over Dosing IntervalNorm by WTAUMC OverDosing IntervalNorm by WTThe area under the first moment curve (AUMC) for thedefined interval between doses (TAU) divided by theweight.AUMC Over Dosing IntervalNormalized by WeightC85569 AUMC to Last Nonzero Conc AUMC to LastNonzero ConcThe area under the moment curve (AUMC) from thetime of dosing to the last measurable concentration.Area Under the First Moment CurveFrom Dosing to Last ConcentrationC92326AUMC to Last Nonzero ConcNorm by BMIAUMC to LastNonzero ConcNorm by BMIThe area under the moment curve (AUMC) from thetime of dosing to the last measurable concentrationdivided by the body mass index.AUMC Dosing to Last ConcentrationNormalized by Body Mass IndexC92327AUMC to Last Nonzero ConcNorm by DoseAUMC to LastNonzero ConcNorm by DoseThe area under the moment curve (AUMC) from thetime of dosing to the last measurable concentrationdivided by the dose.AUMC Dosing to Last ConcentrationNormalized by DoseC92328AUMC to Last Nonzero ConcNorm by SAAUMC to LastNonzero ConcNorm by SAThe area under the moment curve (AUMC) from thetime of dosing to the last measurable concentrationdivided by the surface area.AUMC Dosing to Last ConcentrationNormalized by Surface AreaC92329AUMC to Last Nonzero ConcNorm by WTAUMC to LastNonzero ConcNorm by WTThe area under the moment curve (AUMC) from thetime of dosing to the last measurable concentrationdivided by the weight.AUMC Dosing to Last ConcentrationNormalized by WeightC85768 AURC % Extrapolation Obs AURC %ExtrapolationObsC85792 AURC % Extrapolation Pred AURC %ExtrapolationPredThe area under the urinary excretion rate curve(AURC) from the last observed non-zero rate value toinfinity as a percentage of the area under the urinaryexcretion rate curve extrapolated to infinity.The area under the urinary excretion rate curve(AURC) from the last predicted non-zero rate value toinfinity as a percentage of the area under the urinaryexcretion rate curve extrapolated to infinity.Observed Area Under the UrinaryExcretion Rate Curve PercentExtrapolationPredicted Area Under the UrinaryExcretion Rate Curve PercentExtrapolationC85841 AURC All AURC All The area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate.Area Under Urinary Excretion RateCurve AllC92342 AURC All Norm by BMI AURC All Normby BMIC92343 AURC All Norm by Dose AURC All Normby DoseThe area under the urinary excretion rate curve(AURC) from time zero to the last measurable ratedivided by the body mass index.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable ratedivided by the dose.AURC All Normalized by Body MassIndexAURC All Normalized by DoseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 272 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92344 AURC All Norm by SA AURC All Normby SAC92345 AURC All Norm by WT AURC All Normby WTThe area under the urinary excretion rate curve(AURC) from time zero to the last measurable ratedivided by the surface area.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable ratedivided by the weight.AURC All Normalized by SurfaceAreaAURC All Normalized by WeightC92346AURC Dosing to Last ConcNorm by BMIAURC to LastNonzero RateNorm by BMIThe area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate,divided by the body mass index.AURC Dosing to Last ConcentrationNormalized by Body Mass IndexC92347AURC Dosing to Last ConcNorm by DoseAURC to LastNonzero RateNorm by DoseThe area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate,divided by the dose.AURC Dosing to Last ConcentrationNormalized by DoseC92348AURC Dosing to Last ConcNorm by SAAURC to LastNonzero RateNorm by SAThe area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate,divided by the surface area.AURC Dosing to Last ConcentrationNormalized by Surface AreaC92349AURC Dosing to Last ConcNorm by WTAURC to LastNonzero RateNorm by WTThe area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate,divided by the weight.AURC Dosing to Last ConcentrationNormalized by WeightC85767 AURC Infinity Obs AURC InfinityObsThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate.Observed Area Under the UrinaryExcretion Rate Curve infinityC92354AURC Infinity Obs Norm byBMIAURC InfinityObs Norm byBMIThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate, divided by thebody mass index.AURC Infinity Observed Normalizedby Body Mass IndexC92355AURC Infinity Obs Norm byDoseAURC InfinityObs Norm byDoseThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate, divided by thedose.AURC Infinity Observed Normalizedby DoseC92356AURC Infinity Obs Norm bySAAURC InfinityObs Norm by SAThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate, divided by thesurface area.AURC Infinity Observed Normalizedby Surface AreaC92357AURC Infinity Obs Norm byWTAURC InfinityObs Norm byWTThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate, divided by theweight.AURC Infinity Observed Normalizedby WeightC85791 AURC Infinity Pred AURC InfinityPredThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate.Predicted Area Under the UrinaryExcretion Rate Curve InfinityC92358AURC Infinity Pred Norm byBMIAURC InfinityPred Norm byBMIThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate,divded by the body mass index.AURC Infinity Predicted Normalizedby Body Mass IndexC92359AURC Infinity Pred Norm byDoseAURC InfinityPred Norm byDoseThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate,divded by the dose.AURC Infinity Predicted Normalizedby DoseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 273 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92360AURC Infinity Pred Norm bySAAURC InfinityPred Norm bySAThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate,divded by the surface area.AURC Infinity Predicted Normalizedby Surface AreaC92361AURC Infinity Pred Norm byWTAURC InfinityPred Norm byWTThe area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate,divded by the weight.AURC Infinity Predicted Normalizedby WeightC85572 AURC from T1 to T2 AURC from T1to T2The area under the urinary excretion rate curve(AURC) over the interval from T1 to T2.Area Under the Urinary ExcretionRate Curve from T1 to T2C92350AURC from T1 to T2 Norm byBMIAURC from T1to T2 Norm byBMIThe area under the urinary excretion rate curve(AURC) over the interval from T1 to T2 divided by thebody mass index.AURC from T1 to T2 Normalized byBody Mass IndexC92351AURC from T1 to T2 Norm byDoseAURC from T1to T2 Norm byDoseThe area under the urinary excretion rate curve(AURC) over the interval from T1 to T2 divided by thedose.AURC from T1 to T2 Normalized byDoseC92352AURC from T1 to T2 Norm bySAAURC from T1to T2 Norm bySAThe area under the urinary excretion rate curve(AURC) over the interval from T1 to T2 divided by thesurface area.AURC from T1 to T2 Normalized bySurface AreaC92353AURC from T1 to T2 Norm byWTAURC from T1to T2 Norm byWTThe area under the urinary excretion rate curve(AURC) over the interval from T1 to T2 divided by theweight.AURC from T1 to T2 Normalized byWeightC85571 AURC to Last Nonzero Rate AURC to LastNonzero RateC102356 Accumulation Ratio AUC AccumulationRatio AUCC102357 Accumulation Ratio Cmax AccumulationRatio CmaxC102358 Accumulation Ratio Cmin AccumulationRatio CminC102426 Accumulation Ratio Ctrough AccumulationRatio CtroughC102363 Amt Rec Over Dosing Interval Amt Rec OverDosing IntervalThe area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate.The area under the curve (AUCTAU) at steady statedivided by the area under the curve over the initialdosing interval.The maximum concentration at steady state divided bythe maximum concentration during the initial dosinginterval.The minimum concentration at steady state divided bythe minimum concentration during the initial dosinginterval.The trough concentration at steady state divided by thetrough concentration during the initial dosing interval.The cumulative amount recovered from the specimentype specified in PPSPEC between doses (TAU).Area Under the Urinary ExcretionRate Curve From Dosing to LastConcentrationAccumulation Ratio Area Under theCurveAccumulation Ratio CmaxAccumulation Ratio CminAccumulation Ratio CtroughAmount Recovered Over DosingIntervalC102364Amt Rec Over Dosing IntervalNorm by BMIAmt Rec OverDosing IntervalNorm by BMIThe cumulative amount recovered from the specimentype specified in PPSPEC between doses (TAU)divided by body mass index.Amount Recovered Over DosingInterval Normalized by Body MassIndexC102365Amt Rec Over Dosing IntervalNorm by SAAmt Rec OverDosing IntervalNorm by SAThe cumulative amount recovered from the specimentype specified in PPSPEC between doses (TAU)divided by surface area.Amount Recovered Over DosingInterval Normalized by Surface AreaC102366Amt Rec Over Dosing IntervalNorm by WTAmt Rec OverDosing IntervalNorm by WTThe cumulative amount recovered from the specimentype specified in PPSPEC between doses (TAU)divided by weight.Amount Recovered Over DosingInterval Normalized by WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 274 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102359 Amt Rec from T1 to T2 Amt Rec fromT1 to T2The cumulative amount recovered from the specimentype specified in PPSPEC over the interval from T1 toT2.Amount Recovered from T1 to T2C102360Amt Rec from T1 to T2 Normby BMIAmt Rec fromT1 to T2 Normby BMIThe cumulative amount recovered from the specimentype specified in PPSPEC over the interval from T1 toT2 divided by body mass index.Amount Recovered from T1 to T2Normalized by Body Mass IndexC102361Amt Rec from T1 to T2 Normby SAAmt Rec fromT1 to T2 Normby SAThe cumulative amount recovered from the specimentype specified in PPSPEC over the interval from T1 toT2 divided by surface area.Amount Recovered from T1 to T2Normalized by Surface AreaC102362Amt Rec from T1 to T2 Normby WTAmt Rec fromT1 to T2 Normby WTThe cumulative amount recovered from the specimentype specified in PPSPEC over the interval from T1 toT2 divided by weight.Amount Recovered from T1 to T2Normalized by WeightC85575 Average Conc Average Conc;AverageConcentrationAUCTAU divided by TAU.Average ConcentrationC92367 Average Conc Norm by BMI Average ConcNorm by BMIC92368 Average Conc Norm by Dose Average ConcNorm by DoseAUCTAU divided by TAU and then divided by the bodymass index.Average Concentration Normalizedby Body Mass IndexAUCTAU divided by TAU and then divided by the dose. Average Concentration Normalizedby DoseC92369 Average Conc Norm by SA Average ConcNorm by SAC92370 Average Conc Norm by WT Average ConcNorm by WTAUCTAU divided by TAU and then divided by thesurface area.AUCTAU divided by TAU and then divided by theweight.Average Concentration Normalizedby Surface AreaAverage Concentration Normalizedby WeightC41185 Conc Conc The quantity of a specified substance in a unit volumeor weight of another substance.ConcentrationC102394 Conc Trough Conc Trough Concentration at end of dosing interval. Trough ConcentrationC102395 Conc Trough by BMI Conc Trough byBMIC102396 Conc Trough by Dose Conc Trough byDoseC102397 Conc Trough by SA Conc Trough bySAC102398 Conc Trough by WT Conc Trough byWTThe trough concentration divided by body mass index.The trough concentration divided by dose.The trough concentration divided by surface area.The trough concentration divided by weight.Trough Concentration Divided byBody Mass IndexTrough Concentration Divided byDoseTrough Concentration Divided bySurface AreaTrough Concentration Divided byWeightC102367 Conc by BMI Conc by BMI The concentration divided by body mass index. Concentration Divided by Body MassIndexC102368 Conc by Dose Conc by Dose The concentration divided by dose. Concentration Divided by DoseC102369 Conc by SA Conc by SA The concentration divided by surface area. Concentration Divided by SurfaceAreaC102370 Conc by WT Conc by WT The concentration divided by weight. Concentration Divided by WeightC85821Correlation Between TimeXand Log ConcYCorrelationBetween TimeXand Log ConcYThe correlation between time (X) and logconcentration (Y) for the points used in the estimationof lambda z.Time and Log ConcentrationCorrelationC95007 Effective Half-Life EffectiveHalf-LifeThe drug half-life that quantifies the accumulationratio of a drug following multiple dosing.Effective Half-lifeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 275 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85581 Fluctuation% Fluctuation% The difference between Cmin and Cmax standardizedto Cavg, between dose time and Tau.Concentration Variability BetweenDose Time and TauC85818 Half-Life Lambda z Half-LifeLambda zTerminal half-life.Terminal Half LifeC85644 Initial Conc Initial Conc Initial concentration. Given only for bolus IV models. Initial ConcentrationC92383 Initial Conc Norm by BMI Initial ConcNorm by BMIC92384 Initial Conc Norm by Dose Initial ConcNorm by DoseC92385 Initial Conc Norm by SA Initial ConcNorm by SAC92386 Initial Conc Norm by WT Initial ConcNorm by WTInitial concentration divided by the body mass index.Given only for bolus IV models.Initial concentration divided by the dose. Given onlyfor bolus IV models.Initial concentration divided by the surface area. Givenonly for bolus IV models.Initial concentration divided by the weight. Given onlyfor bolus IV models.Initial Concentration Normalized byBody Mass IndexInitial Concentration Normalized byDoseInitial Concentration Normalized bySurface AreaInitial Concentration Normalized byWeightC85652 Lambda z Lambda z The first order rate constant associated with theterminal (log-linear) portion of the curve.Lambda ZC85653 Lambda z Lower Limit Lambda z LowerLimitC85654 Lambda z Upper Limit Lambda z UpperLimitC85656 Last Meas Excretion Rate Last MeasExcretion RateThe lower limit on time for values to be included in thecalculation of Lambda z.The upper limit on time for values to be included in thecalculation of Lambda z.The last measurable (positive) excretion rate.Lambda Z Time Lower LimitLambda Z Time Upper LimitLast Measurable Observed ExcretionRateC92391Last Meas Excretion RateNorm by BMILast MeasExcretion RateNorm by BMIThe last measurable (positive) excretion rate dividedby the body mass index.Last Measurable Excretion RateNormalized by Body Mass IndexC92392Last Meas Excretion RateNorm by DoseLast MeasExcretion RateNorm by DoseThe last measurable (positive) excretion rate dividedby the dose.Last Measurable Excretion RateNormalized by DoseC92393Last Meas Excretion RateNorm by SALast MeasExcretion RateNorm by SAThe last measurable (positive) excretion rate dividedby the surface area.Last Measurable Excretion RateNormalized by Surface AreaC92394Last Meas Excretion RateNorm by WTLast MeasExcretion RateNorm by WTThe last measurable (positive) excretion rate dividedby the weight.Last Measurable Excretion RateNormalized by WeightC85655 Last Nonzero Conc Last NonzeroConcThe concentration corresponding to Tlast.Last ConcentrationC92387Last Nonzero Conc Norm byBMILast NonzeroConc Norm byBMIThe concentration corresponding to Tlast divided bythe body mass index.Last Concentration Normalized byBody Mass IndexC92388Last Nonzero Conc Norm byDoseLast NonzeroConc Norm byDoseThe concentration corresponding to Tlast divided bythe dose.Last Concentration Normalized byDoseC92389Last Nonzero Conc Norm bySALast NonzeroConc Norm bySAThe concentration corresponding to Tlast divided bythe surface area.Last Concentration Normalized bySurface AreaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 276 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92390Last Nonzero Conc Norm byWTLast NonzeroConc Norm byWTThe concentration corresponding to Tlast divided bythe weight.Last Concentration Normalized byWeightC85769 MRT Infinity Obs MRT InfinityObsC85793 MRT Infinity Pred MRT InfinityPredC85700 MRT to Last Nonzero Conc MRT to LastNonzero ConcThe mean residence time (MRT) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration.The mean residence time (MRT) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration.Mean residence time (MRT) from the time of dosingto the time of the last measurable concentration.Observed Mean Residence TimeInfinityPredicted Mean Residence TimeInfinityMean Residence Time LastC70918 Max Conc Max Conc The maximum concentration occurring at Tmax. CmaxC92371 Max Conc Norm by BMI Max Conc Normby BMIC85698 Max Conc Norm by Dose Max Conc Normby DoseC92372 Max Conc Norm by SA Max Conc Normby SAC92373 Max Conc Norm by WT Max Conc Normby WTC85699 Max Excretion Rate Max ExcretionRateThe maximum concentration occurring at Tmax,divided by the body mass index.The maximum concentration occurring at Tmax,divided by the dose.The maximum concentration occurring at Tmax,divided by the surface area.The maximum concentration occurring at Tmax,divided by the weight.The maximum excretion rate.Maximum Concentration Normalizedby Body Mass IndexMaximum Concentration DoseNormalizedMaximum Concentration Normalizedby Surface AreaMaximum Concentration Normalizedby WeightMaximum Observed Excretion RateC92395Max Excretion Rate Norm byBMIMax ExcretionRate Norm byBMIThe maximum excretion rate divided by the body massindex.Maximum Observed Excretion RateNormalized by Body Mass IndexC92396Max Excretion Rate Norm byDoseMax ExcretionRate Norm byDoseThe maximum excretion rate divided by the dose.Maximum Observed Excretion RateNormalized by DoseC92397Max Excretion Rate Norm bySAMax ExcretionRate Norm by SAThe maximum excretion rate divided by the surfacearea.Maximum Observed Excretion RateNormalized by Surface AreaC92398Max Excretion Rate Norm byWTMax ExcretionRate Norm byWTThe maximum excretion rate divided by the weight.Maximum Observed Excretion RateNormalized by WeightC85580Midpoint of CollectionIntervalMidpoint ofCollectionIntervalThe midpoint of collection interval, which isassociated with last Measurable rate.Collection Interval MidpointC85579 Min Conc Min Conc The minimum concentration between dose time anddose time plus Tau (at Tmin).CminC92374 Min Conc Norm by BMI Min Conc Normby BMIC92375 Min Conc Norm by Dose Min Conc Normby DoseC92376 Min Conc Norm by SA Min Conc Normby SAThe minimum concentration between dose time anddose time plus Tau (at Tmin) divided by the body massindex.The minimum concentration between dose time anddose time plus Tau (at Tmin) divided by the dose.The minimum concentration between dose time anddose time plus Tau (at Tmin) divided by the surfacearea.Minimum Concentration Normalizedby Body Mass IndexMinimum Concentration Normalizedby DoseMinimum Concentration Normalizedby Surface AreaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 277 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92377 Min Conc Norm by WT Min Conc Normby WTThe minimum concentration between dose time anddose time plus Tau (at Tmin) divided by the weight.Minimum Concentration Normalizedby WeightC102376 Nonrenal CL Nonrenal CL The total clearance of a substance from the blood lessthe renal clearance.Nonrenal ClearanceC85816Number of Points for LambdazNumber ofPoints forLambda zThe number of time points used in computing Lambdaz.Sum of Lambda Z TimepointsC102386 Pct Rec Over Dosing Interval Pct Rec OverDosing IntervalThe percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, between doses (TAU).Percent Recovered Over DosingIntervalC102387Pct Rec Over Dosing IntervalNorm by BMIPct Rec OverDosing IntervalNorm by BMIThe percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, between doses (TAU) divided by the bodymass index.Percent Recovered Over DosingInterval Normalized by Body MassIndexC102388Pct Rec Over Dosing IntervalNorm by SAPct Rec OverDosing IntervalNorm by SAThe percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, between doses (TAU) divided by surfacearea.Percent Recovered Over DosingInterval Normalized by Surface AreaC102389Pct Rec Over Dosing IntervalNorm by WTPct Rec OverDosing IntervalNorm by WTThe percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, between doses (TAU) divided by weight.Percent Recovered Over DosingInterval Normalized by WeightC102382 Pct Rec from T1 to T2 Pct Rec from T1to T2The percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, over the interval between T1 and T2.Percent Recovered from T1 to T2C102383Pct Rec from T1 to T2 Normby BMIPct Rec from T1to T2 Norm byBMIThe percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, over the interval between T1 and T2 dividedby body mass index.Percent Recovered from T1 to T2Normalized by Body Mass IndexC102384Pct Rec from T1 to T2 Normby SAPct Rec from T1to T2 Norm bySAThe percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, over the interval between T1 and T2 dividedby surface area.Percent Recovered from T1 to T2Normalized by Surface AreaC102385Pct Rec from T1 to T2 Normby WTPct Rec from T1to T2 Norm byWTThe percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, over the interval between T1 and T2 dividedby weight.Percent Recovered from T1 to T2Normalized by WeightC102381 Peak Trough Ratio Peak TroughRatioThe ratio of Cmax to Ctrough during a dosing interval.Peak Trough RatioC85542 R Squared R Squared The goodness of fit statistic for the terminalelimination phase.R SquaredC85553 R Squared Adjusted R SquaredAdjustedThe goodness of fit statistic for the terminalelimination phase, adjusted for the number of timepoints used in the estimation of Lambda z.Adjusted R SquaredC75913 Renal CL Renal CL The clearance of a substance from the blood by thekidneys.Renal ClearanceC85817 Sum of Urine Vol Sum of UrineVolThe sum of urine volumes that are used for PKparameters.Sum Urine VolumeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 278 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85824Time Until First NonzeroConcTime Until FirstNonzero ConcThe time prior to the first measurable (non-zero)concentration.Time until First NonzeroConcentrationC70919 Time of CMAX Time of CMAX The time of maximum observed concentration sampledduring a dosing interval.TmaxC85825 Time of CMIN Observation Time of CMINObservationC85822 Time of Last Nonzero Conc Time of LastNonzero ConcC85823 Time of Max Excretion Rate Time of MaxExcretion RateThe time of minimum concentration sampled during adosing interval.The time of the last measurable (positive)concentration.The midpoint of collection interval associated with themaximum excretion rate.TminTime of Last Nonzero ConcentrationTime of Maximum ObservedExcretion RateC85773 Total CL Obs Total CL Obs The total body clearance for intravascularadministration, calculated using the observed value ofthe last non-zero concentration.Observed Total Body Clearance RateC92403 Total CL Obs Norm by BMI Total CL ObsNorm by BMIC92405 Total CL Obs Norm by SA Total CL ObsNorm by SAC92406 Total CL Obs Norm by WT Total CL ObsNorm by WTC85772 Total CL Obs by F Total CL Obs byFThe total body clearance for intravascularadministration, calculated using the observed value ofthe last non-zero concentration, divided by the bodymass index.The total body clearance for intravascularadministration, calculated using the observed value ofthe last non-zero concentration, divided by the surfacearea.The total body clearance for intravascularadministration, calculated using the observed value ofthe last non-zero concentration, divided by the weight.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the observed value of thelast non-zero concentration.Total Clearance ObservedNormalized by Body Mass IndexTotal Clearance ObservedNormalized by Surface AreaTotal Clearance ObservedNormalized by WeightObserved Total Body Clearance byFraction of Dose AbsorbedC92399Total CL Obs by F Norm byBMITotal CL Obs byF Norm by BMIThe total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the observed value of thelast non-zero concentration, divided by the body massindex.Total Clearance Observed byFraction Dose Normalized by BodyMass IndexC92401Total CL Obs by F Norm bySATotal CL Obs byF Norm by SAThe total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the observed value of thelast non-zero concentration, divided by the surfacearea.Total Clearance Observed byFraction Dose Normalized bySurface AreaC92402Total CL Obs by F Norm byWTTotal CL Obs byF Norm by WTThe total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the observed value of thelast non-zero concentration, divided by the weight.Total Clearance Observed byFraction Dose Normalized byWeightC85797 Total CL Pred Total CL Pred The total body clearance for intravascularadministration, calculated using the predicted value ofthe last non-zero concentration.Predicted Total Body Clearance RateC92421 Total CL Pred Norm by BMI Total CL PredNorm by BMIThe total body clearance for intravascularadministration, calculated using the predicted value ofthe last non-zero concentration, divided by the bodymass index.Total Clearance PredictedNormalized by Body Mass IndexSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 279 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92423 Total CL Pred Norm by SA Total CL PredNorm by SAC92424 Total CL Pred Norm by WT Total CL PredNorm by WTC85796 Total CL Pred by F Total CL Pred byFThe total body clearance for intravascularadministration, calculated using the predicted value ofthe last non-zero concentration, divided by the surfacearea.The total body clearance for intravascularadministration, calculated using the predicted value ofthe last non-zero concentration, divided by the weight.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the predicted value of thelast non-zero concentration.Total Clearance PredictedNormalized by Surface AreaTotal Clearance PredictedNormalized by WeightPredicted Total Body Clearance byFraction of Dose AbsorbedC92417Total CL Pred by F Norm byBMITotal CL Pred byF Norm by BMIThe total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the predicted value of thelast non-zero concentration, divided by body massindex.Total Clearance Predicted byFraction Dose Normalized by BodyMass IndexC92419Total CL Pred by F Norm bySATotal CL Pred byF Norm by SAThe total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the predicted value of thelast non-zero concentration, divided by the surfacearea.Total Clearance Predicted byFraction Dose Normalized bySurface AreaC92420Total CL Pred by F Norm byWTTotal CL Pred byF Norm by WTThe total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the predicted value of thelast non-zero concentration, divided by the weight.Total Clearance Predicted byFraction Dose Normalized byWeightC102371 Vol Dist Initial Vol Dist Initial The initial volume of distribution for a substanceadministered by bolus intravascular dosing.Initial Volume of DistributionC102372 Vol Dist Initial Norm by BMI Vol Dist InitialNorm by BMIC102373 Vol Dist Initial Norm by Dose Vol Dist InitialNorm by DoseC102374 Vol Dist Initial Norm by SA Vol Dist InitialNorm by SAC102375 Vol Dist Initial Norm by WT Vol Dist InitialNorm by WTC85770 Vol Dist Steady State Obs Vol Dist SteadyState ObsThe initial volume of distribution for a substanceadministered by bolus intravascular dosing divided bythe body mass index.The initial volume of distribution for a substanceadministered by bolus intravascular dosing divided bythe dose.The initial volume of distribution for a substanceadministered by bolus intravascular dosing divided bythe surface area.The initial volume of distribution for a substanceadministered by bolus intravascular dosing divided bythe weight.The volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing.Initial Volume of DistributionNormalized by Body Mass IndexInitial Volume of DistributionNormalized by DoseInitial Volume of DistributionNormalized by Surface AreaInitial Volume of DistributionNormalized by WeightObserved Steady State Volume ofDistributionC102377Vol Dist Steady State ObsNorm by BMIVol Dist SteadyState Obs Normby BMIThe volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing divided by the body mass index.Observed Steady State Volume ofDistribution Normalized by BodyMass IndexC102378Vol Dist Steady State ObsNorm by DoseVol Dist SteadyState Obs Normby DoseThe volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing divided by the dose.Observed Steady State Volume ofDistribution Normalized by DoseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 280 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102379Vol Dist Steady State ObsNorm by SAVol Dist SteadyState Obs Normby SAThe volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing divided by the surface area.Observed Steady State Volume ofDistribution Normalized by SurfaceAreaC102380Vol Dist Steady State ObsNorm by WTVol Dist SteadyState Obs Normby WTThe volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing divided by the weight.Observed Steady State Volume ofDistribution Normalized by WeightC85794 Vol Dist Steady State Pred Vol Dist SteadyState PredThe volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing.Predicted Steady State Volume ofDistributionC102390Vol Dist Steady State PredNorm by BMIVol Dist SteadyState Pred Normby BMIThe volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing divided by the body mass index.Predicted Steady State Volume ofDistribution Normalized by BodyMass IndexC102391Vol Dist Steady State PredNorm by DoseVol Dist SteadyState Pred Normby DoseThe volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing divided by the dose.Predicted Steady State Volume ofDistribution Normalized by DoseC102392Vol Dist Steady State PredNorm by SAVol Dist SteadyState Pred Normby SAThe volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing divided by the surface area.Predicted Steady State Volume ofDistribution Normalized by SurfaceAreaC102393Vol Dist Steady State PredNorm by WTVol Dist SteadyState Pred Normby WTThe volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing divided by the weight.Predicted Steady State Volume ofDistribution Normalized by WeightC85774 Vz Obs Vz Obs The volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration.Observed Volume of DistributionC92407 Vz Obs Norm by BMI Vz Obs Norm byBMIC102683 Vz Obs Norm by Dose Vz Obs Norm byDoseC92408 Vz Obs Norm by SA Vz Obs Norm bySAC92409 Vz Obs Norm by WT Vz Obs Norm byWTThe volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration, divided by the body massindex.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration, divided by the dose.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration, divided by the surface area.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration, divided by the weight.Volume of Distribution ObservedNormalized by Body Mass IndexObserved Volume of DistributionNormalized by DoseVolume of Distribution ObservedNormalized by Surface AreaVolume of Distribution ObservedNormalized by WeightC85775 Vz Obs by F Vz Obs by F The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration.Observed Volume of Distribution ofAbsorbed FractionSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 281 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92410 Vz Obs by F Norm by BM Vz Obs by FNorm by BMC102729 Vz Obs by F Norm by Dose Vz Obs by FNorm by DoseC92411 Vz Obs by F Norm by SA Vz Obs by FNorm by SAC924<strong>12</strong> Vz Obs by F Norm by WT Vz Obs by FNorm by WTThe volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration, divided by the body mass index.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration, divided by the dose.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration, divided by the surface area.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration, divided by the weight.Volume of Distribution of FractionDose Observed Normalized by BodyMass IndexVolume of Distribution of FractionDose Observed Normalized by DoseVolume of Distribution of FractionDose Observed Normalized bySurface AreaVolume of Distribution of FractionDose Observed Normalized byWeightC85798 Vz Pred Vz Pred The volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration.Predicted Volume of DistributionC92425 Vz Pred Norm by BMI Vz Pred Norm byBMIC102696 Vz Pred Norm by Dose Vz Pred Norm byDoseC92426 Vz Pred Norm by SA Vz Pred Norm bySAC92427 Vz Pred Norm by WT Vz Pred Norm byWTThe volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration, divided by the body massindex.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration, divided by the dose.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration, divided by the surface area.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration, divided by the weight.Volume of Distribution PredictedNormalized by Body Mass IndexPredicted Volume of DistributionNormalized by DoseVolume of Distribution PredictedNormalized by Surface AreaVolume of Distribution PredictedNormalized by WeightC85799 Vz Pred by F Vz Pred by F The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration.Predicted Volume of Distribution ofAbsorbed FractionC92428 Vz Pred by F Norm by BMI Vz Pred by FNorm by BMIThe volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration, divided by the body mass index.Volume of Distribution of FractionDose Predicted Normalized by BodyMass IndexSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 282 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85493 - PKPARM - PK ParametersCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102730 Vz Pred by F Norm by Dose Vz Pred by FNorm by DoseC92429 Vz Pred by F Norm by SA Vz Pred by FNorm by SAC92430 Vz Pred by F Norm by WT Vz Pred by FNorm by WTThe volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration, divided by the dose.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration, divided by the surface area.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration, divided by the weight.Volume of Distribution of FractionDose Predicted Normalized by DoseVolume of Distribution of FractionDose Predicted Normalized bySurface AreaVolume of Distribution of FractionDose Predicted Normalized byWeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 283 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102356 ARAUC AccumulationRatio AUCC102357 ARCMAX AccumulationRatio CmaxC102358 ARCMIN AccumulationRatio CminC102426 ARCTROUG AccumulationRatio CtroughThe area under the curve (AUCTAU) at steady statedivided by the area under the curve over the initialdosing interval.The maximum concentration at steady state divided bythe maximum concentration during the initial dosinginterval.The minimum concentration at steady state divided bythe minimum concentration during the initial dosinginterval.The trough concentration at steady state divided by thetrough concentration during the initial dosing interval.Accumulation Ratio Area Under theCurveAccumulation Ratio CmaxAccumulation Ratio CminAccumulation Ratio CtroughC85564 AUCALL AUC All The area under the curve (AUC) from the time ofdosing to the time of the last observation, regardlessof whether the last concentration is measurable or not.Area Under the Curve AllC92362 AUCALLB AUC All Normby BMIC92306 AUCALLD AUC All Normby DoseC92307 AUCALLS AUC All Normby SAC92308 AUCALLW AUC All Normby WTThe area under the curve (AUC) from the time ofdosing to the time of the last observation divided bythe body mass index, regardless of whether the lastconcentration is measurable or not.The area under the curve (AUC) from the time ofdosing to the time of the last observation divided bythe dose, regardless of whether the last concentrationis measurable or not.The area under the curve (AUC) from the time ofdosing to the time of the last observation divided bythe surface area, regardless of whether the lastconcentration is measurable or not.The area under the curve (AUC) from the time ofdosing to the time of the last observation divided bythe weight, regardless of whether the lastconcentration is measurable or not.AUC All Normalized by Body MassIndexAUC All Normalized by DoseAUC All Normalized by SurfaceAreaAUC All Normalized by WeightC85761 AUCIFO AUC Infinity Obs The area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration.Observed Area Under the CurveInfinityC92316 AUCIFOB AUC Infinity ObsNorm by BMIC96695 AUCIFOD AUC Infinity ObsNorm by DoseC92317 AUCIFOS AUC Infinity ObsNorm by SAC92318 AUCIFOW AUC Infinity ObsNorm by WTC85785 AUCIFP AUC InfinityPredThe area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration, divided by the body massindex.The area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration, divided by the dose.The area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration, divided by the surface area.The area under the curve (AUC) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration, divided by the weight.The area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration.AUC Infinity Observed Normalizedby Body Mass IndexAUC Infinity Observed Normalizedby DoseAUC Infinity Observed Normalizedby Surface AreaAUC Infinity Observed Normalizedby WeightPredicted Area Under the CurveInfinitySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 284 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92319 AUCIFPB AUC InfinityPred Norm byBMIC85786 AUCIFPD AUC InfinityPred Norm byDoseC92320 AUCIFPS AUC InfinityPred Norm bySAC92321 AUCIFPW AUC InfinityPred Norm byWTC85566 AUCINT AUC from T1 toT2C923<strong>12</strong> AUCINTB AUC from T1 toT2 Norm by BMIC92313 AUCINTD AUC from T1 toT2 Norm byDoseC92314 AUCINTS AUC from T1 toT2 Norm by SAC92315 AUCINTW AUC from T1 toT2 Norm by WTC85565 AUCLST AUC to LastNonzero ConcC92309 AUCLSTB AUC to LastNonzero ConcNorm by BMIC92310 AUCLSTD AUC to LastNonzero ConcNorm by DoseC92311 AUCLSTS AUC to LastNonzero ConcNorm by SAC92305 AUCLSTW AUC to LastNonzero ConcNorm by WTC85787 AUCPBEP AUC %BackExtrapolationPredC85764 AUCPEO AUC%ExtrapolationObsThe area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration, divided by the body massindex.The area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration, divided by the dose.The area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration, divided by the surface area.The area under the curve (AUC) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration, divided by the weight.The area under the curve (AUC) over the interval fromT1 to T2.The area under the curve (AUC) over the interval fromT1 to T2 divided by the body mass index.The area under the curve (AUC) over the interval fromT1 to T2 divided by the dose.The area under the curve (AUC) over the interval fromT1 to T2 divided by the surface area.The area under the curve (AUC) over the interval fromT1 to T2 divided by the weight.The area under the curve (AUC) from the time ofdosing to the last measurable concentration.The area under the curve (AUC) from the time ofdosing to the last measurable concentration divided bythe body mass index.The area under the curve (AUC) from the time ofdosing to the last measurable concentration divided bythe dose.The area under the curve (AUC) from the time ofdosing to the last measurable concentration divided bythe surface area.The area under the curve (AUC) from the time ofdosing to the last measurable concentration divided bythe weight.The area under the curve (AUC) from the predictedconcentration value at time zero to the first measuredconcentration value as a percentage of the area underthe curve extrapolated to infinity. Applies only forintravascular bolus dosing.The area under the curve (AUC) from the last observednon-zero concentration value to infinity as apercentage of the area under the curve extrapolated toinfinity.AUC Infinity Predicted Normalizedby Body Mass IndexPredicted Area Under the CurveInfinity by DoseAUC Infinity Predicted Normalizedby Surface AreaAUC Infinity Predicted Normalizedby WeightArea Under the Curve from T1 to T2AUC from T1 to T2 Normalized byBody Mass IndexAUC from T1 to T2 Normalized byDoseAUC from T1 to T2 Normalized bySurface AreaAUC from T1 to T2 Normalized byWeightArea Under the Curve From Dosingto Last ConcentrationAUC Dosing to Last ConcentrationNormalized by Body Mass IndexAUC Dosing to Last ConcentrationNormalized by DoseAUC Dosing to Last ConcentrationNormalized by Surface AreaAUC Dosing to Last ConcentrationNormalized by WeightPredicted Area Under the CurvePercent Back ExtrapolationObserved Area Under the CurvePercent ExtrapolationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 285 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85788 AUCPEP AUC%ExtrapolationPredC85567 AUCTAU AUC OverDosing IntervalC92322 AUCTAUB AUC OverDosing IntervalNorm by BMIC92323 AUCTAUD AUC OverDosing IntervalNorm by DoseC92324 AUCTAUS AUC OverDosing IntervalNorm by SAC92325 AUCTAUW AUC OverDosing IntervalNorm by WTC85765 AUMCIFO AUMC InfinityObsC92330 AUMCIFOB AUMC InfinityObs Norm byBMIC92331 AUMCIFOD AUMC InfinityObs Norm byDoseC92332 AUMCIFOS AUMC InfinityObs Norm by SAC92333 AUMCIFOW AUMC InfinityObs Norm byWTC85789 AUMCIFP AUMC InfinityPredC92334 AUMCIFPB AUMC InfinityPred Norm byBMIC92335 AUMCIFPD AUMC InfinityPred Norm byDoseThe area under the curve (AUC) from the last predictednon-zero concentration value to infinity as apercentage of the area under the curve extrapolated toinfinity.The area under the curve (AUC) for the definedinterval between doses (TAU).The area under the curve (AUC) for the definedinterval between doses (TAU) divided by the bodymass index.The area under the curve (AUC) for the definedinterval between doses (TAU) divided by the dose.The area under the curve (AUC) for the definedinterval between doses (TAU) divided by the surfacearea.The area under the curve (AUC) for the definedinterval between doses (TAU) divided by the weight.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration, divided by thebody mass index.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration, divided by thedose.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration, divided by thesurface area.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the observedvalue of the last non-zero concentration, divided by theweight.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration, divided by thebody mass index.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration, divided by thedose.Predicted Area Under the CurvePercent ExtrapolationArea Under the Curve Over DosingIntervalAUC Over Dosing IntervalNormalized by Body Mass IndexAUC Over Dosing IntervalNormalized by DoseAUC Over Dosing IntervalNormalized by Surface AreaAUC Over Dosing IntervalNormalized by WeightObserved Area Under the FirstMoment Curve InfinityAUMC Infinity ObservedNormalized by Body Mass IndexAUMC Infinity ObservedNormalized by DoseAUMC Infinity ObservedNormalized by Surface AreaAUMC Infinity ObservedNormalized by WeightPredicted Area Under the FirstMoment Curve InfinityAUMC Infinity PredictedNormalized by Body Mass IndexAUMC Infinity PredictedNormalized by DoseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 286 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92336 AUMCIFPS AUMC InfinityPred Norm bySAC92337 AUMCIFPW AUMC InfinityPred Norm byWTC85569 AUMCLST AUMC to LastNonzero ConcC92326 AUMCLSTB AUMC to LastNonzero ConcNorm by BMIC92327 AUMCLSTD AUMC to LastNonzero ConcNorm by DoseC92328 AUMCLSTS AUMC to LastNonzero ConcNorm by SAC92329 AUMCLSTW AUMC to LastNonzero ConcNorm by WTC85766 AUMCPEO AUMC %ExtrapolationObsC85790 AUMCPEP AUMC %ExtrapolationPredC85570 AUMCTAU AUMC OverDosing IntervalC92338 AUMCTAUB AUMC OverDosing IntervalNorm by BMIC92339 AUMCTAUD AUMC OverDosing IntervalNorm by DoseC92340 AUMCTAUS AUMC OverDosing IntervalNorm by SAC92341 AUMCTAUW AUMC OverDosing IntervalNorm by WTThe area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration, divided by thesurface area.The area under the moment curve (AUMC)extrapolated to infinity, calculated using the predictedvalue of the last non-zero concentration, divided by theweight.The area under the moment curve (AUMC) from thetime of dosing to the last measurable concentration.The area under the moment curve (AUMC) from thetime of dosing to the last measurable concentrationdivided by the body mass index.The area under the moment curve (AUMC) from thetime of dosing to the last measurable concentrationdivided by the dose.The area under the moment curve (AUMC) from thetime of dosing to the last measurable concentrationdivided by the surface area.The area under the moment curve (AUMC) from thetime of dosing to the last measurable concentrationdivided by the weight.The area under the moment curve (AUMC) from thelast observed non-zero concentration value to infinityas a percentage of the area under the moment curveextrapolated to infinity.The area under the moment curve (AUMC) from thelast predicted non-zero concentration value to infinityas a percentage of the area under the moment curveextrapolated to infinity.The area under the first moment curve (AUMC) for thedefined interval between doses (TAU).The area under the first moment curve (AUMC) for thedefined interval between doses (TAU) divided by thebody mass index.The area under the first moment curve (AUMC) for thedefined interval between doses (TAU) divided by thedose.The area under the first moment curve (AUMC) for thedefined interval between doses (TAU) divided by thesurface area.The area under the first moment curve (AUMC) for thedefined interval between doses (TAU) divided by theweight.AUMC Infinity PredictedNormalized by Surface AreaAUMC Infinity PredictedNormalized by WeightArea Under the First Moment CurveFrom Dosing to Last ConcentrationAUMC Dosing to Last ConcentrationNormalized by Body Mass IndexAUMC Dosing to Last ConcentrationNormalized by DoseAUMC Dosing to Last ConcentrationNormalized by Surface AreaAUMC Dosing to Last ConcentrationNormalized by WeightObserved Area Under the FirstMoment Curve PercentExtrapolationPredicted Area Under the FirstMoment Curve PercentExtrapolationArea Under the First Moment CurveOver Dosing IntervalAUMC Over Dosing IntervalNormalized by Body Mass IndexAUMC Over Dosing IntervalNormalized by DoseAUMC Over Dosing IntervalNormalized by Surface AreaAUMC Over Dosing IntervalNormalized by WeightC85841 AURCALL AURC All The area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate.Area Under Urinary Excretion RateCurve AllC92342 AURCALLB AURC All Normby BMIThe area under the urinary excretion rate curve(AURC) from time zero to the last measurable ratedivided by the body mass index.AURC All Normalized by Body MassIndexSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 287 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92343 AURCALLD AURC All Normby DoseC92344 AURCALLS AURC All Normby SAC92345 AURCALLW AURC All Normby WTC85767 AURCIFO AURC InfinityObsC92354 AURCIFOB AURC InfinityObs Norm byBMIC92355 AURCIFOD AURC InfinityObs Norm byDoseC92356 AURCIFOS AURC InfinityObs Norm by SAC92357 AURCIFOW AURC InfinityObs Norm byWTC85791 AURCIFP AURC InfinityPredC92358 AURCIFPB AURC InfinityPred Norm byBMIC92359 AURCIFPD AURC InfinityPred Norm byDoseC92360 AURCIFPS AURC InfinityPred Norm bySAC92361 AURCIFPW AURC InfinityPred Norm byWTC85572 AURCINT AURC from T1to T2The area under the urinary excretion rate curve(AURC) from time zero to the last measurable ratedivided by the dose.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable ratedivided by the surface area.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable ratedivided by the weight.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate, divided by thebody mass index.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate, divided by thedose.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate, divided by thesurface area.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using theobserved value of the last excretion rate, divided by theweight.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate,divded by the body mass index.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate,divded by the dose.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate,divded by the surface area.The area under the urinary excretion rate curve(AURC) extrapolated to infinity, calculated using thepredicted value of the last non-zero excretion rate,divded by the weight.The area under the urinary excretion rate curve(AURC) over the interval from T1 to T2.AURC All Normalized by DoseAURC All Normalized by SurfaceAreaAURC All Normalized by WeightObserved Area Under the UrinaryExcretion Rate Curve infinityAURC Infinity Observed Normalizedby Body Mass IndexAURC Infinity Observed Normalizedby DoseAURC Infinity Observed Normalizedby Surface AreaAURC Infinity Observed Normalizedby WeightPredicted Area Under the UrinaryExcretion Rate Curve InfinityAURC Infinity Predicted Normalizedby Body Mass IndexAURC Infinity Predicted Normalizedby DoseAURC Infinity Predicted Normalizedby Surface AreaAURC Infinity Predicted Normalizedby WeightArea Under the Urinary ExcretionRate Curve from T1 to T2Source: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 288 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92350 AURCINTB AURC from T1to T2 Norm byBMIC92351 AURCINTD AURC from T1to T2 Norm byDoseC92352 AURCINTS AURC from T1to T2 Norm bySAC92353 AURCINTW AURC from T1to T2 Norm byWTC85571 AURCLST AURC to LastNonzero RateC92346 AURCLSTB AURC to LastNonzero RateNorm by BMIC92347 AURCLSTD AURC to LastNonzero RateNorm by DoseC92348 AURCLSTS AURC to LastNonzero RateNorm by SAC92349 AURCLSTW AURC to LastNonzero RateNorm by WTC85768 AURCPEO AURC %ExtrapolationObsC85792 AURCPEP AURC %ExtrapolationPredThe area under the urinary excretion rate curve(AURC) over the interval from T1 to T2 divided by thebody mass index.The area under the urinary excretion rate curve(AURC) over the interval from T1 to T2 divided by thedose.The area under the urinary excretion rate curve(AURC) over the interval from T1 to T2 divided by thesurface area.The area under the urinary excretion rate curve(AURC) over the interval from T1 to T2 divided by theweight.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate,divided by the body mass index.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate,divided by the dose.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate,divided by the surface area.The area under the urinary excretion rate curve(AURC) from time zero to the last measurable rate,divided by the weight.The area under the urinary excretion rate curve(AURC) from the last observed non-zero rate value toinfinity as a percentage of the area under the urinaryexcretion rate curve extrapolated to infinity.The area under the urinary excretion rate curve(AURC) from the last predicted non-zero rate value toinfinity as a percentage of the area under the urinaryexcretion rate curve extrapolated to infinity.AURC from T1 to T2 Normalized byBody Mass IndexAURC from T1 to T2 Normalized byDoseAURC from T1 to T2 Normalized bySurface AreaAURC from T1 to T2 Normalized byWeightArea Under the Urinary ExcretionRate Curve From Dosing to LastConcentrationAURC Dosing to Last ConcentrationNormalized by Body Mass IndexAURC Dosing to Last ConcentrationNormalized by DoseAURC Dosing to Last ConcentrationNormalized by Surface AreaAURC Dosing to Last ConcentrationNormalized by WeightObserved Area Under the UrinaryExcretion Rate Curve PercentExtrapolationPredicted Area Under the UrinaryExcretion Rate Curve PercentExtrapolationC85644 C0 Initial Conc Initial concentration. Given only for bolus IV models. Initial ConcentrationC92383 C0B Initial ConcNorm by BMIC92384 C0D Initial ConcNorm by DoseC92385 C0S Initial ConcNorm by SAC92386 C0W Initial ConcNorm by WTC85575 CAVG Average Conc;AverageConcentrationInitial concentration divided by the body mass index.Given only for bolus IV models.Initial concentration divided by the dose. Given onlyfor bolus IV models.Initial concentration divided by the surface area. Givenonly for bolus IV models.Initial concentration divided by the weight. Given onlyfor bolus IV models.AUCTAU divided by TAU.Initial Concentration Normalized byBody Mass IndexInitial Concentration Normalized byDoseInitial Concentration Normalized bySurface AreaInitial Concentration Normalized byWeightAverage ConcentrationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 289 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92367 CAVGB Average ConcNorm by BMIC92368 CAVGD Average ConcNorm by DoseAUCTAU divided by TAU and then divided by the bodymass index.Average Concentration Normalizedby Body Mass IndexAUCTAU divided by TAU and then divided by the dose. Average Concentration Normalizedby DoseC92369 CAVGS Average ConcNorm by SAC92370 CAVGW Average ConcNorm by WTC85772 CLFO Total CL Obs byFC92399 CLFOB Total CL Obs byF Norm by BMIC92401 CLFOS Total CL Obs byF Norm by SAC92402 CLFOW Total CL Obs byF Norm by WTC85796 CLFP Total CL Pred byFC92417 CLFPB Total CL Pred byF Norm by BMIC92419 CLFPS Total CL Pred byF Norm by SAC92420 CLFPW Total CL Pred byF Norm by WTAUCTAU divided by TAU and then divided by thesurface area.AUCTAU divided by TAU and then divided by theweight.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the observed value of thelast non-zero concentration.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the observed value of thelast non-zero concentration, divided by the body massindex.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the observed value of thelast non-zero concentration, divided by the surfacearea.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the observed value of thelast non-zero concentration, divided by the weight.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the predicted value of thelast non-zero concentration.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the predicted value of thelast non-zero concentration, divided by body massindex.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the predicted value of thelast non-zero concentration, divided by the surfacearea.The total body clearance for extravascularadministration divided by the fraction of doseabsorbed, calculated using the predicted value of thelast non-zero concentration, divided by the weight.Average Concentration Normalizedby Surface AreaAverage Concentration Normalizedby WeightObserved Total Body Clearance byFraction of Dose AbsorbedTotal Clearance Observed byFraction Dose Normalized by BodyMass IndexTotal Clearance Observed byFraction Dose Normalized bySurface AreaTotal Clearance Observed byFraction Dose Normalized byWeightPredicted Total Body Clearance byFraction of Dose AbsorbedTotal Clearance Predicted byFraction Dose Normalized by BodyMass IndexTotal Clearance Predicted byFraction Dose Normalized bySurface AreaTotal Clearance Predicted byFraction Dose Normalized byWeightC85773 CLO Total CL Obs The total body clearance for intravascularadministration, calculated using the observed value ofthe last non-zero concentration.Observed Total Body Clearance RateC92403 CLOB Total CL ObsNorm by BMIThe total body clearance for intravascularadministration, calculated using the observed value ofthe last non-zero concentration, divided by the bodymass index.Total Clearance ObservedNormalized by Body Mass IndexSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 290 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92405 CLOS Total CL ObsNorm by SAC92406 CLOW Total CL ObsNorm by WTThe total body clearance for intravascularadministration, calculated using the observed value ofthe last non-zero concentration, divided by the surfacearea.The total body clearance for intravascularadministration, calculated using the observed value ofthe last non-zero concentration, divided by the weight.Total Clearance ObservedNormalized by Surface AreaTotal Clearance ObservedNormalized by WeightC85797 CLP Total CL Pred The total body clearance for intravascularadministration, calculated using the predicted value ofthe last non-zero concentration.Predicted Total Body Clearance RateC92421 CLPB Total CL PredNorm by BMIC92423 CLPS Total CL PredNorm by SAC92424 CLPW Total CL PredNorm by WTC85655 CLST Last NonzeroConcC92387 CLSTB Last NonzeroConc Norm byBMIC92388 CLSTD Last NonzeroConc Norm byDoseC92389 CLSTS Last NonzeroConc Norm bySAC92390 CLSTW Last NonzeroConc Norm byWTThe total body clearance for intravascularadministration, calculated using the predicted value ofthe last non-zero concentration, divided by the bodymass index.The total body clearance for intravascularadministration, calculated using the predicted value ofthe last non-zero concentration, divided by the surfacearea.The total body clearance for intravascularadministration, calculated using the predicted value ofthe last non-zero concentration, divided by the weight.The concentration corresponding to Tlast.The concentration corresponding to Tlast divided bythe body mass index.The concentration corresponding to Tlast divided bythe dose.The concentration corresponding to Tlast divided bythe surface area.The concentration corresponding to Tlast divided bythe weight.Total Clearance PredictedNormalized by Body Mass IndexTotal Clearance PredictedNormalized by Surface AreaTotal Clearance PredictedNormalized by WeightLast ConcentrationLast Concentration Normalized byBody Mass IndexLast Concentration Normalized byDoseLast Concentration Normalized bySurface AreaLast Concentration Normalized byWeightC70918 CMAX Max Conc The maximum concentration occurring at Tmax. CmaxC92371 CMAXB Max Conc Normby BMIC85698 CMAXD Max Conc Normby DoseC92372 CMAXS Max Conc Normby SAC92373 CMAXW Max Conc Normby WTThe maximum concentration occurring at Tmax,divided by the body mass index.The maximum concentration occurring at Tmax,divided by the dose.The maximum concentration occurring at Tmax,divided by the surface area.The maximum concentration occurring at Tmax,divided by the weight.Maximum Concentration Normalizedby Body Mass IndexMaximum Concentration DoseNormalizedMaximum Concentration Normalizedby Surface AreaMaximum Concentration Normalizedby WeightC85579 CMIN Min Conc The minimum concentration between dose time anddose time plus Tau (at Tmin).CminSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 291 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92374 CMINB Min Conc Normby BMIC92375 CMIND Min Conc Normby DoseC92376 CMINS Min Conc Normby SAC92377 CMINW Min Conc Normby WTThe minimum concentration between dose time anddose time plus Tau (at Tmin) divided by the body massindex.The minimum concentration between dose time anddose time plus Tau (at Tmin) divided by the dose.The minimum concentration between dose time anddose time plus Tau (at Tmin) divided by the surfacearea.The minimum concentration between dose time anddose time plus Tau (at Tmin) divided by the weight.Minimum Concentration Normalizedby Body Mass IndexMinimum Concentration Normalizedby DoseMinimum Concentration Normalizedby Surface AreaMinimum Concentration Normalizedby WeightC41185 CONC Conc The quantity of a specified substance in a unit volumeor weight of another substance.ConcentrationC102367 CONCB Conc by BMI The concentration divided by body mass index. Concentration Divided by Body MassIndexC102368 CONCD Conc by Dose The concentration divided by dose. Concentration Divided by DoseC102369 CONCS Conc by SA The concentration divided by surface area. Concentration Divided by SurfaceAreaC102370 CONCW Conc by WT The concentration divided by weight. Concentration Divided by WeightC85821 CORRXY CorrelationBetween TimeXand Log ConcYThe correlation between time (X) and logconcentration (Y) for the points used in the estimationof lambda z.Time and Log ConcentrationCorrelationC102394 CTROUGH Conc Trough Concentration at end of dosing interval. Trough ConcentrationC102395 CTROUGHB Conc Trough byBMIC102396 CTROUGHD Conc Trough byDoseC102397 CTROUGHS Conc Trough bySAC102398 CTROUGHW Conc Trough byWTC95007 EFFHL EffectiveHalf-LifeC85656 ERLST Last MeasExcretion RateC92391 ERLSTB Last MeasExcretion RateNorm by BMIC92392 ERLSTD Last MeasExcretion RateNorm by DoseC92393 ERLSTS Last MeasExcretion RateNorm by SAThe trough concentration divided by body mass index.The trough concentration divided by dose.The trough concentration divided by surface area.The trough concentration divided by weight.The drug half-life that quantifies the accumulationratio of a drug following multiple dosing.The last measurable (positive) excretion rate.The last measurable (positive) excretion rate dividedby the body mass index.The last measurable (positive) excretion rate dividedby the dose.The last measurable (positive) excretion rate dividedby the surface area.Trough Concentration Divided byBody Mass IndexTrough Concentration Divided byDoseTrough Concentration Divided bySurface AreaTrough Concentration Divided byWeightEffective Half-lifeLast Measurable Observed ExcretionRateLast Measurable Excretion RateNormalized by Body Mass IndexLast Measurable Excretion RateNormalized by DoseLast Measurable Excretion RateNormalized by Surface AreaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 292 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92394 ERLSTW Last MeasExcretion RateNorm by WTC85699 ERMAX Max ExcretionRateC92395 ERMAXB Max ExcretionRate Norm byBMIC92396 ERMAXD Max ExcretionRate Norm byDoseThe last measurable (positive) excretion rate dividedby the weight.The maximum excretion rate.The maximum excretion rate divided by the body massindex.The maximum excretion rate divided by the dose.Last Measurable Excretion RateNormalized by WeightMaximum Observed Excretion RateMaximum Observed Excretion RateNormalized by Body Mass IndexMaximum Observed Excretion RateNormalized by DoseC92397 ERMAXS Max ExcretionRate Norm by SAThe maximum excretion rate divided by the surfacearea.Maximum Observed Excretion RateNormalized by Surface AreaC92398 ERMAXW Max ExcretionRate Norm byWTC85823 ERTMAX Time of MaxExcretion RateThe maximum excretion rate divided by the weight.The midpoint of collection interval associated with themaximum excretion rate.Maximum Observed Excretion RateNormalized by WeightTime of Maximum ObservedExcretion RateC85581 FLUCP Fluctuation% The difference between Cmin and Cmax standardizedto Cavg, between dose time and Tau.C85652 LAMZ Lambda z The first order rate constant associated with theterminal (log-linear) portion of the curve.Concentration Variability BetweenDose Time and TauLambda ZC85818 LAMZHL Half-LifeLambda zC85653 LAMZLL Lambda z LowerLimitC85816 LAMZNPT Number ofPoints forLambda zC85654 LAMZUL Lambda z UpperLimitC85580 MIDPTLST Midpoint ofCollectionIntervalC85769 MRTIFO MRT InfinityObsC85793 MRTIFP MRT InfinityPredC85700 MRTLST MRT to LastNonzero ConcTerminal half-life.The lower limit on time for values to be included in thecalculation of Lambda z.The number of time points used in computing Lambdaz.The upper limit on time for values to be included in thecalculation of Lambda z.The midpoint of collection interval, which isassociated with last Measurable rate.The mean residence time (MRT) extrapolated toinfinity, calculated using the observed value of the lastnon-zero concentration.The mean residence time (MRT) extrapolated toinfinity, calculated using the predicted value of the lastnon-zero concentration.Mean residence time (MRT) from the time of dosingto the time of the last measurable concentration.Terminal Half LifeLambda Z Time Lower LimitSum of Lambda Z TimepointsLambda Z Time Upper LimitCollection Interval MidpointObserved Mean Residence TimeInfinityPredicted Mean Residence TimeInfinityMean Residence Time LastC102376 NRENALCL Nonrenal CL The total clearance of a substance from the blood lessthe renal clearance.Nonrenal ClearanceC102381 PTROUGHR Peak TroughRatioThe ratio of Cmax to Ctrough during a dosing interval.Peak Trough RatioSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 293 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85542 R2 R Squared The goodness of fit statistic for the terminalelimination phase.R SquaredC85553 R2ADJ R SquaredAdjustedC102359 RCAMINT Amt Rec fromT1 to T2C102360 RCAMINTB Amt Rec fromT1 to T2 Normby BMIC102361 RCAMINTS Amt Rec fromT1 to T2 Normby SAC102362 RCAMINTW Amt Rec fromT1 to T2 Normby WTC102363 RCAMTAU Amt Rec OverDosing IntervalC102364 RCAMTAUB Amt Rec OverDosing IntervalNorm by BMIC102365 RCAMTAUS Amt Rec OverDosing IntervalNorm by SAC102366 RCAMTAUW Amt Rec OverDosing IntervalNorm by WTC102382 RCPCINT Pct Rec from T1to T2C102383 RCPCINTB Pct Rec from T1to T2 Norm byBMIC102384 RCPCINTS Pct Rec from T1to T2 Norm bySAC102385 RCPCINTW Pct Rec from T1to T2 Norm byWTC102386 RCPCTAU Pct Rec OverDosing IntervalThe goodness of fit statistic for the terminalelimination phase, adjusted for the number of timepoints used in the estimation of Lambda z.The cumulative amount recovered from the specimentype specified in PPSPEC over the interval from T1 toT2.The cumulative amount recovered from the specimentype specified in PPSPEC over the interval from T1 toT2 divided by body mass index.The cumulative amount recovered from the specimentype specified in PPSPEC over the interval from T1 toT2 divided by surface area.The cumulative amount recovered from the specimentype specified in PPSPEC over the interval from T1 toT2 divided by weight.The cumulative amount recovered from the specimentype specified in PPSPEC between doses (TAU).The cumulative amount recovered from the specimentype specified in PPSPEC between doses (TAU)divided by body mass index.The cumulative amount recovered from the specimentype specified in PPSPEC between doses (TAU)divided by surface area.The cumulative amount recovered from the specimentype specified in PPSPEC between doses (TAU)divided by weight.The percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, over the interval between T1 and T2.The percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, over the interval between T1 and T2 dividedby body mass index.The percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, over the interval between T1 and T2 dividedby surface area.The percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, over the interval between T1 and T2 dividedby weight.The percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, between doses (TAU).Adjusted R SquaredAmount Recovered from T1 to T2Amount Recovered from T1 to T2Normalized by Body Mass IndexAmount Recovered from T1 to T2Normalized by Surface AreaAmount Recovered from T1 to T2Normalized by WeightAmount Recovered Over DosingIntervalAmount Recovered Over DosingInterval Normalized by Body MassIndexAmount Recovered Over DosingInterval Normalized by Surface AreaAmount Recovered Over DosingInterval Normalized by WeightPercent Recovered from T1 to T2Percent Recovered from T1 to T2Normalized by Body Mass IndexPercent Recovered from T1 to T2Normalized by Surface AreaPercent Recovered from T1 to T2Normalized by WeightPercent Recovered Over DosingIntervalSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 294 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102387 RCPCTAUB Pct Rec OverDosing IntervalNorm by BMIC102388 RCPCTAUS Pct Rec OverDosing IntervalNorm by SAC102389 RCPCTAUW Pct Rec OverDosing IntervalNorm by WTThe percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, between doses (TAU) divided by the bodymass index.The percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, between doses (TAU) divided by surfacearea.The percentage of the administered dose that isrecovered from the specimen type specified inPPSPEC, between doses (TAU) divided by weight.Percent Recovered Over DosingInterval Normalized by Body MassIndexPercent Recovered Over DosingInterval Normalized by Surface AreaPercent Recovered Over DosingInterval Normalized by WeightC75913 RENALCL Renal CL The clearance of a substance from the blood by thekidneys.Renal ClearanceC85824 TLAG Time Until FirstNonzero ConcC85822 TLST Time of LastNonzero ConcThe time prior to the first measurable (non-zero)concentration.The time of the last measurable (positive)concentration.Time until First NonzeroConcentrationTime of Last Nonzero ConcentrationC70919 TMAX Time of CMAX The time of maximum observed concentration sampledduring a dosing interval.TmaxC85825 TMIN Time of CMINObservationThe time of minimum concentration sampled during adosing interval.TminC102371 V0 Vol Dist Initial The initial volume of distribution for a substanceadministered by bolus intravascular dosing.Initial Volume of DistributionC102372 V0B Vol Dist InitialNorm by BMIC102373 V0D Vol Dist InitialNorm by DoseC102374 V0S Vol Dist InitialNorm by SAC102375 V0W Vol Dist InitialNorm by WTC85817 VOLPK Sum of UrineVolC85770 VSSO Vol Dist SteadyState ObsC102377 VSSOB Vol Dist SteadyState Obs Normby BMIC102378 VSSOD Vol Dist SteadyState Obs Normby DoseThe initial volume of distribution for a substanceadministered by bolus intravascular dosing divided bythe body mass index.The initial volume of distribution for a substanceadministered by bolus intravascular dosing divided bythe dose.The initial volume of distribution for a substanceadministered by bolus intravascular dosing divided bythe surface area.The initial volume of distribution for a substanceadministered by bolus intravascular dosing divided bythe weight.The sum of urine volumes that are used for PKparameters.The volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing.The volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing divided by the body mass index.The volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing divided by the dose.Initial Volume of DistributionNormalized by Body Mass IndexInitial Volume of DistributionNormalized by DoseInitial Volume of DistributionNormalized by Surface AreaInitial Volume of DistributionNormalized by WeightSum Urine VolumeObserved Steady State Volume ofDistributionObserved Steady State Volume ofDistribution Normalized by BodyMass IndexObserved Steady State Volume ofDistribution Normalized by DoseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 295 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102379 VSSOS Vol Dist SteadyState Obs Normby SAC102380 VSSOW Vol Dist SteadyState Obs Normby WTC85794 VSSP Vol Dist SteadyState PredC102390 VSSPB Vol Dist SteadyState Pred Normby BMIC102391 VSSPD Vol Dist SteadyState Pred Normby DoseC102392 VSSPS Vol Dist SteadyState Pred Normby SAC102393 VSSPW Vol Dist SteadyState Pred Normby WTThe volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing divided by the surface area.The volume of distribution at steady state based on theobserved CLST for a substance administered byintravascular dosing divided by the weight.The volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing.The volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing divided by the body mass index.The volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing divided by the dose.The volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing divided by the surface area.The volume of distribution at steady state based on thepredicted CLST for a substance administered byintravascular dosing divided by the weight.Observed Steady State Volume ofDistribution Normalized by SurfaceAreaObserved Steady State Volume ofDistribution Normalized by WeightPredicted Steady State Volume ofDistributionPredicted Steady State Volume ofDistribution Normalized by BodyMass IndexPredicted Steady State Volume ofDistribution Normalized by DosePredicted Steady State Volume ofDistribution Normalized by SurfaceAreaPredicted Steady State Volume ofDistribution Normalized by WeightC85775 VZFO Vz Obs by F The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration.Observed Volume of Distribution ofAbsorbed FractionC92410 VZFOB Vz Obs by FNorm by BMC102729 VZFOD Vz Obs by FNorm by DoseC92411 VZFOS Vz Obs by FNorm by SAC924<strong>12</strong> VZFOW Vz Obs by FNorm by WTThe volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration, divided by the body mass index.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration, divided by the dose.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration, divided by the surface area.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the observed value of the last non-zeroconcentration, divided by the weight.Volume of Distribution of FractionDose Observed Normalized by BodyMass IndexVolume of Distribution of FractionDose Observed Normalized by DoseVolume of Distribution of FractionDose Observed Normalized bySurface AreaVolume of Distribution of FractionDose Observed Normalized byWeightC85799 VZFP Vz Pred by F The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration.Predicted Volume of Distribution ofAbsorbed FractionSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 296 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92428 VZFPB Vz Pred by FNorm by BMIC102730 VZFPD Vz Pred by FNorm by DoseC92429 VZFPS Vz Pred by FNorm by SAC92430 VZFPW Vz Pred by FNorm by WTThe volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration, divided by the body mass index.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration, divided by the dose.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration, divided by the surface area.The volume of distribution associated with theterminal slope following extravascular administrationdivided by the fraction of dose absorbed, calculatedusing the predicted value of the last non-zeroconcentration, divided by the weight.Volume of Distribution of FractionDose Predicted Normalized by BodyMass IndexVolume of Distribution of FractionDose Predicted Normalized by DoseVolume of Distribution of FractionDose Predicted Normalized bySurface AreaVolume of Distribution of FractionDose Predicted Normalized byWeightC85774 VZO Vz Obs The volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration.Observed Volume of DistributionC92407 VZOB Vz Obs Norm byBMIC102683 VZOD Vz Obs Norm byDoseC92408 VZOS Vz Obs Norm bySAC92409 VZOW Vz Obs Norm byWTThe volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration, divided by the body massindex.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration, divided by the dose.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration, divided by the surface area.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the observed value of the lastnon-zero concentration, divided by the weight.Volume of Distribution ObservedNormalized by Body Mass IndexObserved Volume of DistributionNormalized by DoseVolume of Distribution ObservedNormalized by Surface AreaVolume of Distribution ObservedNormalized by WeightC85798 VZP Vz Pred The volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration.Predicted Volume of DistributionC92425 VZPB Vz Pred Norm byBMIThe volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration, divided by the body massindex.Volume of Distribution PredictedNormalized by Body Mass IndexSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 297 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85839 - PKPARMCD - PK Parameters CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC102696 VZPD Vz Pred Norm byDoseC92426 VZPS Vz Pred Norm bySAC92427 VZPW Vz Pred Norm byWTThe volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration, divided by the dose.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration, divided by the surface area.The volume of distribution associated with theterminal slope following intravascular administration,calculated using the predicted value of the lastnon-zero concentration, divided by the weight.Predicted Volume of DistributionNormalized by DoseVolume of Distribution PredictedNormalized by Surface AreaVolume of Distribution PredictedNormalized by WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 298 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC66966 /h Per Hour A rate of occurrences of something within a period oftime equal to sixty minutes.Per HourC85645 IU/day IU/d International units per day. International Unit per DayC85646 IU/h IU/h International units per hour. International Unit per HourC85647 IU/min IU/min International units per minute. International Unit per MinuteC85648 IU/s IU/sec; IU/s International units per second. International Unit per SecondC48505 L Liter A unit of volume equal to a cubic decimeter, or onethousandth of cubic meter, or 1000 cubic centimeters,or approximately 61.023 744 cubic inches.(NCI)LiterC69110 L/day Liters per day. Liter per DayC85657 L/g/day mL/mg/day;uL/ug/day;L/day/g; L/g/dC85658 L/g/h L/h/g; mL/mg/h;uL/ug/h;mL/h/mg;uL/h/ug; L/g/hC85659 L/g/min mL/mg/min;uL/ug/min;L/min/g; L/g/minC85660 L/g/s L/g/sec;mL/mg/s;uL/ug/s; L/s/g;mL/mg/sec;uL/ug/sec;L/sec/g; L/g/sLiters per gram per day.Liters per gram per hour.Liters per gram per minute.Liters per gram per second.Liter per Gram per DayLiter per Gram per HourLiter per Gram per MinuteLiter per Gram per SecondC69160 L/h L/h Liters per hour. Liter per HourC73755 L/kg/day Milliliter perGram per Day;mL/g/day;uL/mg/day;L/day/kg;mL/day/g;uL/day/mg;L/kg/dLiters per kilogram per day.Milliliter per Gram per DayC73756 L/kg/h Milliliters per gram per hour. Milliliter per Gram per HourC73757 L/kg/min Milliliters per gram per minute. Milliliter per Gram per MinuteC85664 L/kg/s L/kg/sec; mL/g/s;uL/mg/s; L/s/kg;L/sec/kg; mL/s/g;uL/s/mg; L/kg/sC85672 L/mg/day mL/ug/day;L/day/mg;mL/day/ug;L/mg/dC85673 L/mg/h mL/ug/h; L/h/mg;mL/h/ug; L/mg/hLiters per kilogram per second.Liters per milligram per day.Liters per milligram per hour.Liter per Kilogram per SecondLiter per Milligram per DayLiter per Milligram per HourSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 299 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85674 L/mg/min mL/ug/min;L/min/mg;mL/min/ug;L/mg/minC85675 L/mg/s mL/ug/s; L/s/mg;mL/s/ug;mL/ug/sec;L/sec/mg;mL/sec/ug;L/mg/sC67388 L/min Liters perMinuteC85676 L/mmol/day mL/umol/day;L/day/mmol;mL/day/umol;L/mmol/dC85677 L/mmol/h mL/umol/h;L/h/mmol;mL/h/umol;L/mmol/hC85678 L/mmol/min mL/umol/min;L/min/mmol;mL/min/umol;L/mmol/minC85679 L/mmol/s L/mmol/sec;L/s/mmol;L/sec/mmol;mL/umol/s;mL/umol/sec;mL/s/umol;mL/sec/umoL;L/mmol/sC85680 L/mol mL/mmol;uL/umol; L/molC85681 L/mol/day L/day/mol;mL/mmol/day;uL/umol/day;mL/day/mmol;uL/day/umol;L/mol/dC85682 L/mol/h L/h/mol;mL/mmol/h;uL/umol/h;mL/h/mmol;uL/h/umol;L/mol/hC85683 L/mol/min L/min/mol;mL/mmol/min;uL/umol/min;mL/min/mmol;uL/min/umol;L/mol/minLiters per milligram per minute.Liters per milligram per second.A unit of volumetric flow rate defined as the rate atwhich one liter of matter travels during the period oftime equal to one minute.(NCI)Liters per millimole per day.Liters per millimole per hour.Liters per millimole per minute.Liters per millimole per second.Liters per mole.Liters per mole per day.Liters per mole per hour.Liters per mole per minute.Liter per Milligram per MinuteLiter per Milligram per SecondLiter per MinuteLiter per Millimole per DayLiter per Millimole per HourLiter per Millimole per MinuteLiter per Millimole per SecondLiter per MoleLiter per Mole per DayLiter per Mole per HourLiter per Mole per MinuteSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 300 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85684 L/mol/s L/mol/sec;L/s/mol;L/sec/mol;mL/mmol/s;mL/mmol/sec;mL/s/mmol;mL/sec/mmol;L/mol/sC85685 L/ng/day L/day/ng;mL/pg/day;mL/day/pg;L/ng/dC85686 L/ng/h L/h/ng; mL/pg/h;mL/h/pg; L/ng/hC85687 L/ng/min L/min/ng;mL/pg/min;mL/min/pg;L/ng/minC85688 L/ng/s L/s/ng; L/ng/sec;L/sec/ng;mL/pg/s;mL/s/pg;mL/pg/sec;mL/sec/pg;L/ng/sC85689 L/nmol/day mL/pmol/day;L/day/nmol;mL/day/pmol;L/nmol/dC85690 L/nmol/h mL/pmol/h;L/h/nmol;mL/h/pmol;L/nmol/hC85691 L/nmol/min mL/pmol/min;L/min/nmol;mL/min/pmol;L/nmol/minC85692 L/nmol/s L/nmol/sec;mL/pmol/s;mL/s/pmol;L/s/nmol;L/sec/nmol;L/nmol/sLiters per mole per second.Liters per nanogram per day.Liters per nanogram per hour.Liters per nanogram per minute.Liters per nanogram per second.Liters per nanomole per day.Liters per nanomole per hour.Liters per nanomole per minute.Liters per nanomole per second.Liter per Mole per SecondLiter per Nanogram per DayLiter per Nanogram per HourLiter per Nanogram per MinuteLiter per Nanogram per SecondLiter per Nanomole per DayLiter per Nanomole per HourLiter per Nanomole per MinuteLiter per Nanomole per SecondC85693 L/pg/day L/day/pg; L/pg/d Liters per picogram per day. Liter per Picogram per DayC85694 L/pg/h L/h/pg; L/pg/h Liters per picogram per hour. Liter per Picogram per HourC85695 L/pg/min L/min/pg;L/pg/minC85696 L/pg/s L/pg/sec; L/s/pg;L/sec/pg; L/pg/sLiters per picogram per minute.Liters per picogram per second.Liter per Picogram per MinuteLiter per Picogram per SecondC67390 L/s L/sec Liters per second. Liter per SecondSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 301 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85665 L/ug/day L/mcg/day;L/day/ug;L/day/mcg;mL/day/ng;mL/ng/dayC85662 L/ug/h L/mcg/h; L/h/ug;L/h/mcg;mL/h/ng;mL/ng/h; L/ug/hC85666 L/ug/min L/mcg/min;L/min/ug;L/min/mcg;mL/min/ng;mL/ng/minC85667 L/ug/s L/ug/sec; L/s/ug;L/ug/s; L/sec/ug;L/mcg/sec;L/s/mcg;L/mcg/s;L/sec/mcg;mL/ng/s;mL/s/ngC85668 L/umol/day L/mcmol/day;L/day/umol;L/day/mcmol;L/umol/dC85669 L/umol/h L/mcmol/h;L/h/umol;L/h/mcmol;L/umol/hC85670 L/umol/min L/mcmol/min;L/min/umol;L/min/mcmol;L/umol/minC85671 L/umol/s L/mcmol/s;L/umol/sec;L/s/umol;L/sec/umol;L/mcmol/sec;L/sec/mcmol;mL/nmol/s;mL/s/nmol;L/umol/sC85583 day*fg/mL pg*day/L;day*pg/L;fg*day/mL;d*fg/mLC85584 day*g/mL g*day/mL;day*kg/L;kg*day/L;d*g/mLLiters per microgram per day.Liters per microgram per hour.Liters per microgram per minute.Liters per microgram per second.Liters per micromole per day.Liters per micromole per hour.Liters per micromole per minute.Liters per micromole per second.Days times femtograms per milliliter.Days times grams per milliliter.Liter per Microgram per DayLiter per Microgram per HourLiter per Microgram per MinuteLiter per Microgram per SecondLiter per Micromole per DayLiter per Micromole per HourLiter per Micromole per MinuteLiter per Micromole per SecondDay Times Femtogram per MilliliterDay Times Gram per MilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 302 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85585 day*kg/mL kg*day/mL;g*day/uL;day*g/uL;d*kg/mLC85588 day*mg/mL mg*day/mL;day*g/L;g*day/L;day*ug/uL;ug*day/uL;d*mg/mLC85589 day*mmol/mL mmol*day/mL;day*mol/L;mol*day/L;day*umol/uL;umol*day/uL;d*mmol/mLC85590 day*mol/mL mol*day/mL;d*mol/mLC85591 day*ng/mL ng*day/mL;day*ug/L;ug*day/L;d*ng/mLC85592 day*nmol/mL nmol*day/mL;day*umol/L;umol*day/L;d*nmol/mLC85593 day*pg/mL pg*day/mL;day*ng/L;ng*day/L;d*pg/mLC85594 day*pmol/mL nmol*day/L;day*nmol/L;pmol*day/mL;d*pmol/mLC85586 day*ug/mL ug*day/mL;day*mg/L;mg*day/L;day*mcg/mL;mcg*day/mLC85587 day*umol/mL umol*day/mL;day*mmol/L;mmol*day/L;day*mcmol/mL;mcmol*day/mL;d*umol/mLDays times kilograms per milliliter.Days times milligrams per milliliter.Days times millimoles per milliliter.Days times moles per milliliter.Days times nanograms per milliliter.Days times nanomoles per milliliter.Days times picograms per milliliter.Days times picomoles per milliliter.Days times micrograms per milliliter.Days times micromoles per milliliter.Day Times Kilogram per MilliliterDay Times Milligram per MilliliterDay Times Millimole per MilliliterDay Times Mole per MilliliterDay Times Nanogram per MilliliterDay Times Nanomole per MilliliterDay Times Picogram per MilliliterDay Times Picomole per MilliliterDay Times Microgram per MilliliterDay Times Micromole per MilliliterC85597 fg/mL pg/L; fg/mL Femtograms per milliliter. Femtogram per MilliliterC48155 g Gram A metric unit of mass equal to one one thousandth of akilogram. (NCI)GramSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 303 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC67372 g/day Gram per Day;gram/day; g/d;g/24h; gram/24HoursGrams per day.Gram per DayC85601 g/h gram/h; g/h Grams per hour. Gram per HourC64566 g/mL Kilogram perLiter; g/mL;Gram perMilliliter;gram/mL; kg/L;mg/uLA unit of mass concentration defined as theconcentration of one kilogram of a substance in unitvolume of the mixture equal to one liter. The conceptalso refers to the unit of mass density (volumic mass)defined as the density of substance which mass equalto one kilogram occupies the volume one liter.(NCI)Kilogram per LiterC85602 g/min gram/min; g/min Grams per minute. Gram per MinuteC85603 g/s gram/sec; g/sec;gram/s; g/sC85611 h*fg/mL pg*h/L; h*pg/L;fg*h/mL;h*fg/mLC856<strong>12</strong> h*fmol/mL pmol*h/L;h*pmol/L;fmol*h/mL;h*fmol/mLC85613 h*g/mL g*h/mL; h*kg/L;kg*h/L; h*g/mLC85614 h*kg/mL kg*h/mL;g*h/uL; h*g/uL;h*kg/mLC85620 h*mg/L/mg mg*h/L/mg;h*mg/mg/L;mg*h/mg/L;h*mg/L/mgC85621 h*mg/mL mg*h/mL; h*g/L;g*h/L; ug*h/uL;h*ug/uL;h*mg/mLC85622 h*mmol/mL mmol*h/mL;h*mol/L;mol*h/L;umol*h/uL;h*umol/uL;h*mmol/mLC85623 h*mol/mL mol*h/mL;h*mol/mLC85624 h*ng/mL ng*h/mL;h*ug/L; ug*h/L;h*ng/mLC85630 h*ng/mL*kg ng*h/mL*kg;h*ng/kg*mL;ng*h/kg*mL;h*ng/mL*kgGrams per second.Hours times femtograms per milliliter.Hours times femtomoles per milliliter.Hours times grams per milliliter.Hours times kilograms per milliliter.Hours times milligrams per liter per milligram.Hours times milligrams per milliliter.Hours times millimoles per milliliter.Hours times moles per milliliter.Hours times nanograms per milliliter.Hours times nanograms per milliliter times kilogram.Gram per SecondHour Times Femtogram perMilliliterHour Times Femtomole perMilliliterHour Times Gram per MilliliterHour Times Kilogram per MilliliterHour Times Milligram per Liter perMilligramHour Times Milligram per MilliliterHour Times Millimole per MilliliterHour Times Mole per MilliliterHour Times Nanogram per MilliliterHour Times Nanogram per MilliliterTimes KilogramSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 304 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85625 h*ng/mL/g ng*h/mL/g;h*ng/g/mL;ng*h/g/mL;h*ng/mL/gC85626 h*ng/mL/kg ng*h/mL/kg;ng*h/kg/mL;h*ng/kg/mL;h*ng/mL/kgC85627 h*ng/mL/mg ng*h/mL/mg;h*ng/mg/mL;ng*h/mg/mL;h*ng/mL/mgC85628 h*ng/mL/mg/kg ng*h/mL/mg/kg;h*ng/mL/mg/kgC85629 h*ng/mL/mg/m2 h*ng/mL/mg/m^2;h*ng/mL/mg/m*m;ng*h/mL/mg/m2C85631 h*nmol/L/umol h*nmol/L/mcmol;nmol*h/L/umol;h*nmol/umol/L;nmol*h/umol/LC85632 h*nmol/mL umol*h/L;h*umol/L;nmol*h/mL;h*nmol/mLC85634 h*nmol/mL/mg nmol*h/mL/mg;h*nmol/mg/mL;nmol*h/mg/mL;h*nmol/mL/mgC85635 h*pg/mL pg*h/mL;h*ng/L; ng*h/L;h*pg/mLC85636 h*pg/mL/kg pg*h/mL/kg;h*pg/kg/mL;pg*h/kg/mL;h*pg/mL/kgC85638 h*pg/mL/mg pg*h/mL/mg;h*pg/mg/mL;pg*h/mg/mL;h*pg/mL/mgC85637 h*pg/mL/ug h*pg/mL/mcg;pg*h/mL/ug;h*pg/ug/mL;pg*h/ug/mLC85639 h*pmol/L/ug pmol*h/L/ug;h*pmol/L/mcg;h*pmol/ug/L;pmol*h/ug/LHours times nanograms per milliliter per gram.Hours times nanograms per milliliter per kilogram.Hours times nanograms per milliliter per milligram.Hours times nanograms per milliliter per milligramper kilogram.Hours times nanograms per milliliter per milligramper meter squared.Hours times nanomoles per liter per micromole.Hours times nanomoles per milliliter.Hours times nanomoles per milliliter per milligram.Hours times picograms per milliliter.Hours times picograms per milliliter per kilogram.Hours times picograms per milliliter per milligram.Hours times picograms per milliliter per microgram.Hours times picomoles per liter per microgram.Hour Times Nanogram per Milliliterper GramHour Times Nanogram per Milliliterper KilogramHour Times Nanogram per Milliliterper MilligramHour Times Nanogram per Milliliterper Milligram per KilogramHour Times Nanogram per Milliliterper Milligram per Meter SquaredHour Times Nanomole per Liter perMicromoleHour Times Nanomole per MilliliterHour Times Nanomole per Milliliterper MilligramHour Times Picogram per MilliliterHour Times Picogram per Milliliterper KilogramHour Times Picogram per Milliliterper MilligramHour Times Picogram per Milliliterper MicrogramHour Times Picomole per Liter perMicrogramSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 305 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85640 h*pmol/mL pmol*h/mL;h*nmol/L;nmol*h/L;h*pmol/mLC85641 h*pmol/mL/mg pmol*h/mL/mg;h*pmol/mg/mL;pmol*h/mg/mL;h*pmol/mL/mgC85615 h*ug/mL mg*h/L; h*mg/L;h*ng/uL;ng*h/uL;h*ug/mL;h*mcg/mL;mcg*h/mLC85617 h*ug/mL/mg ug*h/mL/mg;h*mcg/mL/mg;h*ug/mg/mL;ug*h/mg/mLC85618 h*umol/mL mmol*h/L;h*mmol/L;umol*h/mL;h*mcmol/mL;mcmol*h/mL;h*umol/mLC85606 h2*mg/mL mg*h2/mL;h^2*mg/mL;h*h*mg/mL;h2*g/L;h2*ug/uLC85607 h2*mmol/mL mmol*h2/mL;h^2*mmol/mL;h*h*mmol/mL;h2*mol/L;h2*umol/uLC85608 h2*ng/mL ng*h2/mL;h^2*ng/mL;h*h*ng/mL;h2*ug/LC85609 h2*pg/mL pg*h2/mL;h^2*pg/mL;h*h*pg/mL;h2*ng/LC85610 h2*pmol/mL pmol*h2/mL;h^2*pmol/mL;h*h*pmol/mL;h2*nmol/LC85604 h2*ug/mL ug*h2/mL;h^2*ug/mL;h*h*ug/mL;h2*mcg/mL;h2*mg/LHours times picomoles per milliliter.Hours times picomoles per milliliter per milligram.Hours times micrograms per milliliter.Hours times micrograms per milliliter per milligram.Hours times micromoles per milliliter.Hours squared times milligrams per milliliter.Hours squared times millimoles per milliliter.Hours squared times nanograms per milliliter.Hours squared times picogram per milliliter.Hours squared times picomoles per milliliter.Hours squared times micrograms per milliliter.Hour Times Picomole per MilliliterHour Times Picomole per Milliliterper MilligramHour Times Microgram perMilliliterHour Times Microgram perMilliliter per MilligramHour Times Micromole perMilliliterHour Squared Times Milligram perMilliliterHour Squared Times Millimole perMilliliterHour Squared Times Nanogram perMilliliterHour Squared Times Picogram perMilliliterHour Squared Times Picomole perMilliliterHour Squared Times Microgram perMilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 306 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85605 h2*umol/mL umol*h2/mL;h^2*umol/mL;h*h*umol/mL;h2*mcmol/mL;h2*mmol/LC67376 mIU/mL InternationalUnit per Liter;IU/L; mIU/mLHours squared times micromoles per milliliter.Unit of arbitrary substance concentration (biologicactivity concentration) defined as the concentration ofone international unit per one liter of systemvolume.(NCI)Hour Squared Times Micromole perMilliliterInternational Unit per LiterC28254 mL Milliliter; cm3 The unit of volume equal to one thousandth of a liter,one cubic centimeter, 10E-6 cubic meter, orapproximately to 0.061 023 7 cubic inch.(NCI)MilliliterC67410 mL/day Milliliters per day. Milliliter per DayC66962 mL/h Milliliters perHourC73758 mL/kg/day Milliliter perKilogram perDayC73759 mL/kg/h Milliliter perKilogram perHourC73760 mL/kg/min Milliliter perKilogram perMinute;uL/g/min;mL/min/kg;uL/min/g;mL/kg/minC85715 mL/kg/s mL/sec/kg;mL/s/kg;mL/kg/sec;mL/kg/sC64777 mL/min Milliliters perMinuteC85716 mL/mol/day uL/mmol/day;mL/day/mol;uL/day/mmol;mL/mol/dC85717 mL/mol/h uL/mmol/h;mL/h/mol;uL/h/mmol;mL/mol/hC85718 mL/mol/min uL/mmol/min;mL/min/mol;uL/min/mmol;mL/mol/minA unit of volumetric flow rate defined as the rate atwhich one milliliter of matter travels during the periodof time equal to one hour.(NCI)Milliliters per kilogram per day.Milliliters per kilogram per hour.Milliliters per kilogram per minute.Milliliters per kilogram per second.A metric unit of volumetric flow rate defined as therate at which one milliliter of matter travels during theperiod of time equal to one minute.(NCI)Milliliters per mole per day.Milliliters per mole per hour.Milliliters per mole per minute.Milliliter per HourMilliliter per Kilogram per DayMilliliter per Kilogram per HourMilliliter per Kilogram per MinuteMilliliter per Kilogram per SecondMilliliter per MinuteMilliliter per Mole per DayMilliliter per Mole per HourMilliliter per Mole per MinuteSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 307 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85719 mL/mol/s mL/sec/mol;mL/s/mol;mL/mol/sec;mL/mol/sMilliliters per mole per second.Milliliter per Mole per SecondC69073 mL/s mL/sec Milliliters per second. Milliliter per SecondC28253 mg Milligram The unit of mass equal to one thousandth of a gram or1000 micrograms. One milligram equalsapproximately 0.015432 grain or 35.274 x 10E-6ounce.(NCI)MilligramC85710 mg/L/mg mg/mg/L;mg/L/mgC67399 mg/day Milligram perDayC66969 mg/h Milligram perHourC42576 mg/mL g/L; Gram perLiter; kg/m3;Kilogram perCubic Metre;Microgram perMicroliter;Milligram perMilliliter; ug/uL;mg/mLC85711 mg/mL/h g/L/h; ug/uL/h;mg/h/mL; g/h/L;ug/h/uLMilligrams per liter per milligram.A unit of measure referring to the ratio between massexpressed in milligrams per day.(NCI)A unit of mass flow rate equivalent to the rate at whichone thousandth of a gram of matter travels to a givenobject or space over a period of time equal to onehour.(NCI)A unit of mass concentration defined as theconcentration of one kilogram of a substance per unitvolume of the mixture equal to one cubic meter, or theconcentration of one milligram of a substance per unitvolume of the mixture equal to one milliliter, or onegram of a substance per one liter of the mixture. It isalso a unit of mass density (volumic mass) defined asthe density of substance which mass equal to onemilligram occupies the volume one milliliter.(NCI)Milligrams per milliliter per hour.Milligram per Liter per MilligramMilligram per 24 HoursMilligram per HourKilogram per Cubic MeterMilligram per Milliliter per HourC73742 mg/min Milligrams per minute. Milligram per MinuteC857<strong>12</strong> mg/s mg/sec; mg/s Milligrams per second. Milligram per SecondC85724 min*fg/mL pg*min/L;min*pg/L;fg*min/mL;min*fg/mLC85725 min*g/mL g*min/mL;min*kg/L;kg*min/L;min*g/mLC85726 min*kg/mL kg*min/mL;g*min/uL;min*g/uL;min*kg/mLC85729 min*mg/mL mg*min/mL;min*g/L;g*min/L;ug*min/uL;min*ug/uL;min*mg/mLMinutes times femtograms per milliliter.Minutes times grams per milliliter.Minutes times kilograms per milliliter.Minutes times milligrams per milliliter.Minute Times Femtogram perMilliliterMinute Times Gram per MilliliterMinute Times Kilogram perMilliliterMinute Times Milligram perMilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 308 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85730 min*mmol/mL mmol*min/mL;min*mol/L;mol*min/L;min*umol/uL;umol*min/uL;min*mmol/mLC85731 min*mol/mL mol*min/mL;min*mol/mLC85732 min*ng/mL ng*min/mL;min*ug/L;ug*min/L;min*ng/mLC85733 min*nmol/mL nmol*min/mL;min*umol/L;umol*min/L;min*nmol/mLC85734 min*pg/mL pg*min/mL;min*ng/L;ng*min/L;min*pg/mLC85735 min*pmol/mL nmol*min/L;min*nmol/L;pmol*min/mL;min*pmol/mLC85727 min*ug/mL ug*min/mL;min*mg/L;mg*min/L;min*mcg/mL;mcg*min/mLC85728 min*umol/mL umol*min/mL;min*mcmol/mL;min*mmol/L;mmol*min/L;min*umol/mLMinutes times millimoles per milliliter.Minutes times moles per milliliter.Minutes times nanograms per milliliter.Minutes times nanomoles per milliliter.Minutes times picograms per milliliter.Minutes times picomoles per milliliter.Minutes times micrograms per milliliter.Minutes times micromoles per milliliter.Minute Times Millimole perMilliliterMinute Times Mole per MilliliterMinute Times Nanogram perMilliliterMinute Times Nanomole perMilliliterMinute Times Picogram perMilliliterMinute Times Picomole perMilliliterMinute Times Microgram perMilliliterMinute Times Micromole perMilliliterC85720 mmol/h Millimoles per hour. Millimole per HourC48555 mmol/mL Mole per Liter;mol/L; mmol/mLC85721 mmol/mL/h mol/L/h;umol/uL/h;mmol/h/mL;mol/h/L;umol/h/uL;mmol/mL/hA unit of concentration (molarity unit) equal to onemole of solute in one liter of solution.(NCI)Millimoles per milliliter per hour.Mole per LiterMillimole per Milliliter per HourC85722 mmol/min Millimoles per minute. Millimole per MinuteC85723 mmol/s mmol/sec;mmol/sMillimoles per second.Millimole per SecondC85737 mol/day mol/d Moles per day. Mole per DayC85738 mol/h Moles per hour. Mole per HourC85739 mol/min Moles per minute. Mole per MinuteSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 309 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85740 mol/s mol/sec; mol/s Moles per second. Mole per SecondC69188 nL Nanoliter A unit of volume equal to one billionth of a liter(10E-9 liter).(NCI)NanoliterC85760 nU/mL uU/L; mcU/L;nU/mLNanounits per milliliter.Nanounit per MilliliterC48516 ng Nanogram A unit of mass equal to one billionth (10E-9) of agram, or one millionth (10E-6) of a milligram.(NCI)NanogramC85741 ng/day ng/d Nanograms per day. Nanogram per DayC85742 ng/h Nanograms per hour. Nanogram per HourC85743 ng/kg/min ng/min/kg;pg/g/min;pg/min/g;ng/kg/minC67306 ng/mL mg/m3;Microgram perLiter; Milligramper Cubic Meter;Nanogram perMilliliter; ug/L;ng/mL; mcg/LC85748 ng/mL*kg ng/kg*mL;ng/mL*kgC67430 ng/mL/h ug/L/h; ng/h/mL;ng/mL/hC85746 ng/mL/kg ng/kg/mL;ng/mL/kgC85747 ng/mL/mg ng/mg/mL;ng/mL/mgC85744 ng/mg of Creatinine ug/g ofCreatinine;ng/mg ofCreatinineNanograms per kilogram per minute.A unit of mass concentration defined as theconcentration of one microgram of a substance perunit volume of the mixture equal to one liter. Theconcept also refers to the unit of mass density(volumic mass) defined as the density of substancewhich mass equal to one microgram occupies thevolume one liter.(NCI)Nanograms per milliliter times kilogram.Nanograms per milliliter per hour.Nanograms per milliliter per kilogram.Nanograms per milliliter per milligram.Nanograms per milligram of creatinine.Nanogram per Kilogram per MinuteMicrogram per LiterNanogram per Milliliter TimesKilogramNanogram per Milliliter per HourNanogram per Milliliter perKilogramNanogram per Milliliter perMilligramNanogram per Milligram ofCreatinineC85749 ng/min Nanograms per minute. Nanogram per MinuteC85750 ng/s ng/sec; ng/s Nanograms per second. Nanogram per SecondC85755 nmol/L/umol nmol/L/mcmol;nmol/umol/L;nmol/mcmol/L;nmol/L/umolNanomoles per liter per micromole.Nanomole per Liter per MicromoleC85751 nmol/day nmol/d Nanomoles per day. Nanomole per DayC85752 nmol/g umol/kg;pmol/mg; nmol/gNanomoles per gram.Nanomole per GramC85753 nmol/h Nanomoles per hour. Nanomole per HourC85754 nmol/kg pmol/g; nmol/kg Nanomoles per kilogram. Nanomole per KilogramSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 310 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85756 nmol/mL umol/L;mcmol/L;nmol/mLC85757 nmol/mL/h umol/L/h;nmol/h/mL;nmol/mL/hNanomoles per milliliter.Nanomoles per milliliter per hour.Nanomole per MilliliterNanomole per Milliliter per HourC85758 nmol/min Nanomoles per minute. Nanomole per MinuteC85759 nmol/s nmol/sec; nmol/s Nanomoles per second. Nanomole per SecondC67331 pg/dL Picogram perDeciliterPicograms per deciliter.Picogram per DeciliterC85778 pg/day pg/d Picograms per day. Picogram per DayC85779 pg/h Picograms per hour. Picogram per HourC67327 pg/mL ug/m3;Microgram perCubic Meter;ng/L; pg/mLC85781 pg/mL/ug pg/mL/mcg;pg/ug/mL;pg/mcg/mLC67396 pg/mg mcg/kg;Microgram perKilogram; ng/g;ug/kgC85780 pg/mg Creatinine ng/g ofCreatinine;pg/mg ofCreatinineA unit of mass concentration defined as theconcentration of one nanogram of a substance per oneliter of the mixture, or one picogram of a substance inunit volume of the mixture equal to one milliliter, orone microgram of a substance per one cubic meter ofthe mixture. The concept also refers to the metric unitof mass density (volumic mass) defined as the densityof substance which mass equal to one nanogramoccupies the volume one liter.(NCI)Picograms per milliliter per microgram.A unit of a mass fraction expressed as a number ofmicrograms of substance per kilogram of mixture. Theunit is also used as a dose calculation unit.(NCI)Picograms per milligram creatinine.Nanogram per LiterPicogram per Milliliter perMicrogramMicrogram per KilogramPicogram per Milligram ofCreatinineC85782 pg/min Picograms per minute. Picogram per MinuteC85783 pg/s pg/sec; pg/s Picograms per second. Picogram per SecondC85784 pmol/L/ug pmol/L/mcg;pmol/ug/L;pmol/mcg/LC85802 s*fg/mL pg*s/L;sec*fg/mL;s*pg/L; fg*s/mL;fg*sec/mL;s*fg/mLC85803 s*g/mL g*s/mL;sec*g/mL;g*sec/mL;s*kg/L; kg*s/L;sec*kg/L;kg*sec/LPicomoles per liters per microgram.Seconds times femtograms per milliliter.Seconds times grams per milliliter.Picomole per Liter per MicrogramSecond Times Femtogram perMilliliterSecond Times Gram per MilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 311 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85804 s*kg/mL kg*s/mL; g*s/uL;s*g/uL;kg*sec/mL;g*sec/uL;sec*g/uL;s*kg/mLC85807 s*mg/mL mg*s/mL;sec*mg/mL;s*g/L; g*s/L;s*mg/mLC85808 s*mmol/mL mmol*s/mL;sec*mmol/mL;s*mol/L;mol*s/L;sec*mol/L;mol*sec/L;s*mmol/mLC85809 s*mol/mL mol*s/mL;sec*mol/mL;mol*sec/mL;s*mol/mLC85810 s*ng/mL ng*s/mL;sec*ng/mL;ng*sec/mL;s*ug/L; ug*s/L;sec*ug/L;ug*sec/L;s*ng/mLC85811 s*nmol/mL nmol*s/mL;sec*nmol/mL;nmol*sec/mL;s*umol/L;umol*s/L;sec*umol/L;umol*sec/L;s*nmol/mLC858<strong>12</strong> s*pg/mL pg*s/mL;sec*pg/mL;pg*sec/mL;s*ng/L; ng*s/L;sec*ng/L;ng*sec/L;s*pg/mLC85813 s*pmol/mL nmol*s/L;s*nmol/L;sec*pmol/mL;pmol*s/mL;pmol*sec/mL;s*pmol/mLSeconds times kilograms per milliliter.Seconds times milligrams per milliliter.Seconds times millimoles per milliliter.Seconds times moles per milliliter.Seconds times nanograms per milliliter.Seconds times nanomoles per milliliter.Seconds times picograms per milliliter.Seconds times picomoles per milliliter.Second Times Kilogram perMilliliterSecond Times Milligram perMilliliterSecond Times Millimole perMilliliterSecond Times Mole per MilliliterSecond Times Nanogram perMilliliterSecond Times Nanomole perMilliliterSecond Times Picogram perMilliliterSecond Times Picomole perMilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 3<strong>12</strong> of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85805 s*ug/mL ug*s/mL;s*mcg/mL;ug*sec/mL;sec*ug/mL;sec*mcg/mL;mcg*sec/mL;s*mg/L; mg*s/LC85806 s*umol/mL umol*s/mL;s*mcmol/mL;sec*umol/mL;umol*sec/mL;mcmol*sec/mL;sec*mcmol/mL;s*mmol/L;mmol*s/L;s*umol/mLC67405 uIU/mL Micro-International Unit permilliliter; mIU/L;mcIU/mL;uIU/mLC48153 uL mcL; Microliter;mm3Seconds times micrograms per milliliter.Seconds times micromoles per milliliter.Microinternational units per milliliter.A unit of volume accepted for use with the SI and equalto one millionth of a liter (10E-6 liter).(NCI)Second Times Microgram perMilliliterSecond Times Micromole perMilliliterMicrointernational Unit perMilliliterMicroliterC48152 ug Microgram; mcg A unit of mass equal to one millionth of a gram or onethousandth of a milligram.(NCI)MicrogramC7<strong>12</strong>05 ug/day Microgram perDay; mcg/dayC67394 ug/h Micrograms perHour; mcg/hA unit of mass flow rate equivalent to the rate at whichone millionth of a gram of matter travels to a givenobject or space over a period of time equal to twentyfour hours. Microgram per day is also a doseadministration rate unit equal to the rate at which onemillionth of a gram of a product is administered perunit of time equal to twenty four hours.(NCI)A unit of mass flow rate equivalent to the rate at whichone millionth of a gram of matter travels to a givenobject or space over a period of time equal to onehour.(NCI)Microgram per DayMicrogram per HourC85703 ug/inh mcg/inh Micrograms per inhalation. Microgram per InhalationC64572 ug/mL g/m3; Gram perCubic Meter;mg/L;Microgram perMilliliter;Milligram perLiter; mcg/mL;ng/uL; ug/mLC7<strong>12</strong>11 ug/min Micrograms perMinute; mcg/minA unit of mass concentration equal to theconcentration of one gram of a substance per unitvolume of the mixture equal to one cubic meter. Theconcept also refers to the metric unit of mass density(volumic mass) defined as the density of substancewhich mass equal to one gram occupies the volumeone cubic meter.(NCI)A unit of mass flow rate equivalent to the rate at whichone millionth of a gram of matter travels to a givenobject or space over a period of time equal to oneminute. Microgram per minute is also a doseadministration rate unit equal to the rate at which onemillionth of a gram of a product is administered perunit of time equal to one minute.(NCI)Microgram per MilliliterMicrogram per MinuteC85704 ug/puff mcg/puff Micrograms per puff. Microgram per PuffSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 313 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C85494 - PKUNIT - PK Parameter Units of MeasureCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC85705 ug/s mcg/sec; mcg/s;ug/sec; ug/sC67406 umol/day Micromoles perDay; mcmol/dayMicrograms per second.A unit of amount of substance flow rate equivalent tothe rate at which one millionth of a mole of substancetravels to a given object or space over a period of timeequal to 24 hours.(NCI)Microgram per SecondMicromole per 24 HoursC85707 umol/h mcmol/h; umol/h Micromoles per hour. Micromole per HourC64387 umol/mL Millimole perLiter; umol/mL;Micromole perMilliliter;mol/m3; Moleper Cubic Meter;mcmol/mL;mmol/L;nmol/uLC85708 umol/min mcmol/min;umol/minC85709 umol/s umol/sec;mcmol/s;mcmol/sec;umol/sA unit of concentration (molarity unit) equal to onethousandth of a mole (10E-3 mole) of solute per oneliter of solution.(NCI)Micromoles per minute.Micromoles per second.Millimole per LiterMicromole per MinuteMicromole per SecondSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 314 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C99075 - PORTOT - Portion/TotalityCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64916 ALL Being or representing the total number of individualentities.C25326 ENTIRE Whole Being or representing the complete extent of a singleentity.AllWholeC78728 MULTIPLE Many; Several More than one. (NCI) ManyC25378 PARTIAL Being or representing an incomplete extent of a singleentity.PartialC453<strong>12</strong> SEGMENT One of the parts into which something is divided. SegmentC48440 SINGLE One. SingleSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 315 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71148 - POSITION - PositionCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC62173 FOWLERS Fowlers A semi-sitting position whereby the head of anadjustable bed is elevated to the desired height, about60-90 cm, to produce angulation of the body, usually45 degrees to 60 degrees. Knees may or may not bebent. (NCI)Fowler's PositionC100758 LATERAL DECUBITUS LateralDecubitusLying down on one side.Lateral Decubitus PositionC62172LEFT LATERAL DECUBITUS Left lateraldecubitusA recumbent left lateral side position. (NCI)Left Lateral Decubitus PositionC62165 PRONE Prone An anterior recumbent body position whereby theperson lies on its stomach and faces downward. (NCI)Prone PositionC62169REVERSETRENDELENBURGReverseTrendelenburgA supine position with the person inclined at an angleof 45 degrees so that the head is higher than the pelvis.(NCI)Reverse TrendelenburgC62171RIGHT LATERALDECUBITUSRight lateraldecubitusA recumbent right lateral side position. (NCI)Right Lateral Decubitus PositionC62174 SEMI-FOWLERS Semi-Fowlers A semi-sitting or semi-reclined body position wherebythe head is elevated on an angle of approximately 30degrees. (NCI)C62<strong>12</strong>2 SITTING Sitting The state or act of one who sits; the posture of onewho occupies a seat. (NCI)C92604 SLING Sling A position in which the subject's body is supported bya sling.C62166 STANDING Standing The act of assuming or maintaining an erect uprightposition. (NCI)C62167 SUPINE Supine A posterior recumbent body position whereby theperson lies on its back and faces upward. (NCI)C62168 TRENDELENBURG Trendelenburg A supine position with the person inclined at an angleof 45 degrees so that the pelvis is higher than the head.(NCI)C90480 UNCONSTRAINED Unconstrained The ability to move body parts and limbs withoutphysical restriction. (NCI)Semi-Fowler's PositionSittingPatient in Body SlingStandingSupine PositionTrendelenburgUnconstrained Body MovementSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 316 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C78737 - RELTYPE - Relationship TypeCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC78728 MANY Many; Several More than one. (NCI) ManyC66832 ONE A textual representation of the numeral 1. OneSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 317 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66729 - ROUTE - Route of AdministrationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC38192 AURICULAR (OTIC) Administration to or by way of the ear. (FDA) Auricular Route of AdministrationC38193 BUCCAL Administration directed toward the cheek, generallyfrom within the mouth. (FDA)C38194 CONJUNCTIVAL Administration to the conjunctiva, the delicatemembrane that lines the eyelids and covers theexposed surface of the eyeball. (FDA)Buccal Route of AdministrationConjunctival Route ofAdministrationC38675 CUTANEOUS Administration to the skin. (FDA) Cutaneous Route of AdministrationC38197 DENTAL Administration to a tooth or teeth. (FDA) Dental Route of AdministrationC78373 DIETARY Administration by way of food stuff. (NCI) Dietary Route of AdministrationC38633 ELECTRO-OSMOSIS Administration of through the diffusion of substancethrough a membrane in an electric field. (FDA)C38205 ENDOCERVICAL Administration within the canal of the cervix uteri.Synonymous with the term intracervical. (FDA)C38206 ENDOSINUSIAL Administration within the nasal sinuses of the head.(FDA)Electro-osmosis Route ofAdministrationEndocervical Route ofAdministrationEndosinusial Route ofAdministrationC38208 ENDOTRACHEAL IntratrachealRoute ofAdministrationAdministration directly into the trachea. Synonymouswith the term intratracheal. (FDA)Endotracheal Route ofAdministrationC38209 ENTERAL Administration directly into the intestines. (FDA) Enteral Route of AdministrationC38210 EPIDURAL Administration upon or over the dura mater. (FDA) Epidural Route of AdministrationC38211 EXTRA-AMNIOTIC Administration to the outside of the membraneenveloping the fetus. (FDA)Extraamniotic Route ofAdministrationC382<strong>12</strong> EXTRACORPOREAL Administration outside of the body. (FDA) Extracorporeal Circulation Route ofAdministrationC38200 HEMODIALYSIS Administration through hemodialysate fluid. (FDA) Administration Via HemodialysisC38215 INFILTRATION Administration that results in substances passing intotissue spaces or into cells. (FDA)C38219 INTERSTITIAL Administration to or in the interstices of a tissue.(FDA)Infiltration Route of AdministrationInterstitial Route of AdministrationC38220 INTRA-ABDOMINAL Administration within the abdomen. (FDA) Intraabdominal Route ofAdministrationC38221 INTRA-AMNIOTIC Administration within the amnion. (FDA) Intraamniotic Route ofAdministrationC38222 INTRA-ARTERIAL Administration within an artery or arteries. (FDA) Intraarterial Route of AdministrationC38223 INTRA-ARTICULAR Administration within a joint. (FDA) Intraarticular Route ofAdministrationC38224 INTRABILIARY Administration within the bile, bile ducts orgallbladder. (FDA)Intrabiliary Route of AdministrationC38225 INTRABRONCHIAL Administration within a bronchus. (FDA) Intrabronchial Route ofAdministrationC38226 INTRABURSAL Administration within a bursa. (FDA) Intrabursal Route of AdministrationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 318 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66729 - ROUTE - Route of AdministrationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64984 INTRACAMERAL Administration by injection directly into the anteriorchamber of the eye.Intracameral Route ofAdministrationC38227 INTRACARDIAC Administration within the heart. (FDA) Intracardiac Route of AdministrationC38228 INTRACARTILAGINOUS Administration within a cartilage; endochondral. (FDA) Intracartilaginous Route ofAdministrationC38229 INTRACAUDAL Administration within the cauda equina. (FDA) Intracaudal Route of AdministrationC38230 INTRACAVERNOUS Administration within a pathologic cavity, such asoccurs in the lung in tuberculosis. (FDA)C38231 INTRACAVITARY Administration within a non-pathologic cavity, such asthat of the cervix, uterus, or penis, or such as that isformed as the result of a wound. (FDA)Intracavernous Route ofAdministrationIntracavitary Route of AdministrationC38232 INTRACEREBRAL Administration within the cerebrum. (FDA) Intracerebral Route ofAdministrationC38233 INTRACISTERNAL Administration within the cisterna magnacerebellomedularis. (FDA)C38234 INTRACORNEAL Administration within the cornea (the transparentstructure forming the anterior part of the fibrous tunicof the eye). (FDA)C38217 INTRACORONAL, DENTAL Administration of a drug within a portion of a toothwhich is covered by enamel and which is separatedfrom the roots by a slightly constricted region knownas the neck. (FDA)Intracisternal Route ofAdministrationIntracorneal Route of AdministrationIntracoronal Dental Route ofAdministrationC38218 INTRACORONARY Administration within the coronary arteries. (FDA) Intracoronary Route ofAdministrationC38235INTRACORPORUSCAVERNOSUMAdministration within the dilatable spaces of thecorporus cavernosa of the penis. (FDA)Intracorporus Cavernosum Route ofAdministrationC38238 INTRADERMAL Administration within the dermis. (FDA) Intradermal Route of AdministrationC38239 INTRADISCAL Administration within a disc. (FDA) Intradiscal Route of AdministrationC38240 INTRADUCTAL Administration within the duct of a gland. (FDA) Intraductal Route of AdministrationC38241 INTRADUODENAL Administration within the duodenum. (FDA) Intraduodenal Route ofAdministrationC38242 INTRADURAL Administration within or beneath the dura. (FDA) Intradural Route of AdministrationC38243 INTRAEPIDERMAL Administration within the epidermis. (FDA) Intraepidermal Route ofAdministrationC38245 INTRAESOPHAGEAL Administration within the esophagus. (FDA) Intraesophageal Route ofAdministrationC38246 INTRAGASTRIC Administration within the stomach. (FDA) Intragastric Route of AdministrationC38247 INTRAGINGIVAL Administration within the gingivae. (FDA) Intragingival Route of AdministrationC38249 INTRAILEAL Administration within the distal portion of the smallintestine, from the jejunum to the cecum. (FDA)Intraileal Route of AdministrationC102399 INTRAJEJUNAL Administration into the jejunum. Intrajejunal Route of AdministrationC38250 INTRALESIONAL Administration within or introduced directly into alocalized lesion. (FDA)Intralesional Route ofAdministrationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 319 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66729 - ROUTE - Route of AdministrationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC38251 INTRALUMINAL Administration within the lumen of a tube. (FDA) Intraluminal Route of AdministrationC38252 INTRALYMPHATIC Administration within the lymph. (FDA) Intralymphatic Route ofAdministrationC38253 INTRAMEDULLARY Administration within the marrow cavity of a bone.(FDA)C38254 INTRAMENINGEAL Administration within the meninges (the threemembranes that envelope the brain and spinal cord).(FDA)Intramedullary Route ofAdministrationIntrameningeal Route ofAdministrationC28161 INTRAMUSCULAR Administration within a muscle. (FDA) Intramuscular Route ofAdministrationC38255 INTRAOCULAR Administration within the eye. (FDA) Intraocular Route of AdministrationC38256 INTRAOVARIAN Administration within the ovary. (FDA) Intraovarian Route of AdministrationC102400 INTRAPALATAL Administration into the palate. Intrapalatal Route of AdministrationC38257 INTRAPERICARDIAL Administration within the pericardium. (FDA) Intrapericardial Route ofAdministrationC38258 INTRAPERITONEAL Administration within the peritoneal cavity. (FDA) Intraperitoneal Route ofAdministrationC38259 INTRAPLEURAL Administration within the pleura. (FDA) Intrapleural Route of AdministrationC38260 INTRAPROSTATIC Administration within the prostate gland. (FDA) Intraprostatic Route ofAdministrationC38261 INTRAPULMONARY Administration within the lungs or its bronchi. (FDA) Intrapulmonary Route ofAdministrationC38262 INTRASINAL Administration within the nasal or periorbital sinuses.(FDA)Intrasinal Route of AdministrationC38263 INTRASPINAL Administration within the vertebral column. (FDA) Intraspinal Route of AdministrationC65138 INTRASTOMAL Administration into a stoma. Administration via StomaC38264 INTRASYNOVIAL Administration within the synovial cavity of a joint.(FDA)Intrasynovial Route ofAdministrationC38265 INTRATENDINOUS Administration within a tendon. (FDA) Intratendinous Route ofAdministrationC38266 INTRATESTICULAR Administration within the testicle. (FDA) Intratesticular Route ofAdministrationC38267 INTRATHECAL Administration within the cerebrospinal fluid at anylevel of the cerebrospinal axis, including injection intothe cerebral ventricles. (FDA)C38207 INTRATHORACIC Administration within the thorax (internal to the ribs);synonymous with the term endothoracic. (FDA)Intrathecal Route of AdministrationEndothoracic Route ofAdministrationC38268 INTRATUBULAR Administration within the tubules of an organ. (FDA) Intratubular Route of AdministrationC38269 INTRATUMOR Intratumor RouteofAdministrationAdministration within a tumor. (FDA)Intratumoral Route of AdministrationC38270 INTRATYMPANIC Administration within the auris media. (FDA) Intratympanic Route ofAdministrationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 320 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66729 - ROUTE - Route of AdministrationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC38272 INTRAUTERINE Administration within the uterus. (FDA) Intrauterine Route of AdministrationC38273 INTRAVASCULAR Administration within a vessel or vessels. (FDA) Intravascular Route ofAdministrationC38276 INTRAVENOUS Administration within or into a vein or veins. (FDA) Intravenous Route of AdministrationC38274 INTRAVENOUS BOLUS Administration within or into a vein or veins all atonce. (FDA)C38279 INTRAVENOUS DRIP Administration within or into a vein or veins over asustained period of time. (FDA)Intravenous BolusIntravenous DripC38277 INTRAVENTRICULAR Administration within a ventricle. (FDA) Intraventricular Route ofAdministrationC38278 INTRAVESICAL Administration within the bladder. (FDA) Intravesical Route of AdministrationC38280 INTRAVITREAL Administration within the vitreous body of the eye.(FDA)C38203 IONTOPHORESIS Administration by means of an electric current whereions of soluble salts migrate into the tissues of thebody. (FDA)C38281 IRRIGATION Administration to bathe or flush open wounds or bodycavities. (FDA)Intravitreal Route of AdministrationIontophoresis Route ofAdministrationIrrigation-Route of AdministrationC38282 LARYNGEAL Administration directly upon the larynx. (FDA) Laryngeal Route of AdministrationC38284 NASAL Intranasal RouteofAdministrationAdministration to the nose; administered by way of thenose. (FDA)Nasal Route of AdministrationC38285 NASOGASTRIC Administration through the nose and into the stomach,usually by means of a tube. (FDA)Nasogastric Route of AdministrationC48623 NOT APPLICABLE Routes of administration are not applicable. (FDA) Route of Administration NotApplicableC38286OCCLUSIVE DRESSINGTECHNIQUEAdministration by the topical route which is thencovered by a dressing which occludes the area. (FDA)Occlusive Dressing TechniqueC38287 OPHTHALMIC Administration to the external eye. (FDA) Ophthalmic Route of AdministrationC38288 ORAL PO; IntraoralRoute ofAdministrationAdministration to or by way of the mouth. (FDA)Oral Route of AdministrationC78374 ORAL GAVAGE Administration through the mouth and into thestomach, usually by means of a tube. (NCI)Oral Gavage Route of AdministrationC64906 OROMUCOSAL Administration across the mucosa of the oral cavity. Oromucosal Route of AdministrationC38289 OROPHARYNGEAL Administration directly to the mouth and pharynx.(FDA)C38290 OTHER Administration is different from others on this list.(FDA)C38291 PARENTERAL Administration by injection, infusion, or implantation.(FDA)Oropharyngeal Route ofAdministrationOther Route of AdministrationParenteral Route of AdministrationC38676 PERCUTANEOUS Administration through the skin. (FDA) Percutaneous Route ofAdministrationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 321 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66729 - ROUTE - Route of AdministrationCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC38292 PERIARTICULAR Administration around a joint. (FDA) Periarticular Route ofAdministrationC38677 PERIDURAL Administration to the outside of the dura mater of thespinal cord. (FDA)Peridural Route of AdministrationC38293 PERINEURAL Administration surrounding a nerve or nerves. (FDA) Perineural Route of AdministrationC38294 PERIODONTAL Administration around a tooth. (FDA) Periodontal Route of AdministrationC38295 RECTAL Administration to the rectum. (FDA) Rectal Route of AdministrationC38216RESPIRATORY(INHALATION)Administration within the respiratory tract by inhalingorally or nasally for local or systemic effect. (FDA)Inhalation Route of AdministrationC38296 RETROBULBAR Administration behind the pons or behind the eyeball.(FDA)Retrobulbar Route of AdministrationC38198 SOFT TISSUE Administration into any soft tissue. (FDA) Soft Tissue Route Of AdministrationC38297 SUBARACHNOID Administration beneath the arachnoid. (FDA) Subarachnoid Route ofAdministrationC38298 SUBCONJUNCTIVAL Administration beneath the conjunctiva. (FDA) Subconjunctival Route ofAdministrationC38299 SUBCUTANEOUS SC Administration beneath the skin; hypodermic.Synonymous with the term SUBDERMAL. (FDA)Subcutaneous Route ofAdministrationC38300 SUBLINGUAL Administration beneath the tongue. (FDA) Sublingual Route of AdministrationC38301 SUBMUCOSAL Administration beneath the mucous membrane. (FDA) Submucosal Route of AdministrationC94636 SUBTENON Administration by injection through the membranecovering the muscles and nerves at the back of theeyeball.C38304 TOPICAL TOP Administration to a particular spot on the outer surfaceof the body. The E2B term TRANSMAMMARY is asubset of the term TOPICAL. (FDA)C38305 TRANSDERMAL Administration through the dermal layer of the skin tothe systemic circulation by diffusion. (FDA)Subtenon Route of AdministrationTopical Route of AdministrationTransdermal Route of AdministrationC38283 TRANSMUCOSAL Administration across the mucosa. (FDA) Mucosal Route of AdministrationC38307 TRANSPLACENTAL Administration through or across the placenta. (FDA) Transplacental Route ofAdministrationC38308 TRANSTRACHEAL Administration through the wall of the trachea. (FDA) Transtracheal Route ofAdministrationC38309 TRANSTYMPANIC Administration across or through the tympanic cavity.(FDA)C38310 UNASSIGNED Route of administration has not yet been assigned.(FDA)Transtympanic Route ofAdministrationUnassigned Route of AdministrationC38311 UNKNOWN Route of administration is unknown. (FDA) Unknown Route of AdministrationC383<strong>12</strong> URETERAL Administration into the ureter. (FDA) Ureteral Route of AdministrationC38271 URETHRAL Administration into the urethra. (FDA) Intraurethral Route of AdministrationC38313 VAGINAL Administration into the vagina. (FDA) Vaginal Route of AdministrationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 322 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89981 - SBCCDSND - <strong>SEND</strong> Subject Characteristics Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90353 ALTID AlternateIdentifierA secondary sequence of characters used to identify asubject.Alternate IdentifierC90383 FEEDREG Feeding Regimen A plan that specifies a diet, amount and schedule ofnutritional intake.Feeding RegimenC90392 HAIRCOLR Hair Coat Color The hue of a subject's hair or fur. (NCI) Hair Coat ColorC90435 PHYMARK Physical Marking A distinctive observable characteristic of an object.(NCI)Physical MarkingC90474 SPLRLOC Test SubjectSupplier SiteC90473 SPLRNAM Test SubjectSupplier; TestSubject SupplierNameThe geographic location of the organization thatsupplied the test subjects.The name of the organization that supplied the testsubjects.Test Subject Supplier SiteTest Subject SupplierC68551 USDANUM USDA Number Numeric ID assignment by United States Departmentof Agriculture for test facilities.USDA_IDSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 323 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89980 - SBCSND - <strong>SEND</strong> Subject Characteristics Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90353 Alternate Identifier AlternateIdentifierA secondary sequence of characters used to identify asubject.Alternate IdentifierC90383 Feeding Regimen Feeding Regimen A plan that specifies a diet, amount and schedule ofnutritional intake.Feeding RegimenC90392 Hair Coat Color Hair Coat Color The hue of a subject's hair or fur. (NCI) Hair Coat ColorC90435 Physical Marking Physical Marking A distinctive observable characteristic of an object.(NCI)Physical MarkingC90473 Test Subject Supplier Name Test SubjectSupplier; TestSubject SupplierNameC90474 Test Subject Supplier Site Test SubjectSupplier SiteThe name of the organization that supplied the testsubjects.The geographic location of the organization thatsupplied the test subjects.Test Subject SupplierTest Subject Supplier SiteC68551 USDA Number USDA Number Numeric ID assignment by United States Departmentof Agriculture for test facilities.USDA_IDSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 324 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90000 - SEV - SeverityCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC48662 MARKED The fourth level of severity in an ordered list based ona five-level scale of minimal, mild, moderate, marked,and severe.C70666 MILD Slight The second level of severity in an ordered list based ona five-level scale of minimal, mild, moderate, marked,and severe.C25570 MINIMAL Trace The first (lowest) level of severity in an ordered listbased on a five-level scale of minimal, mild, moderate,marked, and severe.C61376 MODERATE The third level of severity in an ordered list based on afive-level scale of minimal, mild, moderate, marked,and severe.C70667 SEVERE The fifth (highest) level of severity in an ordered listbased on a five-level scale of minimal, mild, moderate,marked, and severe.MarkedMildMinimumModerateSevereSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 325 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66731 - SEX - SexCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC16576 F Female A person who belongs to the sex that normallyproduces ova. The term is used to indicate biologicalsex distinctions, or cultural gender role distinctions,or both. (NCI)C20197 M Male A person who belongs to the sex that normallyproduces sperm. The term is used to indicatebiological sex distinctions, cultural gender roledistinctions, or both. (NCI)C17998 U U; Unknown Not known, not observed, not recorded, or refused.(NCI)C45908 UN Undifferentiated A person (one of unisexual specimens) who is bornwith genitalia and/or secondary sexual characteristicsof indeterminate sex, or which combine features ofboth sexes. (NCI)FemaleMaleUnknownIntersexSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 326 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66732 - SEXPOP - Sex of ParticipantsCodelist extensible: NoNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49636 BOTH One and the other; relating to or being two inconjunction. (NCI)C16576 F Female A person who belongs to the sex that normallyproduces ova. The term is used to indicate biologicalsex distinctions, or cultural gender role distinctions,or both. (NCI)C20197 M Male A person who belongs to the sex that normallyproduces sperm. The term is used to indicatebiological sex distinctions, cultural gender roledistinctions, or both. (NCI)BothFemaleMaleSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 327 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C89982 - SNDIGVER - <strong>SEND</strong> Implementation Guide VersionCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC96371<strong>SEND</strong> Implementation GuideVersion 3.0<strong>SEND</strong> IGVersion 3.0The 3.0 version of the standard for exchange ofnonclinical data (<strong>SEND</strong>) implementation guide. (NCI)Standard for the Exchange ofNonclinical Data ImplementationGuide Version 3.0Source: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 328 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77608 ABDOMINAL WALL The tissues that surround the organs that are presentwithin the abdominal cavity. The abdominal wall tissueis composed of layers of fat, parietal peritoneum,fascia, and muscles. (NCI)Abdominal WallC98702 ABOMASUM The glandular stomach of the ruminant. (NCI) AbomasumC<strong>12</strong>472 ADIPOSE TISSUE Adipose Tissue;Body Fat; FatTissueA specialized form of connective tissue consistingprimarily of adipocytes (fat cells), surrounded by ameshwork of collagen fibers. (NCI)Adipose TissueC32235 ADIPOSE TISSUE, BROWN BAT; Brown Fat Brown-colored adipose tissue that contains numeroussmall droplets of lipids and high numbers ofmitochondria as its primary function is to generatebody heat.C33889 ADIPOSE TISSUE, WHITE White Fat Adipose tissue that collects, stores and releases fat inthe form of triglycerides. It functions as a heatinsulator, mechanical cushion and as a source ofenergy. White adipose tissue is composed ofadipocytes that secrete leptin and other substances thateffect energy homeostasis. Its distribution in the bodyis dependent on the sex of the individual. (NCI)C32057 AIR SAC The terminal dilation of an alveolar duct that gives riseto alveoli in the lung. (NCI)Brown Adipose TissueWhite Adipose TissueAlveolar SacC90476 ALL TISSUES The total collection of tissues within a sample. (NCI) Total TissueC<strong>12</strong>440 AMYGDALOID BODY AmygdaloidNucleusAn almond-shaped group of basal nuclei anterior to theinferior horn of the lateral ventricle of the brain,within the temporal lobe. The amygdala is part of thelimbic system. (MESH)AmygdalaC<strong>12</strong>372 ARTERY Artery A blood vessel that carries blood away from the heart.(NCI)C<strong>12</strong>669 ARTERY, AORTA Aorta The major arterial trunk that carries oxygenated bloodfrom the left ventricle into the ascending aorta behindthe heart, the aortic arch, through the thorax as thedescending aorta and through the abdomen as theabdominal aorta; it bifurcates into the left and rightcommon iliac arteries. (NCI)C52849 ARTERY, AURICULAR An artery that supplies oxygenated blood to the ear.(NCI)C<strong>12</strong>681 ARTERY, BRACHIAL Brachial Artery An artery originating at the axillary artery andbranching into the radial and ulnar arteries. (NCI)C<strong>12</strong>687 ARTERY, CAROTID Carotid Artery One of two arteries that supply oxygenated blood tothe head and neck; the left common carotid branchesfrom the aorta and the right common carotid from theinnominate (brachiocephalic artery). (NCI)C<strong>12</strong>843 ARTERY, CORONARY Coronary Artery A principal artery that originates in the aorta. Itsupplies blood to the muscular tissue of the heart.(NCI)C<strong>12</strong>715 ARTERY, FEMORAL Femoral Artery An artery that starts within the inguinal region andextends to the lower extremities. (NCI)C<strong>12</strong>733 ARTERY, ILIAC A branch of the abdominal aorta that supplies blood tothe lower trunk and the legs. (NCI)ArteryAortaAuricular ArteryBrachial ArteryCarotid ArteryCoronary ArteryFemoral ArteryIliac ArterySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 329 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC52941 ARTERY, MAMMARY The blood vessel that supplies the breast and theanterior chest wall with arterial blood. It is located inthe chest wall. (NCI)C52975 ARTERY, MESENTERIC A branch of the abdominal aorta that supplies blood tothe intestines. (NCI)C<strong>12</strong>774 ARTERY, PULMONARY An artery arising from the right ventricle of the heartthat carries deoxygenated blood to the lungs. (NCI)C33581 ARTERY, SPERMATIC A branch of the abdominal aorta that suppliesoxygenated blood to the testis. (NCI)C33587 ARTERY, SPINAL A branch of the intercostal artery that suppliesoxygenated blood to the spinal cord and the pia mater.(NCI)C33643 ARTERY, SUBCLAVIAN An artery located below the clavicle that suppliesblood to the head and the arms. The right subclavianartery originates from the brachiocephalic artery andthe left subclavian artery originates from the aorticarch. (NCI)C13347 ASPIRATE Fluid withdrawn from a body cavity, cyst, or tumor.(NCI)C13192 BILE Fluid composed of waste products, bile acids, salts,cholesterol, and electrolytes. It is secreted by the liverparenchyma and stored in the gallbladder. (NCI)Internal Mammary ArteryMesenteric ArteryPulmonary ArterySpermatic ArterySpinal ArterySubclavian ArteryAspirateBileC70699 BIOSPECIMEN BiologicalSpecimen;BiologicalSample; SampleAny material sample taken from a biological entity fortesting, diagnostic, propagation, treatment or researchpurposes, including a sample obtained from a livingorganism or taken from the biological object afterhalting of all its life functions. Biospecimen cancontain one or more components including but notlimited to cellular molecules, cells, tissues, organs,body fluids, embryos, and body excretory products.(NCI)BiospecimenC25444 BODY CAVITY A natural hollow or sinus within the body. (NCI) Body CavityC<strong>12</strong>664BODY CAVITY,ABDOMINALAbdomen;AbdominalCavityThe portion of the body that lies between the thoraxand the pelvis. (NCI)Abdominal CavityC77638 BODY CAVITY, CRANIAL The atrium that is formed by the bones of the skull, andcontains the brain.Cranial CavityC92208BODY CAVITY,EXTRAPERITONEALExtraperitonealSpace;ExtraperitonealAreaThe space of the abdominal and pelvic cavities outsidethe peritoneum. (NCI)Extraperitoneal SpaceC<strong>12</strong>424 BODY CAVITY, NASAL Nasal Cavity The proximal portion of the respiratory passages oneither side of the nasal septum lying between the floorof the cranium and the roof of the mouth and extendingfrom the face to the pharynx. The nasal cavity is linedwith ciliated mucosa, extending from the nares to thepharynx. (NCI)Nasal CavitySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 330 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>421 BODY CAVITY, ORAL Oral Cavity The cavity located at the upper end of the alimentarycanal, behind the teeth and gums that is bounded on theoutside by the lips, above by the hard and soft palatesand below by the tongue. (NCI)Oral CavityC<strong>12</strong>347 BODY CAVITY, ORBITAL Eye Socket;Ocular Orbit;Orbital Cavity;OrbitC<strong>12</strong>767 BODY CAVITY, PELVIC Pelvic Cavity;Pelvis; PelvicRegionThe bony cavity of the skull which contains the eye,anterior portion of the optic nerve, ocular muscles andocular adnexa. Seven bones contribute to the structureof the orbit: the frontal, maxillary, zygomatic,sphenoid, lacrimal, ethmoid, and palatine bones. (NCI)The bony, basin-shaped structure formed by thehipbones and the base of the backbone supporting thelower limbs in humans. (NCI)OrbitPelvisC38662BODY CAVITY,PERICARDIALPericardialCavityThe potential body space formed between the parietaland visceral layers of the pericardial sac. (NCI)Pericardial CavityC<strong>12</strong>769BODY CAVITY,PERITONEALPeritoneal CavityThe lower part of the abdomen that contains theintestines (the last part of the digestive tract), thestomach, and the liver. It is bound by thin membranes.(NCI)Peritoneal CavityC<strong>12</strong>840 BODY CAVITY, PLEURAL Pleural Cavity The cavity lined by the pleura, located in the thorax andcontains heart and lungs. (NCI)C<strong>12</strong>905 BODY CAVITY, THORACIC Thoracic Cavity The cavity in the vertebrate body enclosed by the ribsbetween the diaphragm and the neck and containing thelungs and heart. (NCI)C13076 BONE The mineralized osseous tissue that gives rigidity tothe bones and forms its honeycomb-likethree-dimensional internal structure. (NCI)C<strong>12</strong>431 BONE MARROW The tissue occupying the spaces of bone. It consists ofblood vessel sinuses and a network of hematopoieticcells which give rise to the red cells, white cells, andmegakaryocytes. (NCI)C77609 BONE MARROW SMEAR A laboratory procedure in which cells from bonemarrow aspirate are spread thinly on a glass slide formicroscopic examination. The study of bone marrowsmears is routinely used for the investigation,diagnosis, and staging of hematopoietic disorders.Pleural CavityThoracic CavityBone TissueBone MarrowBone Marrow SmearC77686 BONE MARROW, FEMUR Bone Marrow,FemoralBone marrow in the femoral bone. (NCI)Bone Marrow, FemurC77687BONE MARROW,HUMERUSBone marrow in the humerus bone. (NCI)Bone Marrow, HumerusC77688 BONE MARROW, RIB Bone marrow in the rib. (NCI) Bone Marrow, RibC77689 BONE MARROW, SCAPULA Bone marrow in the scapula. (NCI) Bone Marrow, ScapulaC77690BONE MARROW,STERNUMBone Marrow,SternalBone marrow in the sternum. (NCI)Bone Marrow, SternumC77691 BONE MARROW, TIBIA Bone marrow in the tibia bone. (NCI) Bone Marrow, TibiaC77692BONE MARROW,VERTEBRUMBone Marrow,VertebralBone marrow in a vertebral bone. (NCI)Bone Marrow, VertebralC83002 BONE, CONDYLE A rounded bony projection at the end of the bone.(NCI)CondyleSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 331 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>717 BONE, FEMUR Bone, Femoral;FemurThe upper leg bone positioned between the pelvis andthe knee. (NCI)FemurC<strong>12</strong>718 BONE, FIBULA Fibula The small, lateral calf bone extending from the knee tothe ankle. (NCI)FibulaC<strong>12</strong>731 BONE, HUMERUS Bone, Humeral;HumerusThe upper arm bone between the shoulder and elbow.(NCI)HumerusC32765 BONE, ILIUM Ilium The broad, dorsal, upper, and widest of the threeprincipal bones composing either half of the pelvis.(NCI)IliumC<strong>12</strong>290 BONE, MANDIBLE InferiorMaxillary Bone;Bone,Mandibular; Jaw;MandibleThe lower jaw bone holding the lower teeth. (NCI)MandibleC26470 BONE, MAXILLA Maxilla The upper jawbone in vertebrates: it is fused to thecranium. (NCI)C33282 BONE, PATELLA Patella A small flat triangular bone in front of the knee thatarticulates with the femur and protects the knee joint.(NCI)MaxillaPatellaC33287 BONE, PELVIS Pelvic Bone;PelvisC<strong>12</strong>777 BONE, RADIUS Radius; RadiusBoneThe caudal portion of the trunk, bounded anteriorly andlaterally by the two hip bones incorporating the socketportion of the hip joint for each leg. Posteriorly it isbounded by the sacrum and coccyx. (NCI)The long bone that extends from the lateral aspect ofthe elbow to the medial side of the carpus.Pelvic BoneRadius BoneC<strong>12</strong>782 BONE, RIB Rib Any one of the paired bones, <strong>12</strong> on either side,extending from the thoracic vertebrae toward themedian line on the ventral aspect of the trunk. The longcurved bones which form the rib cage. Generally, ribs1 to 7 are connected to the sternum by their costalcartilages and are called true ribs, whereas ribs 8 to <strong>12</strong>are termed false ribs. (NCI)RibC<strong>12</strong>783 BONE, SCAPULA Scapula;Shoulder BladeC<strong>12</strong>789 BONE, SKULL Bone, Cranial;Cranium; SkullThe flat triangle-shaped bone that connects thehumerus with the clavicle in the back of the shoulder.(NCI)The bones that form the head, made up of the bones ofthe braincase and face. (NCI)ScapulaSkullC<strong>12</strong>793 BONE, STERNUM Sternum; Sterna The long, flat bone connecting with the cartilages ofthe first seven ribs and the clavicle. (NCI)SternumC<strong>12</strong>796 BONE, TARSUS Bone, Tarsal;Tarsus Bone;TarsusAny one of the seven bones forming the instep of thefoot. (NCI)Tarsal BoneC<strong>12</strong>800 BONE, TIBIA Tibia A bone located between the femur and the tarsus, beingpart of the lower leg. (NCI)C<strong>12</strong>809 BONE, ULNA Ulna Bone The long bone that extends from the elbow to thelateral side of the carpus.TibiaUlnaC33868 BONE, VERTEBRA Vertebral Bone;VertebraOne of the bones that make up the vertebral column.Vertebral BoneSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 332 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>439 BRAIN Brain; NervousSystem, BrainAn organ composed of grey and white mattercontaining billions of neurons that is the center forintelligence and reasoning. It is protected by the bonycranium. (NCI)BrainC<strong>12</strong>447 BRAIN, BASAL GANGLIA Basal Ganglia Clusters of neurons comprising the globus pallidus,putamen, caudate, nucleus accumbens, substantia nigraand subthalamic nucleus. They are involved with highlevel aspects of inhibitory motor activity incoordination with the excitation commands issuedfrom the cerebellum. (NCI)C<strong>12</strong>441 BRAIN, BRAIN STEM Brain Stem The part of the brain that connects the cerebralhemispheres with the spinal cord. It consists of themesencephalon, pons, and medulla oblongata. (NCI)Basal GangliaBrain StemC49137 BRAIN, CEREBELLUM Cerebellar;CerebellumC49136 BRAIN, CEREBRUM CerebralHemispheres;CerebrumThe cortical cell layer of the cerebellum. It consists ofthe granular layer, molecular layer, and Purkinje celllayer. (NCI)Cerebral tissue composed of cells with distinctivecharacteristics. The cerebral cortex contains six celllayers. (NCI)Cortical Cell Layer of theCerebellumCortical Cell Layer of the CerebralCortexC<strong>12</strong>694 BRAIN, CHOROID PLEXUS Choroid Plexus Blood vessels forming villous structures in the third,fourth, and lateral ventricles of the brain. (NCI)C40185 BRAIN, FOREBRAIN The largest part of the brain composed of the cerebralhemispheres, thalamus, hypothalamus, and the limbicsystem. (NCI)C<strong>12</strong>444 BRAIN, HIPPOCAMPUS Hippocampus A curved gray matter structure of the temporal lobelying on the floor of the lateral ventricle of the brain.(NCI)C<strong>12</strong>458 BRAIN, HYPOTHALAMUS Hypothalamus An important supervisory center in the brain, rich inganglia, nerve fibers, and synaptic connections. It iscomposed of several sections called nuclei, each ofwhich controls a specific function. The hypothalamusregulates body temperature, blood pressure, heartbeat,metabolism of fats and carbohydrates, and sugar levelsin the blood. Through direct attachment to the pituitarygland, the hypothalamus also meters secretionscontrolling water balance and milk production in thefemale. The role of the hypothalamus in awareness ofpleasure and pain is well established in the laboratory.It is involved in the expression of emotions, such asfear and rage, and in sexual behaviors.Choroid PlexusForebrainHippocampusHypothalamusC<strong>12</strong>442BRAIN, MEDULLAOBLONGATAMedullaOblongataThe lower portion of the brainstem located betweenthe pons and brainstem. This structure contains severaldescending and ascending tracts, lower cranial nervenuclei, a significant proportion of the reticular systemof the brainstem and other structures. (NCI)Medulla OblongataC<strong>12</strong>510 BRAIN, MIDBRAIN Mesencephalon;MidbrainThe uppermost portion of the brainstem locatedbetween the pons and the diencephalon. The midbraincontains the cerebral peduncles, oculomotor, trochlearand red nuclei, substantia nigra and various other nucleiand tracts.MesencephalonSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 333 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92592 BRAIN, OBEX The location of the brain that corresponds to thenarrowing of the fourth ventricle into the central canalof the spinal column. (NCI)C28401 BRAIN, OLFACTORY BULB Olfactory Bulb Anatomical structure located in the vertebral forebrainthat receives neural input regarding odors that havebeen detected by cells within the nasal cavity. Theaxons of olfactory receptor cells extend into theolfactory bulb. (NCI)C<strong>12</strong>511 BRAIN, PONS Pons Varolii The middle portion of the brainstem located betweenthe midbrain and the medulla oblongata.Obex RegionOlfactory BulbPons VaroliiC<strong>12</strong>453BRAIN, SUBSTANTIANIGRASubstantia NigraA large cell mass extending forward, over the dorsalsurface of the crus cerebri, from the rostral border ofthe pons into the subthalamic region. It is composed ofa dorsal stratum of closely spaced pigmented cells, thepars compacta, and a larger ventral region of widelyscattered cells, the pars reticulata. The pars compactaincludes numerous cells that project forward to thestriatum (caudate nucleus and putamen) and containdopamine, which acts as the primary neurotransmitterat the synaptic endings. Other, apparentlynon-dopaminergic cells project to portions of theventral nucleus of thalamus, the superior colliculus andreticular formation. The nigrostriatal projection isreciprocated by a striatonigral fiber system withmultiple neurotransmitters, chief among which isgamma-aminobutyric acid (GABA). The substantianigra is involved in the metabolic disturbancesassociated with Parkinson's disease and Huntington'sdisease. (NCI)Substantia NigraC<strong>12</strong>459 BRAIN, THALAMUS Thalamus An ovoid mass composed predominantly of graysubstance and associated laminae of white substance.The thalamus is divided into anterior, medial, andlateral parts. The function of the thalamus is to relaysensory impulses and cerebellar and basal gangliaprojections to the cerebral cortex. The thalamus ispositioned within the posterior part of thediencephalon forming most of each lateral wall of thethird ventricle. (NCI)ThalamusC<strong>12</strong>683 BRONCHUS Bronchi;BronchusTubular structure in continuation with the trachea,serving as air passage. It terminates in the lung(terminal bronchiole). (NCI)BronchusC32234BRONCHUS-ASSOCIATEDLYMPHOID TISSUEBALT Lymphoid tissue located with the bronchi. Bronchus-Associated LymphoidTissueC13010 BRUNNER'S GLAND Small, flat, granular glands embedded in thesubmucous areolar tissue of the duodenum. (MeSH)(NCI)C84507 BUFFY COAT The upper clear layer of fluid which is separated froma blood clot following density gradient centrifugation.It contains most of the white blood cells. (NCI)C25264 CARINA Carina, Tracheal A ridge or ridge-like structure. The carina of trachea ispart of the lowest tracheal cartilage which is placedbetween the orifices of the two bronchi.Brunner's GlandBuffy CoatCarinaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 334 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC66852 CAROTID BODY A cluster of cells that function as chemo-receptors,located at the bifurcation of the carotid artery. Its mainpurpose is to detect changes in the composition ofarterial blood. (NCI)C32268 CARTILAGE Cartilaginous A type of connective tissue composed of chondrocytesand an extracellular matrix, composed of collagen,elastin, and ground substance. There are three types ofcartilage; namely elastic, hyaline, and fibrocartilage.(NCI)Carotid BodyCartilaginous TissueC33732 CAUDA EPIDIDYMIS The lower part of the epididymis. (NCI) Tail of the EpididymisC<strong>12</strong>311 CERVIX Cervix Uteri;Uterine CervixThe lower part of the uterus occupying the regionbetween the isthmus of the uterus and the vagina. It isdivided into supravaginal and vaginal portions. (NCI)Cervix UteriC13070 CHEEK The fleshy part of the face bounded by the eyes, nose,ear, and jaw line. (NCI)C<strong>12</strong>308 CLITORIS The erectile tissue in the vulva. It is composed of thecorpora cavernosa and the glans clitoris.C34<strong>12</strong>7 CLOACA The singular posterior opening of the intestinal andurinary tracts of birds, reptiles, amphibians, marsupialsand monotremes. (NCI)C<strong>12</strong>837 COCHLEAR NUCLEI Located at the brainstem, this sensory organ receivesauditory signals from the cochlear auditory nervefibers. It is composed of the dorsal cochlear nucleuslocated on the dorsolateral surface of the inferiorpeduncle and the ventral (or accessory) cochlearnucleus located on the ventral aspect of the inferiorpeduncle. (NCI)C<strong>12</strong>341 CONJUNCTIVA Conjunctiva A thin, transparent tissue divided into the palpebralconjunctiva (covering the inner side of the eye lid) andthe bulbar conjunctiva (covering the eyeball). (NCI)C<strong>12</strong>374 CONNECTIVE TISSUE The supporting or framework tissue of the body,formed of fibrous and ground substance with more orless numerous cells of various kinds; it is derived fromthe mesenchyme, and this in turn from the mesoderm;the varieties of connective tissue are: areolar or loose;adipose; dense, regular or irregular, white fibrous;elastic; mucous; and lymphoid tissue; cartilage; andbone; the blood and lymph may be regarded asconnective tissues the ground substance of which is aliquid.C<strong>12</strong>446 CORPUS CALLOSUM A white matter structure within the cleft that separatesthe left and right cerebral hemispheres in themammalian brain. It is composed of a wide, flat bundleof 200-250 million axonal projections. (NCI)CheekClitorisCloacaCochlear NucleusConjunctivaConnective TissueCorpus CallosumC<strong>12</strong>316 CORPUS UTERI Uterine Body;Uterus, CorpusC32392 COSTOCHONDRAL JOINT CostochondralJunction;CostochondralJunction, RibThe Corpus uteri, or body of uterus, is the part of theuterus above the isthmus, comprising about two thirdsof the non-pregnant organ. (NCI)Hyaline cartilaginous joint between the ribs and costalcartilage. (NCI)Corpus UteriCostochondral JointSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 335 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>948 DUCT A tube that carries various secretions from one part ofthe body to another. (NCI)C43615 DUCT, BILE The tissue that forms the bile duct wall. It is lined byepithelium. (NCI)C32421 DUCT, CYSTIC A short duct attached to the gallbladder through whichthe bile from the gallbladder is secreted in thecommon bile duct. (NCI)C33161 DUCT, NASOLACRIMAL Duct, Nasolac A tube-like structure that conveys tears from thelacrimal sac to the nasal cavity. (NCI)C49783 DUCT, THYROGLOSSAL A tube-like structure present in the developing embryothat connects the area in which the thyroid glandinitially develops, the oropharynx, to the final positionof the thyroid gland in the newborn. This structurenormally atrophies prior to birth but may persist insome cases. (NCI)C<strong>12</strong>394 EAR Ear A sense organ needed for the detection of sound andfor establishing balance. The outer ear consists of theauricle, the ear canal as well as the tympanicmembrane. The middle ear is made up of an air-filledcavity behind the tympanic membrane that contains theossicles (malleus, incus and stapes). The inner ear ismade up of the cochlea needed for hearing and thevestibular apparatus required for balance. (NCI)C<strong>12</strong>395 EAR, COCHLEA The snail shell-shaped auditory component of the innerear. It contains the sensory organ of hearing. (NCI)C13164 EPICARDIUM The outer membranous connective tissue layer of theheart tissue. (NCI)C<strong>12</strong>328 EPIDIDYMIS A crescent-like structure located in the upper andposterior surfaces of the testis. It consists of theefferent ductules and the duct of the epididymis. Itfacilitates the maturation of sperm that is produced inthe testis. (NCI)DuctBile Duct TissueCystic DuctNasolacrimal DuctThyroglossal DuctEarCochleaEpicardiumEpididymisC32529 EPIPHYSIS Junction,Bone-Cartilage;Growth Plate;Epiphyseal PlateThe round end of the long bones. (NCI)Epiphysis of the BoneC32543 ESOPHAGUS The tissue of the esophageal wall. It is composed ofmucosa, a muscular coat, and a serosal surface. (NCI)Esophageal TissueC<strong>12</strong>500 EUSTACHIAN TUBE Pharyngotympanic Tube; AuditoryTube; TubaAuditoriaA tubular structure that runs from the middle ear to thenasopharynx and is approximately 3-4 cm length. Itslumen is roughly triangular and has average diameter of2-3 mm. The lumen is lined by ciliatedpseudostratified, columnar epithelium, which sweepsmaterial from the middle ear to the nasopharynx. It isfunctionally collapsed at rest, with slight negativepressure present in the middle ear, and opens duringswallowing, sneezing, and yawning. It serves toventilate pressure differences between the middle earand nasopharynx. This tube also allows middle earsecretions to drain into the nasopharynx.Eustachian TubeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 336 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC13233 EXUDATE Fluid, cells or cellular debris that escapes from theblood vessels and accumulates in nearby tissues. It isthe result of inflammation and is rich in proteins.ExudateC<strong>12</strong>401 EYE Eyeball; Eye The organ of sight or vision. (NCI) EyeC<strong>12</strong>667 EYE, ANTERIOR CHAMBER The space in the eye, filled with aqueous humor,bounded anteriorly by the cornea and a small portionof the sclera and posteriorly by a small portion of theciliary body, the iris, and that part of the crystallinelens which presents through the pupil. (Cline et al.,Dictionary of Visual Science, 4th ed, p109) (NCI)C<strong>12</strong>344 EYE, CHOROID A blood vessel-containing membrane of the eye thatlies between the retina and the sclera. (NCI)C<strong>12</strong>345 EYE, CILIARY BODY Tissue located behind the iris and composed of muscleand epithelium. Its functions include the production ofaqueous humor and changing the shape of thecrystalline lens. (NCI)C<strong>12</strong>342 EYE, CORNEA Cornea A dome-shaped, transparent, avascular tissue coveringthe front of the eye. It is composed of five layers:squamous epithelium, Bowman's membrane, stroma,Descemet's membrane, and endothelium. Refraction oflight contributing to eye's focusing ability is itscharacteristic function. It contains unmyelinated nerveendings which are responsible for the high sensitivityof the tissue. (NCI)C<strong>12</strong>737 EYE, IRIS The colored disc of the eye composed of connectivetissue, epithelium, and endothelium. It separates theanterior chamber from the posterior chamber. (NCI)Anterior Chamber of the EyeChoroidCiliary BodyCorneaIrisC<strong>12</strong>743 EYE, LENS Crystalline Lens;Lens Of Eye;Lens; OcularLensA biconvex transparent structure of the eye throughwhich light is focused on the retina. The lens sitsbehind the iris and is supported by the zonule, whichconnects it to the ciliary body. The lens is an avascularstructure. (NCI)LensC<strong>12</strong>900EYE, POSTERIORCHAMBEREye, PosteriorCompartmentA space within the eye located between the iris and thelens. It is filled with aqueous humor. (NCI)Posterior Chamber of the EyeC49328 EYE, RETINA The tissue that constitutes the retina. It is composed ofthe following layers: ganglion cell layer, inner limitingmembrane, inner nuclear layer, inner plexiform layer,layer of the ophthalmic nerve fibers, layer of the rodsand cones, neural retina, outer limiting membrane,outer nuclear layer, outer plexiform layer, and retinalpigment epithelium. (NCI)C<strong>12</strong>784 EYE, SCLERA The white, opaque, fibrous, outer tunic of the eyeball,covering it entirely excepting the segment coveredanteriorly by the cornea. It is essentially avascular butcontains apertures for vessels, lymphatics, and nerves.It receives the tendons of insertion of the extraocularmuscles and at the corneoscleral junction contains thecanal of Schlemm. (From Cline et al., Dictionary ofVisual Science, 4th ed)Retina LayerScleraSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 337 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC33884 EYE, VITREOUS A clear, avascular, gelatinous body that occupies theposterior chamber of the eye. It is bounded by theretina except anteriorly where it lies adjacent to thelens and iris.C<strong>12</strong>713 EYELID Eyelid; Palpebra A thin membrane of skin with the purpose of coveringand protecting an eye. (NCI)C13071 FACE Face; Facial The anterior portion of the head extending from theforehead to the chin and ear to ear. The facialstructures contain the eyes, nose and mouth, cheeksand jaws. (NCI)C13234 FECES Feces The material discharged from the bowel duringdefecation. It consists of undigested food, intestinalmucus, epithelial cells, and bacteria. (NCI)Vitreous BodyEyelidFaceFecesC17730 FETUS Any tissue from a fetus. (NCI) Fetal TissueC77611 FLUID, ABDOMINAL The fluid that is abnormally collected in the abdominalcavity as a result of vascular pressure, liver disorder,or malignancy. (NCI)C13188 FLUID, AMNIOTIC Aqua Amnii The fluid within the amniotic cavity which surroundsand protects the developing embryo. (NCI)Abdominal FluidAmniotic FluidC13195FLUID,BRONCHOALVEOLARLAVAGEBronchial LavageFluid; Fluid,Bronchial LavageFluid introduced into, and collected from, the lungs bya bronchoalveolar lavage procedure. (NCI)Bronchoalveolar Lavage FluidC<strong>12</strong>692 FLUID, CEREBROSPINAL CSF The fluid that is contained within the brain ventricles,the subarachnoid space and the central canal of thespinal cord. (NCI)C3319 FLUID, PERICARDIAL Fluid collection within the pericardial sac, usually dueto inflammation. (NCI)C776<strong>12</strong> FLUID, PERITONEAL Fluid, Ascites The small amount of fluid that is generated in theabdominal cavity to lubricate the peritoneum. (NCI)Cerebrospinal FluidPericardial EffusionPeritoneal FluidC33718 FLUID, SYNOVIAL Synovia; SynovialFluidA viscid fluid secreted by the synovial membrane,serving as a lubricant. (NCI)Synovial FluidC77613 FLUID, THORACIC The fluid that is abnormally collected in the pleuralcavity. (NCI)C32622 FOOT Feet; Foot The structure found below the ankle joint required forlocomotion. (NCI)C77671 FOOT/FOOTPAD A laboratory specimen consisting of the foot andfootpad. (NCI)C92654 FOOTPAD A thick, spongy layer of tissue located under themetacarpal and metatarsal joints of the foot. It consistsof a pad of adipose tissue covered by a thick epidermiscontaining dermal sweat glands.C<strong>12</strong>671 FORELIMB The region of the body that includes the arm, theforearm, and hand. (NCI)C77956 FORESTOMACH Non-glandular portion of the stomach of somemammals that is connected to the esophagus. It isutilized as a holding chamber during digestion forun-digested and partially digested food. (NCI)Pleural FluidFootFoot/FootpadFootpadUpper ExtremityForestomachSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 338 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>377 GALLBLADDER Gallbladder A pear-shaped organ located under the liver that storesand concentrates bile secreted by the liver. From thegallbladder the bile is delivered through the bile ductsinto the intestine thereby aiding the digestion offat-containing foods. (NCI)GallbladderC<strong>12</strong>719 GANGLION Ganglia;Ganglion; NeuralGanglionA cluster of nervous tissue principally composed ofneuronal cell bodies external to the central nervoussystem (CNS). (NCI)GanglionC98713 GANGLION, CERVICAL Any one of the three sympathetic ganglia of thecervical vertebrae. (NCI)C<strong>12</strong>462 GANGLION, DORSAL ROOT Dorsal Root Sensory ganglia located on the dorsal spinal rootswithin the vertebral column. (MeSH)Cervical GangliaDorsal Root GanglionC62642 GANGLION, TRIGEMINAL GasserianGanglionLarge sensory ganglion of the trigeminal nerve thatlies adjacent to the cavernous sinus in the trigeminalcavity of the dura mater. (NCI)Trigeminal GanglionC92214GANGLION,TRIGEMINAL/NERVE,TRIGEMINALA tissue sample that contains the trigeminal ganglionand branches of the trigeminal nerve. (NCI)Trigeminal Ganglion/TrigeminalNerveC77614 GASTRIC CONTENTS Ingesta;DigestiveContentsThe contents of the stomach that may includeundigested food mixed with juices secreted by thegastric mucosal glands. (NCI)Gastric ContentC92593 GILLS A respiratory organ found in aquatic animals thatallows for the exchange of dissolved oxygen fromwater into the blood stream. (NCI)C32677 GINGIVA Gingiva; Gum The soft tissue surrounding the neck of individual teethas well as covering the alveolar bone. The tissue isfibrous and continuous with the periodontal ligamentand mucosal covering. (NCI)C77615 GIZZARD The thick-walled secondary stomach of birds used togrind the food prior to digestion. (NCI)GillGingivaGizzardC77616GLAND OF THE THIRDEYELIDNictitans GlandA gland producing tears in a third eyelid in the cornerof each eye, as seen in animals. (NCI)Gland of the Third EyelidC74586GLAND, ACCESSORYOCULARSerous gland with distended lumen. It includes thegland of Krause in the lacrimal sac and the gland ofWolfring on the inner surface of the upper eyelid.(NCI)Accessory Lacrimal GlandC32051 GLAND, ADRENAL The tissue that forms the adrenal gland. It consists ofthe outer adrenal cortex and the inner adrenal medulla.(NCI)C77955 GLAND, AMPULLARY Paired exocrine glands of the male reproductivesystem located at the medial termination of the ductusdeferens that produce and secrete lipids and glycogen,components of the seminal fluid. (NCI)Adrenal Gland TissueAmpullary GlandC32395 GLAND, BULBOURETHRAL Cowper's Gland;Gland, Cowper'sPaired exocrine glands located at the base of the penis,positioned posterior and lateral to the urethra, thatproduce and secrete a clear viscous liquid thatlubricates the urethra in preparation for the passage ofsperm. (NCI)Cowper GlandSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 339 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77610 GLAND, CIRCUMANAL A large apocrine gland located in the area surroundingthe anal orifice. (NCI)C77617 GLAND, CLITORAL A female reproductive gland located on the side of theclitoris. (NCI)C77618 GLAND, COAGULATING The anterior lobe of the prostate gland in animals.(NCI)C<strong>12</strong>313 GLAND, ENDOMETRIAL The endometrium, or tunica mucosa, is the mucousmembrane comprising the inner layer of the uterinewall; it consists of a simple columnar epithelium and alamina propria that contains simple tubular uterineglands. The structure, thickness, and state of theendometrium undergo marked change with themenstrual cycle.Circumanal GlandClitoral GlandCoagulating GlandEndometriumC77619 GLAND, HARDERIAN An accessory gland of the orbit. (NCI) Harderian GlandC<strong>12</strong>346 GLAND, LACRIMAL Paired, almond-shaped exocrine glands situatedsuperior and posterior to each orbit of the eye thatproduce and secrete the watery serous component oftears. (NCI)C<strong>12</strong>370 GLAND, MAMMARY The glandular tissue of the breast. In females, it iscomposed of numerous lobules with alveolar ducts andalveoli. In males, it is composed of rudimentaryglands.C33075 GLAND, MEIBOMIAN A sebaceous gland in the eyelid that produces aspecific type of sebum. (NCI)C33270 GLAND, PARATHYROID Parathyroid gland tissue consists of three primary celltypes - chief cells, oxyphil cells and clear cells. Theseglands are usually located in close proximity to thethyroid gland. (NCI)Lacrimal GlandMammary Gland TissueMeibomian GlandParathyroid Gland TissueC77620 GLAND, PERIANAL A gland located in the area around the anus. (NCI) Perianal GlandC77621 GLAND, PERINEAL A gland near the anus producing an odorous secretion.(NCI)C41834 GLAND, PINEAL Pineal Body The tissue of the pineal gland. The pineal gland is asmall reddish-gray body, about 8 mm. in length whichlies in the depression between the superior colliculi. Itis attached to the roof of the third ventricle near itsjunction with the mid-brain. It develops as anoutgrowth from the third ventricle of the brain. Thepineal parenchyma consists of follicles lined byepithelium and enveloped by connective tissues. Itproduces and secretes melatonin. (NCI)Perineal GlandPineal ParenchymaC<strong>12</strong>399 GLAND, PITUITARY Hypophysis;HypophysisCerebriPea-sized endocrine gland located at the base of thebrain in the pituitary fossa. It produces and secreteshormones such as oxytocin and vasopressin, toregulate the activities of the hypothalamus. (NCI)Pituitary GlandC79432 GLAND, PREPUTIAL Exocrine glands located at the anterior aspect of thegenitals of some mammals, such as mice and rats, thatproduce and secrete pheromones. (NCI)Preputial GlandSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 340 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC33414 GLAND, PROSTATE Tissue composed of tubuloalveolar glands embeddedin fibromuscular stroma. The stroma is smooth muscleseparated by strands of connective tissue rich incollagenous and elastic fibers. The secretory alveoli ofthe prostate are irregularly shaped with papillaryprojections of the mucosa into the lumen of the gland.(NCI)Prostatic TissueC77622GLAND, PROSTATEDORSOLATERALThe dorsolateral lobe of the prostate gland in animals.(NCI)Dorsolateral Prostate GlandC77623GLAND, PROSTATEVENTRALThe ventral lobe of the prostate gland in animals. (NCI)Ventral Prostate GlandC77670GLAND,PROSTATE/GLAND,SEMINAL VESICLEA laboratory specimen consisting of the prostate andseminal vesicles. (NCI)Prostate/Seminal VesiclesC33509 GLAND, SALIVARY Tissue consisting of mucous or serous secretingepithelial tissue, connective tissue, blood vessels,nerves and lymphatic tissue. (NCI)Salivary Gland TissueC33141GLAND, SALIVARY,MUCOUSSalivary glands that produce and secrete a saliva madeup exclusively of mucous. (NCI)Mucous Salivary GlandC<strong>12</strong>427GLAND, SALIVARY,PAROTIDParotid GlandThe largest of the three paired salivary glands, locatedin front of the ear. (NCI)Parotid GlandC33539GLAND, SALIVARY,SEROUSSalivary glands that produce and secrete a saliva madeup exclusively of a pale-yellow transparent fluidcontaining amylase. (NCI)Serous Salivary GlandC<strong>12</strong>234GLAND, SALIVARY,SUBLINGUALA salivary gland located under the tongue in the floorof the oral cavity. (NCI)Sublingual Salivary GlandC<strong>12</strong>233GLAND, SALIVARY,SUBMANDIBULARGland, Salivary,Mandibular;SubmaxillaryGlandOne of two salivary glands in the neck, located in thespace bound by the two bellies of the digastric muscleand the angle of the mandible. It discharges through thesubmandibular duct. (MeSH) (NCI)Submandibular GlandC92215GLAND, SALIVARY,SUBMANDIBULAR/GLAND,SALIVARY, SUBLINGUALA tissue sample that contains the submandibular andsublingual salivary glands. (NCI)Submandibular Gland/SublingualGlandC77624GLAND, SALIVARY,ZYGOMATICA salivary gland located in the upper portion of themouth. (NCI)Zygomatic GlandC33519 GLAND, SEBACEOUS Small glands located within the epidermis, andassociated with the hair follicle, that produce andsecrete an oily substance that lubricates the skin andhair. (NCI)C<strong>12</strong>787 GLAND, SEMINAL VESICLE Seminal Sacs One of the two paired glands in the male genitourinarysystem, posterior to the bladder and superior to theprostate gland, that produces fructose-rich seminalfluid which is a component of semen. These glandsjoin the ipsilateral ductus deferens to form theejaculatory duct. (NCI)Sebaceous GlandSeminal VesicleC92216GLAND, SEMINALVESICLE/GLAND,COAGULATINGA tissue sample that contains a seminal vesicle andcoagulating gland. (NCI)Seminal Vesicle/Coagulating GlandSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 341 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC33785 GLAND, THYROID The tissue that forms the thyroid gland. Its maincomponent is the thyroid follicle. (NCI)Thyroid Gland TissueC77667GLAND, THYROID/GLAND,PARATHYROIDA laboratory specimen consisting of the thyroid andparathyroid. (NCI)Thyroid/ParathyroidC49311 GLAND, URETHRAL Gland Of Littre Any of the glands located at the wall of the urethra ofmale mammals that produce and secrete mucus, amajor component of semen. (NCI)C77954 GLAND, ZYMBAL A sebaceous gland located at the base of the rodentexternal ear that produces and secretes oil, whichlubricates the auditory canal. (NCI)Gland of LittreZymbal GlandC<strong>12</strong>725 GONAD The gamete-producing tissues, ovary or testis. (MeSH) GonadC77639 GRAVID UTERUS The uterus during pregnancy. (NCI) Gravid UterusC3824 GROSS LESION A localized pathological or traumatic structuralchange, damage, deformity, or discontinuity of tissue,organ, or body part. (NCI)LesionC<strong>12</strong>936GUT-ASSOCIATEDLYMPHOID TISSUENon-encapsulated accumulations of lymphoid tissue inthe alimentary tract that form a secretory immunesystem containing cells committed to IgA or IgEsynthesis; It includes Peyer's patches and the lymphoidtissue which is present throughout the gastrointestinalmucosa. (NCI)Gut-Associated Lymphoid TissueC32705 HAIR Hair The filamentous outgrowth of the epidermis. (NCI) HairC327<strong>12</strong> HAND Hand The distal portion of the upper extremity. It consists ofthe carpus, metacarpus, and digits. (NCI)C<strong>12</strong>419 HEAD Head The anterior and superior part of a human or animalbearing the mouth, the brain and sensory organs.C<strong>12</strong>727 HEART Cardiac; Heart A hollow muscular organ which receives the bloodfrom the veins and propels it into the arteries. It isdivided by a musculomembranous septum into twohalves -- right or venous and left or arterial -- each ofwhich consists of a receiving chamber (atrium) and anejecting chamber (ventricle). (NCI)HandHeadHeartC41168HEMOLYMPHORETICULARTISSUEHematopoieticAnd LymphoidTissueThe tissue that contains hematopoietic cells and/orlymphocytes and immune system cells.Hematopoietic and Lymphoid TissueC77625 HINDLIMB The posterior limb of an animal. (NCI) Hind LimbC77626 HOOF WALL The keratinized, outer portion of the foot of a ungulatemammal.Hoof WallC<strong>12</strong>499 INNER EAR Cochlea; InternalEar; LabyrinthThe portion of the ear located within the temporalbone that is involved in both hearing and balance andincludes the semicircular canals, vestibule, andcochlea. (from American Heritage Dictionary online)(NCI)Internal EarC32874INTERVENTRICULARSEPTUMThe wall that separates the left and right ventricles ofthe heart. (NCI)Interventricular SeptumC49571 INTERVERTEBRAL DISC Spongy discs located between the vertebrae of thespinal column; composed of the outer annulus fibrosusand inner nucleus pulposus. (NCI)Intervertebral DiscSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 342 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49478 INTESTINE The tissue that forms the wall of the small and largeintestine. It consists of mucosa, submucosa, muscularcoat, and serosal surface. (NCI)Intestinal Wall TissueC13044 JOINT Articulation;JointThe connection point between two bones or skeletalelements. The joint may be fixed or movable. (NCI)JointC32264 JOINT, CARPAL A joint formed between carpal bones. (NCI) Carpal JointC32742 JOINT, HIP CoxofemoralJoint; Hip JointC32898 JOINT, KNEE Knee Joint;TibiofemoralJointA ball-and-socket joint between the head of the femurand the acetabulum. (NCI)A joint connecting the lower part of the femur with theupper part of the tibia. The lower part of the femur andthe upper part of the tibia are attached to each other byligaments. Other structures of the knee joint includethe upper part of the fibula, located below and parallelto the tibia, and the patella which moves as the kneebends. (NCI)Hip JointKnee JointC98784 JOINT, STIFLE Stifle Joint A synovial joint located in the hind limb of quadrupeds,consisting of the femorotibial articulation,femoropatellar articulation and the proximaltibiofibular articulation. (NCI)Stifle JointC33735 JOINT, TARSUS A joint formed by the union of tarsal bones. Tarsal JointC33461 KIDNEY Tissue composed of the renal cortex and the renalmedulla. The basic functional unit of renal tissue is thenephron. (NCI)C<strong>12</strong>227 LABIAL JUNCTION The junction of the upper and lower lips at the cornerof the mouth. (NCI)C<strong>12</strong>306 LABIUM MAJUS One of the two longitudinal folds of skin that form thelateral boundary of the vulva. It extends from the monspubis to the perineum. (NCI)C<strong>12</strong>307 LABIUM MINUS One of the two longitudinal folds of skin locatedbetween the labia majora. (NCI)Renal TissueCommissure of the LipLabium MajusLabium MinusC92439 LARGE COLON The ascending colon of the horse. (NCI) Large ColonC32931 LARGE INTESTINE Large Bowel The tissue of the large intestine. It is composed of fourlayers - mucosa, submucosa, smooth muscle with innercircular and outer longitudinal layers, and serosa. Themucosa has a large number of goblet cells but does nothave any villi. The longitudinal muscle layer isincomplete. The longitudinal muscle is limited to threedistinct bands, called teniae coli that run the entirelength of the colon. Contraction of the teniae coliexerts pressure on the wall and creates a series ofpouches, called haustra, along the colon. Epiploicappendages, pieces of fat-filled connective tissue, areattached to the outer surface of the colon. (NCI)C43362 LARGE INTESTINE, ANUS Anus The lower opening of the digestive tract, lying in thecleft between the buttocks, through which fecal matteris extruded. (NCI)Large Intestinal Wall TissueAnusC<strong>12</strong>380LARGE INTESTINE,APPENDIXAppendixSmall tissue projection existing as a cecaldiverticulum with a questionable history of vestigialversus specialized organ. (NCI)AppendixSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 343 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>381 LARGE INTESTINE, CECUM Cecal; Cecum A blind pouch-like commencement of the colon in theright lower quadrant of the abdomen at the end of thesmall intestine and the start of the large intestine.(OMD)C<strong>12</strong>382 LARGE INTESTINE, COLON Colon The part of the large intestine measured from thececum to the rectum consisting of ascending,transverse, descending and sigmoid portions. Thepurpose of the colon is to remove water from digestedfood prior to excretion. (NCI)CecumColonC<strong>12</strong>390LARGE INTESTINE,RECTUMThe terminal portion of the gastrointestinal tract,extending from the rectosigmoid junction to the analcanal. (NCI)RectumC92217LARGE INTESTINE,RECTUM/LARGEINTESTINE, ANUSA tissue sample that contains the rectum and anus.(NCI)Rectum/AnusC<strong>12</strong>420 LARYNX Larynx The cartilaginous structure of the respiratory tractbetween the pharynx and the trachea. It contains elasticvocal cords required for sound production. (NCI)C13046 LIGAMENT Ligament Band of fibrous tissue connecting bone to bone orcartilage to bone thereby supporting or strengthening ajoint. (NCI)LarynxLigamentC<strong>12</strong>429 LIMB Extremities;LimbA body region referring to an upper or lowerextremity. (NCI)LimbC<strong>12</strong>220 LIP Lip Fleshy fold which surrounds the opening of the mouth.(NCI)C32735 LIVER The tissue of the liver. It includes the hepatic lobules,hepatic sinusoids, perisinusoidal spaces, and portaltriad. The hepatic lobules are composed ofhepatocytes. (NCI)C77669 LIVER/GALLBLADDER A laboratory specimen consisting of the liver andgallbladder. (NCI)C33024 LUNG Tissue consisting of an external serous coat, subserousareolar tissue and lung parenchyma. The parenchyma ismade up of lobules wound together by connectivetissue. A primary lobule consists of a terminalbronchiole, respiratory bronchioles, and alveolarducts, which communicate with many alveoli, eachalveolus being surrounded by a network of capillaryblood vessels. (NCI)C92218 LUNG/BRONCHUS A sample that contains lung and bronchial tissues.(NCI)C33034 LYMPH NODE Lymph Gland Tissue composed of specialized cells that areorganized as a definite organ, situated along the courseof lymphatic vessels, and consisting of an outercortical and an inner medullary part. The lymph nodesare the main source of lymphocytes of the peripheralblood and serve as a defense mechanism by removingnoxious agents, such as bacteria and toxins, andprobably play a role in antibody production. (NCI)LipHepatic TissueLiver/GallbladderLung TissueLung/BronchusLymph Node TissueSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 344 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>904 LYMPH NODE, AXILLARY Axillary LymphNodeOne of approximately 20-30 lymph nodes in chainformation that traverse the concavity of the underarmto the clavicle. (NCI)Axillary Lymph NodeC92221 LYMPH NODE, BRACHIAL An axillary lymph node along the brachial vein. (NCI) Brachial Lymph NodeC32232LYMPH NODE,BRONCHIALLymph node located in the bronchi. (NCI)Bronchial Lymph NodeC32298 LYMPH NODE, CERVICAL Cervical LymphNodeAny of the lymph nodes located in the neck. (NCI)Cervical Lymph NodeC92222 LYMPH NODE, GASTRIC A lymph node along the left gastric artery or greateromentum. (NCI)C77640 LYMPH NODE, HEPATIC Any of the lymph nodes adjacent to the stomach andduodenum. (NCI)Gastric Lymph NodeHepatic Lymph NodeC77653LYMPH NODE,ILEOCECOCOLICA lymph node at the ileocecocolic junction. (NCI)Ileocecocolic Lymph NodeC32761 LYMPH NODE, ILIAC Iliac LymphNodeC32801 LYMPH NODE, INGUINAL Inguinal LymphNodeOne of the three lymph nodes of the pelvis: thesuperior gluteal, interior gluteal or sacral. (NCI)A superficial or deep lymph node located in theinguinal area. (NCI)Iliac Lymph NodeInguinal Lymph NodeC77652LYMPH NODE,INTERCOSTALA lymph node located in the intercostal space of thethoracic wall. (NCI)Intercostal Lymph NodeC77643 LYMPH NODE, LUMBAR Lymph Node,Para-AorticA lymph node located adjacent to the lumbar region ofthe spine. (NCI)Paraaortic Lymph NodeC32853LYMPH NODE, MAMMARYGLANDAny of the lymph nodes of the breast located under theribcage, near the sternum. (NCI)Internal Mammary Lymph NodeC33073LYMPH NODE,MEDIASTINALMediastinalLymph NodeA lymph node located in the mediastinum. Mediastinallymph nodes are arranged in three groups, one on thelateral, another on the medial, and a third on theanterior aspect of the vessels; the third group is,however, sometimes absent. (NCI)Mediastinal Lymph NodeC77641LYMPH NODE,MESENTERICA lymph node located in the mesentery. (NCI)Mesenteric Lymph NodeC77642LYMPH NODE,PANCREATICA lymph node of the pancreas. (NCI)Pancreatic Lymph NodeC33278 LYMPH NODE, PAROTID Parotid GlandLymph NodeA lymph node located close to, on, or within theparotid gland. (NCI)Parotid Gland Lymph NodeC77644LYMPH NODE,PERITONEALA lymph node located in the peritoneum. (NCI)Peritoneal Lymph NodeC53146 LYMPH NODE, POPLITEAL Lymph node located within the fat layer of the kneejoint. (NCI)Popliteal Lymph NodeC77645 LYMPH NODE, PORTAL Lymph nodes surrounding the portal vein. (NCI) Portal Lymph NodeC77654LYMPH NODE,PRESCAPULARA lymph node located in front of the shoulder. (NCI)Prescapular Lymph NodeC49018 LYMPH NODE, REGIONAL Regional LymphNodeA lymph node that drains lymph from a region ofinterest. (NCI)Regional Lymph NodeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 345 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77646 LYMPH NODE, RENAL A lymph node located in the hilar area of the kidney orthe fat surrounding the kidney. (NCI)Renal Lymph NodeC77649LYMPH NODE,RETROPHARYNGEALA lymph node located in the retropharyngeal spacebehind the upper part of the pharynx. (NCI)Retropharyngeal Lymph NodeC77648LYMPH NODE,RETROSACRALA lymph node located in the retrosacral region. (NCI)Retrosacral Lymph NodeC77647 LYMPH NODE, SACRAL A lymph node located in the sacral region. (NCI) Sacral Lymph NodeC92594 LYMPH NODE, SUBILIAC A lymph node located below the ilium. (NCI) Subiliac Lymph NodeC92434LYMPH NODE,SUBLINGUALA lymph node located under the tongue in the floor ofthe mouth. (NCI)Sublingual Lymph NodeC77655LYMPH NODE,SUBLUMBARA lymph node located under the spine. (NCI)Sublumbar Lymph NodeC77650LYMPH NODE,SUBMANDIBULARLymph Node,MandibularA lymph node located beneath the floor of the oralcavity. (NCI)Submandibular Lymph NodeC77656LYMPH NODE,SUPRAPHARYNGEALA lymph node located in the roof of the pharynx. (NCI)Suprapharyngeal Lymph NodeC33769 LYMPH NODE, THORACIC Lymph node located in the thoracic cavity. (NCI) Thoracic Lymph NodeC77651LYMPH NODE,TRACHEOBRONCHIALA lymph node located near the bifurcation of thetrachea. (NCI)Tracheobronchial Lymph NodeC34808 MASS A benign or malignant pathologic structure in any partof the body resulting from cystic changes oraccumulation of inflammatory or neoplastic cells.C<strong>12</strong>748 MEDIASTINUM Mediastinum A group of organs surrounded by loose connectivetissue, separating the two pleural sacs, between thesternum anteriorly and the vertebral columnposteriorly as well as from the thoracic inletsuperiorly to the diaphragm inferiorly. Themediastinum contains the heart and pericardium, thebases of the great vessels, the trachea and bronchi,esophagus, thymus, lymph nodes, thoracic duct,phrenic and vagus nerves, and other structures andtissues. (NCI)C77657 MEMBRANE, NICTITATING A translucent membrane present in the eye of someanimals, also called the third eyelid.C<strong>12</strong>348 MENINGES Any one of three membranes that surround the brainand spinal cord. (NCI)C33096 MENISCUS A crescent-shaped wedge of cartilaginous material thatserves as a cushion in the knee, between thetuberosities of the femur and the tibia. There are twolocations, the medial meniscus located on the inneraspect of the knee and the lateral which is located onthe outer aspect. (NCI)C33097 MENISCUS, LATERAL A semicircular meniscus located towards the outeraspect of the knee joint. (NCI)C33098 MENISCUS, MEDIAL A semicircular meniscus located towards the inneraspect of the knee joint. (NCI)MassMediastinumNictitating MembraneMeningesMeniscusMeniscus LateralisMeniscus MedialisSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 346 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC33103 MESENTERY A double layer of peritoneum that attaches to the backwall of the abdominal cavity and supports the smallintestines. (NCI)C92435 MESENTERY/PERITONEUM A tissue sample that contains mesenteric fat andperitoneal tissue. (NCI)C92440 MESOVARIAN LIGAMENTS The peritoneal fold that covers and attaches the ovaryto the broad ligament. (NCI)C<strong>12</strong>274 MIDDLE EAR Middle Ear The part of the ear including the eardrum and ossicles.The middle ear leads to the inner ear. (NCI)C13257 MILK Milk produced by females for the purpose of feedingtheir young. (NCI)C77658 MILK SERUM The fluid that remains after removing the fat and caseinfrom the milk. (NCI)C<strong>12</strong>505 MUCOSA, BUCCAL The mucosal membranes located on the inside of thecheek, in the buccal cavity. (NCI)MesenteryMesentery/PeritoneumMesovariumMiddle EarBreast MilkMilk SerumBuccal MucosaC33205 MUCOSA, NASAL OlfactoryMucosaThe part of the nasal mucosa composed ofneuroepithelial tissue and mucus-producing Bowman'sglands. The mucus moistens the epithelium and helpsdissolve odor-containing gases. (NCI)Olfactory MucosaC77637 MUCOSA, ORAL Moist tissue lining the oral cavity, containing mucussecreting glands.C13259 MUCUS The thick fluid secreted by the mucus glands in theaerodigestive tract and the vagina. (NCI)C53039 MUSCLE, ADDUCTOR A group of muscles causing movement of the legtowards the midline of the body. (NCI)C32200 MUSCLE, BICEPS BRACHII Biceps A muscle in the upper limb. Its action involves therotation of the limb and the flexion of thehumeralradial joint.C53147 MUSCLE, BICEPS FEMORIS A muscle in the back of the thigh. Its action involvesthe knee flexion and hip extension. (NCI)C<strong>12</strong>702 MUSCLE, DIAPHRAGM Fibromuscular tissue that separates the thoracic fromthe abdominal cavity. It increases the volume of thethoracic cavity through contractions, thus facilitatingrespiration.C33199 MUSCLE, EXTRAOCULAR A group of six muscles that are responsible for movingthe eye. (NCI)Oral MucosaMucusAdductor Group of the LegBicepsBiceps FemorisDiaphragmOculomotor MuscleC32666MUSCLE,GASTROCNEMIUSA large muscle in the back of the lower limb. Its actioninvolves the flexion of the tarsis.Gastrocnemius MuscleC78205 MUSCLE, GLUTEUS Any of the skeletal muscles that form the buttocks.These muscles are responsible for hip abduction andextension as well as movement of the thigh. (NCI)Gluteal MuscleC32824 MUSCLE, INTERCOSTAL A muscle located between two ribs. (NCI) Intercostal MuscleC33150 MUSCLE, LATISSIMUS MusculusLatissimus DorsiA triangular muscle in the back, connected to thevertebral column, arm, and shoulder. Its action involvesadduction, medial rotation, and extension of the arm.(NCI)Musculus Latissimus DorsiSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 347 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC13074 MUSCLE, MASSETER Muscles arising in the zygomatic arch that close thejaw. (MeSH) (NCI)C33286 MUSCLE, PECTORALIS Muscle, Pectoral Muscles of the upper chest. The term may refer to oneof two muscles, the pectoralis major and pectoralisminor. The former is a thick muscle in the anteriorportion of the chest. Its action involves flexion, medialrotation, and adduction of the humerus. The latter is athin muscle located beneath the pectoralis major. Itsaction involves lowering the scapula and raising theribs. (NCI)C33422 MUSCLE, PSOAS Muscles of the lower back whose actions involve theflexion of the hips and lumbar spine. The term mayrefer to psoas major muscle and psoas minor muscle,both located in the lower back. (NCI)Masseter MusclePectoralis MusclePsoas MuscleC33441MUSCLE, QUADRICEPSFEMORISMuscle,Quadriceps;Muscle, ThighA group of four powerful muscles in the front of thethigh. Their actions involve the extension of the kneejoint. (NCI)Quadriceps Muscle of the ThighC53175MUSCLE, RECTUSFEMORISOne of the four quadriceps muscles, located in themiddle of the front of the thigh. Its actions involve theextension of the knee joint and flexion of the hip.(NCI)Rectus FemorisC53176MUSCLE,SEMITENDINOSUSA muscle located at the posterior and medial aspect ofthe thigh that extends the hip joint, flexes the kneejoint and assists in medially rotating the knee.SemitendinosusC13050 MUSCLE, SKELETAL Skeletal MuscleTissueStriated muscles that are under voluntary control of theorganism. They are connected at either or both ends toa bone and are utilized for locomotion and othermovements. (NCI)Skeletal Muscle TissueC<strong>12</strong>437 MUSCLE, SMOOTH Involuntary muscle tissue of the internal organs. It iscomposed of elongated muscle cells or fibers that arenot arranged in a striated pattern and form layers ofmuscle tissue. The smooth muscle cells contain acontractile apparatus composed of thin and thickfilaments and a cytoskeleton composed ofintermediate filaments. (NCI)C53075 MUSCLE, SOLEUS A muscle located in the back of the lower leg beneaththe gastrocnemius muscle. Its actions involve theflexion of the ankle joint and standing. (NCI)Smooth Muscle TissueSoleusC53079MUSCLE, TIBIALISCRANIALISAn extensor muscle in the anterior surface of the tibia.(NCI)Tibialis CranialisC90604MUSCLE, TRICEPSBRACHIIThe muscle of the upper limb that extends the limb.Triceps BrachiiC49594 NASAL TURBINATE One of three (Inferior, Middle, Superior) paired bonyshelves located within the nasal cavity through whichinhaled air is taken into the nasopharynx. It is linedwith ciliated, pseudostratified columnar epithelium andfunctions to humidify, heat, and filter incoming air, aswell as protecting the olfactory bulb and sinuses fromincorrectly pressurized incoming air. (NCI)Nasal TurbinateC77659NASAL-ASSOCIATEDLYMPHOID TISSUENALTThe lymphocytic cell population present in the mucosaof the nasopharyngeal duct.Nasal-Associated Lymphoid TissueSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 348 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>423 NASOPHARYNX Nasopharynx The part of the pharynx in the back of the throat, at andabove the soft palate. The nasopharynx is continuouswith the nasal passages. (NCI)C13052 NERVE The tissue that generates and conducts electricalsignals in the body. It contains the neurons.C54024 NERVE ROOT One of the two bundles of nerve fibers (dorsal andventral roots) emerging from the spinal cord that jointo form each spinal nerve. (NCI)C<strong>12</strong>682 NERVE, BRACHIAL Brachial Plexus A nerve network originating from C5 to T1 thatsupplies cutaneous and muscular innervation to the armand hand. (NCI)C<strong>12</strong>700 NERVE, CRANIAL Any of the <strong>12</strong> paired nerves that originate in the brainstem. (NCI)NasopharynxNerve TissueNerve RootBrachial PlexusCranial NerveC<strong>12</strong>714 NERVE, FACIAL Seventh CranialNerveThe 7th cranial nerve. The facial nerve has two parts,the larger motor root which may be called the facialnerve proper, and the smaller intermediate or sensoryroot. Together they provide efferent innervation to themuscles of facial expression and to the lacrimal andsalivary glands, and convey afferent information fortaste from the anterior two-thirds of the tongue and fortouch from the external ear. (MeSH)Facial NerveC52815 NERVE, MEDIAN A branch of the brachial plexus that extends along theanterior aspect of the forearm and the hand. (NCI)Median NerveC<strong>12</strong>761 NERVE, OPTIC Optic Nerve The second cranial nerve. (NCI) Optic NerveC<strong>12</strong>768 NERVE, PERIPHERAL Peripheral Nerve Any nerve outside the brain or spinal cord thatconnects with peripheral receptors or effectors. (NCI)C52814 NERVE, PERONEAL Nerve, Fibular Any one of the three (Common, Deep, and Superficial)peroneal nerves.Peripheral NervePeroneal NerveC92601NERVE, PERONEAL,COMMONA terminal branch of the sciatic nerve. It innervates thecalf and foot.Common Peroneal NerveC92602 NERVE, PERONEAL, DEEP A branch of the common peroneal nerve. It innervatesthe ankle and toes.Deep Peroneal NerveC92603NERVE, PERONEAL,SUPERFICIALA branch of the common peroneal nerve. It innervatesthe surface of the calf and foot.Superficial Peroneal NerveC77674 NERVE, PLANTAR A nerve that innervates the sole of the foot. This termmay refer to the medial plantar nerve or lateral plantarnerve, both of which originate from the tibial nerve.(NCI)C528<strong>12</strong> NERVE, RADIAL A large nerve that arises from the brachial plexus andenervates the extensor muscles and skin of the entireupper limb's posterior aspect. (NCI)C52810 NERVE, SCIATIC Sciatic Nerve The longest single nerve that is formed by the mergingof the ventral rami of the L4, L5, and S1 in the pelvisand passes down the lower limb where it divides intothe common peroneal and tibial nerves. (NCI)Plantar NerveRadial NerveSciatic NerveC<strong>12</strong>792 NERVE, SPINAL Spinal Roots Paired nerves that arise from the spinal cord. Spinal NerveSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 349 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77675 NERVE, SURAL A peripheral nerve that runs close to the smallsaphenous vein. It supplies innervation to the skin ofthe lower back of the leg and sends a branch to thelateral side of the foot and little toe. (NCI)C52809 NERVE, TIBIAL One of two major branches of the sciatic nerve. Itinnervates the muscles of the lower limb and themuscles of the plantar aspect of the foot.Sural NerveTibial NerveC<strong>12</strong>806 NERVE, TRIGEMINAL NervusTrigeminus; FifthCranial Nerve;Trigeminal NerveThe fifth set of paired nerves of the face that emergefrom the brain steam. These nerves have sensory andmotor functions in the face, oral cavity, and nasalcavity. (NCI)Trigeminal NerveC52807 NERVE, ULNAR A nerve in the arm that runs along the inside of theupper arm down to the palm and little finger.C<strong>12</strong>8<strong>12</strong> NERVE, VAGUS The 10th cranial nerve. The vagus is a mixed nervewhich contains somatic afferents (from skin in back ofthe ear and the external auditory meatus), visceralafferents (from the pharynx, larynx, thorax, andabdomen), parasympathetic efferents (to the thorax andabdomen), and efferents to striated muscle (of thelarynx and pharynx). (MSH2001)C<strong>12</strong>299 NIPPLE Nipple The pigmented protuberance on the surface of thebreast through which milk is drawn from the breast.(NCI)C<strong>12</strong>756 NOSE Nose A structure of special sense serving as an organ of thesense of smell and as an entrance to the respiratorytract. (NCI)C13197 NUCLEUS Cell Nucleus A body within the cell, surrounded by a membrane,within which lie the chromosomes, one or morenucleoli, combined with proteins, and exhibits mitosis.(NCI)C98765 OLFACTORY REGION The area of mucosa in the nose lined by olfactoryepithelium and containing olfactory glands. (NCI)C98766 OMASUM The third compartment of the forestomach ofruminants with many long folds of mucosa (resemblinga book). (NCI)C33209 OMENTUM Omental Fat A fold of peritoneum originating at the stomach andsupporting the viscera. (NCI)Ulnar NerveVagus NerveNippleNoseNucleusOlfactory RegionOmasumOmentumC<strong>12</strong>760 OPTIC DISC Optic Papilla;Optic NerveHead; SecondCranial NerveA portion of the retina at which the axons of theganglion cells exit the eyeball to form the optic nerve.No light-sensitive photoreceptors are contained withinthis portion of the retina. (NCI)Optic DiscC92600 OROPHARYNX Tissue that occupies the area between the soft palateand upper portion of the epiglottis. (NCI)C<strong>12</strong>404 OVARY Ovary One of the paired female reproductive glandscontaining the ova or germ cells; the ovary's stroma isa vascular connective tissue containing numbers ofovarian follicles enclosing the ova. (NCI)C92595 OVARY/OVIDUCT A laboratory specimen consisting of the ovaries andoviduct. (NCI)Oropharyngeal TissueOvaryOvary/OviductSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 350 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>403 OVIDUCT Fallopian Tube One of a pair of tubes that extend from the uterus toeach of the ovaries. Following ovulation the egg travelsdown the fallopian tube to the uterus wherefertilization may or may not occur. (NCI)C<strong>12</strong>229 PALATE The roof of the oral cavity. It separates the oral cavityfrom the nasal cavity.C<strong>12</strong>230 PALATE, HARD Hard Palate The bony anterior part of the roof of the mouthseparating the nose from the mouth. (NCI)C<strong>12</strong>231 PALATE, SOFT A movable fold suspended from the posterior borderof the hard palate. (MeSH) (NCI)C<strong>12</strong>393 PANCREAS Pancreas An organ behind the lower part of the stomach that isthe shape of a fish and about the size of a hand. It is acompound gland composed of both exocrine andendocrine tissues. The endocrine pancreas makesinsulin so that the body can use glucose (sugar) forenergy. The exocrine pancreas makes enzymes thathelp the body digest food. Spread all over the pancreasare areas called the Islets of Langerhans. The cells inthese areas each have a special purpose. The alpha cellsmake glucagon, which raises the level of glucose in theblood; the beta cells make insulin; the delta cells makesomatostatin. There are also PP cells and D1 cells,about which little is known. (NCI)Fallopian TubePalateHard PalateSoft PalatePancreasC<strong>12</strong>763 PARANASAL SINUS AccessorySinuses; NasalCavity; NasalSinuses;Paranasal SinusAn air-filled cavity adjacent to the nasal cavity lined bya mucous membrane and located in the bones of theskull. There are four paranasal sinuses on each side ofthe face: ethmoid, frontal, maxillary, and sphenoidsinus. (NCI)Paranasal SinusC77660 PAW The foot of a quadruped animal, including canines,felines, mice, rats, and bears. The paw contains clawsor nails and pads composed of a thick keratinizeddermis covering collagenous adipose tissue. (NCI)C49269 PENIS The erectile tissues of the penis. It includes the dorsalcorpora cavernosa and the ventral corpus spongiosum.Their structure consists of multiple vascular spacesthat are surrounded by smooth muscle. (NCI)C13005 PERICARDIUM Pericardium A conical membranous sac filled with serous fluid inwhich the heart as well as the roots of the aorta andother large blood vessels are contained. (NCI)C33301 PERINEUM Perineum The area located between the anus and vulva in females,and anus and scrotum in males. (NCI)C<strong>12</strong>770 PERITONEUM Peritoneum The tissue that lines the wall of the abdominal cavity,intestine, and mesentery. It consists of the parietalperitoneum that covers the inside of the abdominalwall and the visceral peritoneum that covers thesurface of the intestine and mesentery. (NCI)C<strong>12</strong>771 PEYER'S PATCH Lymphoid tissue on the mucosa of the small intestine.(MeSH) (NCI)C<strong>12</strong>425 PHARYNX Pharynx A hollow tube that starts posterior to the mouth andnasal cavity and ends superior to the trachea andesophagus. (NCI)PawPenis Erectile TissuePericardiumPerineumPeritoneumPeyer PatchPharynxSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 351 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>292 PINNA External Ear;AuricleThe external part of the ear. (NCI)External EarC13272 PLACENTA An organ present in some vertebrates duringembryonic gestation that surrounds the fetus andprovides it with nutrients and oxygen, facilitates gasand waste exchange between the fetus and mother, andprovides parasitic cloaking from the mother's immunesystem by excretion of neurokinin B. (NCI)C13356 PLASMA Plasma is the fluid (acellular) portion of thecirculating blood, as distinguished from the serum thatis the fluid portion of the blood obtained by removal ofthe fibrin clot and blood cells after coagulation.C<strong>12</strong>469 PLEURA Pleural Tissue The tissue that lines the wall of the thoracic cavity andthe surface of the lungs. (NCI)C<strong>12</strong>323 PREPUCE Preputium Penis A covering fold of skin, often used alone to designatethe preputium penis. (NCI)C<strong>12</strong>887 RENAL PELVIS The funnel-shaped proximal portion of the ureterlocated within the kidney into which urine is secreted,from the collecting duct system of the kidney. (NCI)C33467 RETE TESTIS A network of tubules that convey sperm from theseminiferous tubules within the testicles to theefferent ducts. (NCI)C98777 RETICULUM Smallest forestomach of ruminants with complexhoneycomb folding of mucosa. (NCI)PlacentaPlasmaPleuraPrepuceRenal PelvisRete TestisReticulumC<strong>12</strong>298 RETROPERITONEUM RETROPERITONEAL CAVITYThe back of the abdomen where the kidneys lie and thegreat blood vessels run. (NCI)RetroperitoneumC98778 RUMEN Paunch Largest forestomach of ruminants where bacterialfermentation occurs. (NCI)C32068 SAC, ANAL Paired glands located on either side of the anus ofmost carnivores that secrete a noxious-smelling liquid,thus allowing members of the same species to identifyone another. (NCI)C14<strong>12</strong>8 SAC, YOLK Membranous sac on the ventral aspect of thedeveloping embryo that acts as a primitive circulatorysystem as well as providing nourishment. (NCI)C13275 SALIVA A clear liquid secreted into the mouth by the salivaryglands and mucous glands of the mouth; moistens themouth and starts the digestion of starches. (NCI)C<strong>12</strong>785 SCROTUM Scrotum The musculocutaneous pouch that encloses thetesticles. (NCI)C13277 SEMEN Seminal Plasma The thick, whitish secretion of the male reproductiveorgans. It is composed of spermatozoa in their nutrientplasma, secretions from the prostate, seminal vesicles,and various other glands, epithelial cells, and minorconstituents. (NCI)C13325 SERUM Sera The clear portion of the blood that remains after theremoval of the blood cells and the clotting proteins.(NCI)RumenAnal GlandYolk SacSalivaScrotumSemenSerumSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 352 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC33556 SINUS Sinus A recess, cavity, or channel. (NCI) SinusC77676 SITE, APPLICATION Site, Exposure The anatomic site at which medical intervention isadministered. (NCI)C77677 SITE, BIOPSY The anatomic site targeted for a biopsy procedure.(NCI)C92596 SITE, CATHETER The anatomic site through which fluid is transferredinto or out of the body using a catheter. (NCI)C77685 SITE, EXTERIORIZATION The site of the surgical exposure of an internal organor tissue. (NCI)C77678 SITE, IMPLANTATION The anatomic site at which a material such as a tissue,graft, device or radioactive material is inserted withsome intended degree of permanence. This term mayalso refer to the site of the uterus at which the earlyembryo is attached.C77679 SITE, INFUSION The anatomic site through which fluid is introducedinto the body. (NCI)C77680 SITE, INJECTION The anatomic site at which a medication or a vaccine isinjected. (NCI)C77681 SITE, INJURY The anatomic site at which damage or harm wassuffered. (NCI)C77682 SITE, MICROCHIP The anatomic site at which a microchip is implanted.(NCI)C77683 SITE, SURGICAL Incision Site The anatomic site of a cut made during surgery. Theterm may also refer to the resultant scar from thesurgical procedure. (NCI)Application SiteBiopsy SiteCatheter SiteExteriorization SiteImplantation SiteInfusion SiteInjection SiteInjury SiteMicrochip SiteIncision SiteC77684 SITE, TATTOO The anatomic site at which a tattoo is present. (NCI) Tattoo SiteC48322SITE, UNCERTAINPRIMARYReferring to the fact that the original site of growth ofa metastatic cancer is unknown or uncertain. (NCI)Primary Site UnknownC<strong>12</strong>470 SKIN Integument; Skin An organ that constitutes the external surface of thebody. It consists of the epidermis, dermis, and skinappendages. (NCI)C92441 SKIN/SUBCUTIS A tissue sample that contains the epidermis, dermis,and subcutaneous adipose tissue. (NCI)C92437 SMALL COLON The terminal part of the colon of the horse with areduced diameter. (NCI)SkinSkin/Subcutaneous TissueSmall ColonC33573 SMALL INTESTINE Small Bowel;Duodenum/Jejunum/IleumThe tissue of the small intestine. It comprises fourlayers - mucosa with simple columnar epithelium,submucosa, smooth muscle with inner circular andouter longitudinal layers, and serosa. (NCI)Small Intestinal Wall TissueC<strong>12</strong>263SMALL INTESTINE,DUODENUMA jointed tube 25-30 cm long that connects thestomach to the jejunum. (NCI)DuodenumC<strong>12</strong>387 SMALL INTESTINE, ILEUM Ileum The final section of the small intestine. (NCI) IleumC<strong>12</strong>388SMALL INTESTINE,JEJUNUMJejunumThe portion of the small intestine that extends fromthe duodenum to the ileum. (NCI)JejunumSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 353 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC88024SMALL INTESTINE,SACCULUS ROTUNDUSAn anatomic structure exclusive to rabbits that islocated at the terminal part of the ileum. It is rich inlymphoid tissue.Sacculus RotundusC<strong>12</strong>329 SPERMATIC CORD A tube-like structure composed of the vas deferens andsurrounding tissue layers, that runs from the abdomento each of the testicles. (NCI)Spermatic CordC<strong>12</strong>998 SPINAL COLUMN VertebralColumnA series of bones, muscles, tendons, and other tissuesreaching from the base of the skull to the tailbone. Thevertebral column forms the axis of the skeleton andencloses as well as protects the spinal cord and thefluid surrounding the spinal cord. (NCI)Vertebral ColumnC<strong>12</strong>464 SPINAL CORD Medulla Spinalis The elongated, approximately cylindrical part of thecentral nervous system of vertebrates that lies in thevertebral canal and from which the spinal nervesemerge. (NCI)C<strong>12</strong>892 SPINAL CORD, CERVICAL The portion of the spinal cord located in the cervicalregion. (NCI)C<strong>12</strong>895 SPINAL CORD, LUMBAR The portion of the spinal cord located in the lumbarregion. (NCI)C<strong>12</strong>896 SPINAL CORD, SACRAL The portion of the spinal cord located in the sacralregion. (NCI)C<strong>12</strong>894 SPINAL CORD, THORACIC The portion of the spinal cord located in the thoracicregion. (NCI)Spinal CordCervical Spinal CordLumbar Spinal CordSacral Spinal CordThoracic Spinal CordC92597SPINAL CORD,THORACOLUMBARThe region of the spinal cord pertaining to the thoracicand lumbar vertebrae. (NCI)Thoracolumbar RegionC92438 SPIRAL COLON The ascending colon of the ruminants and pigs. (NCI) Spiral ColonC34086 SPLEEN The largest mass of lymphatic tissue in the body. Itconsists of cells and vessels contained within a capsulelined by mesothelium, from which trabecula enter thesplenic parenchyma. The parenchyma is supported by aframework of reticular fibers. The spleen consists oftwo types of parenchymal tissue, the white and redpulp. The white pulp is composed of elongated cordsof compact lymphatic tissue containing nodules. Thered pulp is composed of pulp cords and splenicsinusoids. (NCI)C32664 STOMACH The tissue of the stomach. It is composed of a fibrousouter layer bounded by smooth muscle, thesubmucosa, and the mucosa. The mucosa of thestomach consists of secretory cells that producehydrochloric acid and gastric enzymes. (NCI)C<strong>12</strong>256 STOMACH, CARDIA Gastric Cardia The area around the esophagogastric mucosal junctionwhere the esophageal mucosa transitions into thegastric mucosa. (NCI)Splenic TissueGastric TissueGastric CardiaC<strong>12</strong>257 STOMACH, FUNDUS Fundus of theStomachThe portion of the stomach that lies above the cardiacnotch. It allows for the accumulation of gasesproduced by chemical digestion. (NCI)Fundus of the StomachSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 354 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77661 STOMACH, GLANDULAR The part of the stomach that is lined with glandularmucosa and receives the food from the esophagus. Themucosa contains gastric glands that secret gastric acid.(NCI)Glandular StomachC77662STOMACH,NONGLANDULARThe portion of the animal stomach that is responsiblefor storage and digestion of food particles but does notcontain the gastric mucosa-associated glands.Nonglandular StomachC<strong>12</strong>260 STOMACH, PYLORUS The lower part of the stomach that connects to theduodenum. (NCI)PylorusC33645 SUBCUTIS SubcutaneousTissue;SubcutaneousAdipose TissueAdipose tissue located under the dermis. It bindsunderlying structures with the skin. The subcutis isimportant in the regulation of temperature of the skinitself and the body. The size of this layer variesthroughout the body and from subject to subject.SubcutisC13280 SWEAT Sweat The liquid secreted by the sweat glands. (NCI) SweatC77663 TAIL The flexible prolongated structure of the posterior partof an animal's body. (NCI)C33739 TEAR A fluid containing oils, mucin, and proteins, which issecreted by the lacrimal gland and cleans andlubricates the eye. (NCI)C77664 TEAT The outer projections from the mammary organ of ananimal. They are the structures through which the milkis discharged. (NCI)C13045 TENDON A band of fibrous connective tissue that joins bone tomuscle. (NCI)C32043 TENDON, CALCANEAL The tendon that connects the heel bone to the calfmuscles at the back of the lower leg. (NCI)C<strong>12</strong>4<strong>12</strong> TESTIS Testicle; Testis Either of the paired male reproductive glands thatproduce the male germ cells and the male hormones.(NCI)C77668 TESTIS/EPIDIDYMIS A laboratory specimen consisting of the testis andepididymis. (NCI)C62484 THORACIC WALL Chest Wall The total system of structures outside the lungs thatmove as a part of breathing; it includes all structuresfrom the skin to the parietal pleura. (NCI)C33952 THYMUS Tissue composed of a highly cellular outer cortex, andless cellular inner medulla. Both cortex and medullacontain epithelial cells, interdigitating dendritic cells,macrophages, and lymphocytes. (NCI)C<strong>12</strong>801 TISSUE An anatomical structure consisting of similarlyspecialized cells and intercellular matrix, aggregatedaccording to genetically determined spatialrelationships, performing a specific function. (NCI)C<strong>12</strong>473 TISSUE, SYNOVIAL The inner layer of the connective tissue that seals thejoint. (NCI)C<strong>12</strong>422 TONGUE Tongue The muscular organ located in the floor of the mouthand serving as the principal organ of taste andmodification of the voice in speech. (NCI)TailTearTeatTendonAchilles TendonTestisTestis/EpididymisChest WallThymic TissueTissueSynovial MembraneTongueSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 355 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC38516 TONSIL Nodules of lymphoid tissue located on either side ofthe back of the nasal canal and throat. (NCI)C32988 TONSIL, LINGUAL A mass of lymphoid tissue located at the base of thedorsal surface of the tongue.C33250 TONSIL, PALATINE Paired small tissue masses located on opposite sidesof the oropharynx between the faucial pillars.Tonsillar TissueLingual TonsilPalatine TonsilC33318 TONSIL, PHARYNGEAL Adenoid;PharyngealTonsilA fold of lymphatic tissue covered by ciliatedepithelium at the very back of the nose, in the roof ofthe nasopharynx. (NCI)Pharyngeal TonsilC33794 TOOTH Teeth The tissue that forms the tooth. It consists of thedental pulp, dentin, enamel, cementum, odontogenicepithelium, and periodontium. (NCI)Odontogenic TissueC32201 TOOTH, BICUSPID Premolar Tooth;PremolarOne of the two permanent teeth located in front of themolars and behind each cuspid. These teeth have twocusps (points) and are used to tear and grind food.(NCI)Bicuspid ToothC32258 TOOTH, CANINE Canine Tooth A single-cusped (pointed) and usually single-rootedtooth located between the incisors and premolars.(NCI)C32769 TOOTH, INCISOR Incisor One of the teeth in front of the canines in either jawdesigned for cutting or gnawing. (NCI)C33136 TOOTH, MOLAR A grinding tooth with a broad crown; located behindthe premolars. (NCI)Canine ToothIncisorMolar ToothC<strong>12</strong>428 TRACHEA Trachea;WindpipeThe fibrocartilaginous, mucous-lined tube passingfrom the larynx to the bronchi. (NCI)TracheaC33822 TUNICA VAGINALIS Serous membrane that covers the testicles. (NCI) Tunica VaginalisC<strong>12</strong>416 URETER Ureter The thick-walled tube that carries urine from eachkidney to the bladder. (NCI)C<strong>12</strong>417 URETHRA Urethra The tube carrying urine from the bladder to outside ofthe body. (NCI)UreterUrethraC32209 URINARY BLADDER Urinary System,Bladder; BladderThe tissue that forms the bladder wall. It consists ofmucosa, muscular coat, and serosal surface. (NCI)Bladder TissueC13283 URINE The fluid that is excreted by the kidneys. It is stored inthe bladder and discharged through the urethra. (NCI)C<strong>12</strong>405 UTERUS Womb; Uterus A hollow, thick-walled, muscular organ located withinthe pelvic cavity of a woman. Within the uterus thefertilized egg implants and the fetus develops duringpregnancy. (NCI)C92436 UTERUS/CERVIX A tissue sample that contains the body of uterus andthe cervix. (NCI)C77672 UTERUS/OVARY A laboratory specimen consisting of the uterus andovaries. (NCI)C<strong>12</strong>407 VAGINA Vagina The female genital canal, extending from the uterus tothe vulva. (NCI)UrineUterusUterus/CervixUterus/OvariesVaginaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 356 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC<strong>12</strong>670 VALVE, AORTIC A valve that is located between and controls the flowof blood from the left ventricle of the heart and theaorta. (NCI)C<strong>12</strong>729 VALVE, CARDIAC Any of the four heart valves, including the twoatrioventricular valves and the two semilunar valves,that regulate the flow of blood through the chambersof the heart. (NCI)C<strong>12</strong>753 VALVE, MITRAL A dual-flap valve of the heart that regulates the flow ofblood between the left atrium and the left ventricle ofthe heart. (NCI)C<strong>12</strong>775 VALVE, PULMONARY A valve that is located between and controls the flowof blood from the right ventricle of the heart and thepulmonary artery. (NCI)C<strong>12</strong>805 VALVE, TRICUSPID A dual-flap valve of the heart that regulates the flow ofblood between the right atrium and the right ventricleof the heart. (NCI)Aortic ValveCardiac ValveMitral ValvePulmonary ValveTricuspid ValveC<strong>12</strong>813 VAS DEFERENS Duct carrying spermatozoa. (NCI) Vas DeferensC<strong>12</strong>814 VEIN Vein A blood vessel that carries blood towards the heart. VeinC77673 VEIN, AURICULAR A vein located in the ear region. It may pertain to theanterior or posterior auricular veins. (NCI)C<strong>12</strong>883 VEIN, BRACHIAL Deep vein of the upper arm that forms at the junctionof the radial and ulnar veins and ends at the inferiorborder of the teres major. (NCI)C92598 VEIN, CAUDAL Tail Vein A vein located in the tail of vertebrate animals thatleads to the inferior vena cava in mammals and theposterior cardinal vein in the trunk of fish. (NCI)Auricular VeinBrachial VeinCaudal VeinC32286 VEIN, CEPHALIC Cephalic Vein;Vena CephalicaOne of five superficial veins of the upper extremity,beginning at the radial aspect of the wrist area,wrapping around the forearm and emptying into theaxillary vein. (NCI)Cephalic VeinC<strong>12</strong>716 VEIN, FEMORAL Femoral Vein A vein that starts within the inguinal region and extendsto the lower extremities. (NCI)C<strong>12</strong>738 VEIN, JUGULAR Vena Jugularis Veins in the neck which drain the brain, face, and neckinto the brachiocephalic or subclavian veins. (NCI)C53055 VEIN, MESENTERIC One of two veins (inferior or superior) that returnsdeoxygenated blood from the intestines. (NCI)Femoral VeinJugular VeinMesenteric VeinC33343 VEIN, PORTAL Hepatic PortalVein; Portal VeinA short thick trunk vein that transports bloodcontaining the absorbed products of digestion from theintestine directly to the liver. (NCI)Portal VeinC<strong>12</strong>776 VEIN, PULMONARY One of four veins that carries oxygen-rich blood fromthe lungs to the cardiac left atrium. (NCI)C33462 VEIN, RENAL The veins which return blood from the kidneys to thevena cava. (NCI)C33511 VEIN, SAPHENA Saphenous Vein One of two (Greater, Small) superficial veins of theleg and thigh. (NCI)C<strong>12</strong>817 VEIN, VENA CAVA Vena Cava A large vein which returns blood from the head, neckand extremities to the heart. (NCI)Pulmonary VeinRenal VeinSaphenous VeinVena CavaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 357 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77529 - SPEC - SpecimenCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC32213 VESSEL, BLOOD Vessel The tissue component of a blood vessel. Large vesselscontain three layers of tissue, the tunica intima, tunicamedia, and tunica adventitia. Capillary tissue iscomposed of a single layer of endothelium andconnective tissue. (NCI)Blood Vessel TissueC33038 VESSEL, LYMPHATIC Vessel, Lymph A tubule that carries lymph throughout the body. (NCI) Lymphatic VesselC77666 VOMITUS Emesis; Vomit Stomach contents that have returned to the mouth orare ejected beyond the mouth. (NCI)VomitusC<strong>12</strong>408 VULVA ExternalGenitalia; VulvaThe external, visible part of the female genitaliasurrounding the urethral and vaginal opening. The vulvaincludes the clitoris and inner as well as outer labia.(NCI)VulvaC77665 WHOLE ANIMAL Carcass Referring to the entire body of an animal. (NCI) Whole AnimalC41067 WHOLE BLOOD Blood that has not been separated into its variouscomponents; blood that has not been modified exceptfor the addition of an anticoagulant. (NCI)Whole BloodSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 358 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77808 - SPECIES - SpeciesCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC14252 BABOON Multiple species of large terrestrial monkeys in thegenus Papio, including P. hamadryas, P. papio, P.anubis, P. cynocephalus and P. ursinus.C14192 BOVINE Cattle The domesticated ungulates, Bos primigenius taurusand Bos primigenius indicus.BaboonCowC14191 CAT The domestic cat, Felis catus. (NCI) CatC14193 CHICKEN The common domestic fowl, Gallus gallus. (NCI) ChickenC14297 CHIMPANZEE The anthropoid ape, Pan troglodytes. ChimpanzeeC91815 CHINCHILLA A member of the Chinchillidae family of crepuscularrodents.ChinchillaC14201 DOG The domestic dog, Canis familiaris. (NCI) DogC77097 FERRET The common domestic mammal, Mustela putorious. FerretC14207 FISH Any jawed or jawless organisms in the phylumChordata including the jawless fish, armored fish,cartilaginous fish, ray-finned fish and lobe-finned fish.C77807 GERBIL Any of the small mammals belonging to theGerbillinae subfamily.C14210 GOAT Any one of several species in the genus Capra, mostcommonly Capra hircus.FishGerbilGoatC14211 GUINEA PIG The domesticated guinea pig, Cavia porcellus. (NCI) Guinea PigC142<strong>12</strong> HAMSTER Any member of the subfamily cricetinae and thegenuses Mesocricetus, Phodopus, Cricetus,Cricetulus, Allocricetulus, Cansumys and Tscherskia.HamsterC14222 HORSE The domestic horse, Equus caballus. (NCI) HorseC77115 MARMOSET The common marmoset, Callithrix jacchus. MarmosetC91816 MASTOMYS A genus of rodent in the family muridae. MastomysC14243 MONKEY Any haplorhine primate not belonging to the familyTarsiidae, Hylobatidae, Pongidae, or Hominidae; thisdoes not correspond to any taxon. This group is dividedinto Old World monkeys (Cercopithecidae) and NewWorld monkeys (Callitrichidae and Cebidae).C14238 MOUSE Any of numerous species of small rodents belongingto the genus Mus and various related genera of thefamily Muridae. (NCI)MonkeyMouseC14280 PIG The domestic pig Sus scrofa domestica. (NCI) PigC918<strong>12</strong> PIGEON A member of the Columbidae family of birds, mostcommonly referring to the species Columba livia.C91813 QUAIL A member of the Phasianidae family of pheasants thatincludes several genera, including Cotumix,Anurophasis, Perdicula and Ophrysia.C14264 RABBIT Various members of the family Leporidae, especiallythose of the genus Sylvilagus. (NCI)PigeonQuailRabbitSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 359 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77808 - SPECIES - SpeciesCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC14266 RAT Any one of several species in the genus Rattus. RatC14273 SHEEP Any one of several species in the genus Ovis, mostcommonly Ovis aries.SheepSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 360 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90003 - SSTYP - Study TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC79369 ABSORPTION FDA RPSPharmacokinetics: AbsorptionThe branch of pharmacokinetics that studies theprocess by which a drug is absorbed by the body.Pharmacokinetics: AbsorptionC79368ANALYTICAL METHODSAND VALIDATIONREPORTSFDA RPSAnalyticalMethods AndValidationReportsAn indication or description of the process by whichthe truth of something is tested or found.Analytical Methods And ValidationReportsC79391 ANTIGENICITY FDA RPS OtherToxicity Studies:AntigenicityA toxicity study that assesses the ability of a substanceto induce an antigenic response in an animal.Other Toxicity Studies: AntigenicityC49664 BIOAVAILABILITY A study of the degree to which or rate at which a drugor other substance is absorbed or becomes available atthe site of physiological activity after administration.(NCI)Bioavailability StudyC79380 CARCINOGENICITY FDA RPSToxicology:CarcinogenicityA study that assesses the toxic effects of a compoundin animals after repeated administrations withparticular emphasis on determining the carcinogenicityof the compound.Toxicology: CarcinogenicityC18079CARDIOVASCULARPHARMACOLOGYThe study of the effects of drugs upon the heart orcirculatory system.Cardiovascular PharmacologyC90370 CNS PHARMACOLOGY The branch of pharmacology that deals with the centralnervous system. (NCI)Central Nervous SystemPharmacologyC79394 DEPENDENCE FDA RPS OtherToxicity Studies:DependenceC79370 DISTRIBUTION FDA RPSPharmacokinetics: DistributionA study that assesses the capacity of a substance tobecome an abuse liability.The branch of pharmacokinetics that studies theprocess by which a drug is distributed by the body.Other Toxicity Studies: DependencePharmacokinetics: DistributionC79386EMBRYO FETALDEVELOPMENTFDA RPSReproductiveAndDevelopmentalToxicity:EmbryofetalDevelopmentA toxicity study that assesses the effects of asubstance on embryonic and fetal development.Reproductive And DevelopmentalToxicity: Embryofetal DevelopmentC79372 EXCRETION FDA RPSPharmacokinetics: ExcretionThe branch of pharmacokinetics that studies theprocess by which a drug is eliminated by the body.Pharmacokinetics: ExcretionC79385FERTILITY AND EARLYEMBRYONICDEVELOPMENTFDA RPSReproductiveAndDevelopmentalToxicity:Fertility AndEarly EmbryonicDevelopmentA study that assesses the effects of a substance on anorganism's fertility and/or embryonic development.Reproductive And DevelopmentalToxicity: Fertility And EarlyEmbryonic DevelopmentC90388GASTROINTESTINALPHARMACOLOGYThe branch of pharmacology that deals with thegastrointestinal system. (NCI)Gastrointestinal PharmacologySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 361 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90003 - SSTYP - Study TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC79378 GENOTOXICITY IN VITRO FDA RPSGenotoxicity: InVitroC79379 GENOTOXICITY IN VIVO FDA RPSGenotoxicity: InVivoC79392 IMMUNOTOXICITY FDA RPS OtherToxicity Studies:ImmunotoxicityC79396 IMPURITIES FDA RPS OtherToxicity Studies:ImpuritiesA genotoxicity study that tests the ability of asubstance to cause DNA damage not in intact animals,but in cells or other systems.A genotoxicity study that tests the ability of asubstance to cause DNA damage within the body.A toxicity study that assesses potential harm to theimmune system.A study that assesses the effects of impurities that maybe found in a substance.Genotoxicity: In VitroGenotoxicity: In VivoOther Toxicity Studies:ImmunotoxicityOther Toxicity Studies: ImpuritiesC79388 JUVENILE STUDIES FDA RPSStudies In WhichThe Offspring(JuvenileAnimals) AreDosed And/OrFurther EvaluatedA toxicology study that assesses the and effects of asubstance on juveniles before reaching puberty.Studies In Which The Offspring(Juvenile Animals) Are DosedAnd/Or Further EvaluatedC79389 LOCAL TOLERANCE FDA RPSToxicology:Local ToleranceC79393 MECHANISTIC STUDIES FDA RPS OtherToxicity Studies:MechanisticStudiesC79371 METABOLISM FDA RPSPharmacokinetics: MetabolismC79395 METABOLITES FDA RPS OtherToxicity Studies:MetabolitesA toxicology study that assesses the effects of asubstance when administered to a restricted portion ofthe body.A study that investigates the process by which asubstance induces its effects.The branch of pharmacokinetics that studies theprocess by which a drug is metabolized by the body.A study that evaluates the effects of a metabolite of asubstance.Toxicology: Local ToleranceOther Toxicity Studies: MechanisticStudiesPharmacokinetics: MetabolismOther Toxicity Studies: MetabolitesC79367PHARMACODYNAMICDRUG INTERACTIONSFDA RPSPharmacology:Pharmacodynamic DrugInteractionsThe branch of pharmacology that deals with themechanism of action and biochemical andphysiological effects of drug-drug interactions.Pharmacology: PharmacodynamicDrug InteractionsC79373PHARMACOKINETIC DRUGINTERACTIONSFDA RPSPharmacokinetics: DrugInteractionsThe branch of pharmacokinetics that studies theprocess by which two or more drugs in a system areabsorbed, distributed, metabolized, and eliminated bythe body.Pharmacokinetics: Drug InteractionsC79387PRENATAL ANDPOSTNATALDEVELOPMENTFDA RPSReproductiveAndDevelopmentalToxicity:Prenatal AndPostnatalDevelopmentIncludingMaternalFunctionA toxicity study that assesses the effects of asubstance on an organism's development shortlybefore and after birth.Reproductive And DevelopmentalToxicity: Prenatal And PostnatalDevelopment Including MaternalFunctionSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 362 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90003 - SSTYP - Study TypeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC79364PRIMARYPHARMACODYNAMICSFDA RPSPharmacology:PrimaryPharmacodynamicsThe branch of pharmacology that deals with thebiochemical and physiological effects of a drug andthe mechanism of drug action in relation to its desiredtherapeutic target.Pharmacology: PrimaryPharmacodynamicsC18996 RENAL PHARMACOLOGY The science concerned with drugs and their actions anduses in kidney biology and the treatment of kidneydisease. (NCI)Renal PharmacologyC79376 REPEAT DOSE TOXICITY FDA RPSToxicology:Repeat DoseToxicityA study that assesses the toxic effects of a compoundin animals after repeated administrations.Toxicology: Repeat Dose Toxicity[Species, Route, Duration]C90449RESPIRATORYPHARMACOLOGYThe branch of pharmacology that deals with therespiratory system. (NCI)Respiratory PharmacologyC79365SECONDARYPHARMACODYNAMICSFDA RPSPharmacology:SecondaryPharmacodynamicsThe branch of pharmacology that deals with thebiochemical and physiological effects of a drug andthe mechanism of drug action not related to its desiredtherapeutic target.Pharmacology: SecondaryPharmacodynamicsC79375 SINGLE DOSE TOXICITY FDA RPSToxicology:Single DoseToxicityA study that assesses the toxic effects of a compoundin animals after a single administration.Toxicology: Single Dose Toxicity[Species And Route]C90478 TOXICOKINETICS Evaluation of the absorption, distribution, metabolismand excretion of a substance in relation to its toxicityin an animal.ToxicokineticsSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 363 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90002 - STCAT - Study CategoryCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC18809 GENTOX GeneticToxicologyThe branch of toxicology that deals with adverseeffects of chemical, physical or biological agents ongenetic material.Genetic ToxicologyC16974 P Pharmacology Science that deals with the characteristics, effects, anduses of drugs and their interactions with livingorganisms.PharmacologyC15299 PK PharmacokineticsC90448 REPRO Reproductive andDevelopmentalToxicology;DART;Developmentaland ReproductiveToxicologyC90452 SP SafetyPharmacologyThe characteristic movements of drugs withinbiological systems, as affected by absorption,distribution, binding, elimination, biotransformation,and excretion; particularly the rates of suchmovements. (NCI)The branch of toxicology that deals with adverseeffects of chemical, physical or biological agents onreproduction and development.A branch of pharmacology that investigates thepotential undesirable pharmacodynamic effects of asubstance on physiological functions in relation toexposure in the therapeutic range and above.(safetypharmacology.org) (NCI)PharmacokineticsReproductive and DevelopmentalToxicologySafety PharmacologyC17206 TOX Toxicology Toxicology is the study of the adverse effects ofchemical, physical or biological agents on people,animals, and the environment.ToxicologySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 364 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77530 - STRAIN - Strain/SubstrainCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC37320 <strong>12</strong>9/SV Derived by Dunn (1928) from a mouse/chinchillacross, the <strong>12</strong>9/Sv substrain has been recognized as amember of the Parental subgroup of substrains.C14650 A/J Derived by Strong (1921) from a cross between theCold Spring harbor and Bagg Albino stocks. The Astrain mouse has an albino coat (genotype a,b,c) and issusceptible to carcinogen-induced lung adenomas andcleft palate formation in response to cortisone. Also,the strain has defective macrophage functionreminiscent of lps mutation common to other strains.C76184 ACI:SEG Derived by Curtiss and Dunning (1926) at ColumbiaUniversity by crossing an inbred August rat with aninbred Copenhagen rat, to Heston (1945) and then tothe NIH in 1950. Disseminated to HarlanSprague-Dawley from laboratory of Segaloff (AltonOchsner Medican Foundation) in 1956. The ACI/Segrat is agouti in color, with a white belly and feet,genotype A hi.<strong>12</strong>9/Sv MouseA/J MouseACI/Seg, Rat StrainC76360 AFRICAN GREEN The diurnal primate, Chlorocebus sabaeus. African Green MonkeyC14505 AKR/J Originally disseminated by Detweiler and carried byFurth (1928-1936) and the Rockefeller Institute forsubsequent generations. The AKR mouse has an albinocoat (genotype a, B, c) and is highly susceptible toleukemias. The strain is viremic from birth in that alltissues express the AKV retrovirus and copies of theAKV genome are integrated in the mouse genome,which is associated with leukemia development. TheAKR strain is also a source of the Thy1.1 thymocyteantigen, which is expressed on thymocyte, bonemarrow and T cell progenitors and is used as a markerfor a variety of stem cells.C98707 B6.<strong>12</strong>9-Trp53tm1Brd N5 A partial congenic mouse with background strain ofC57BL/6 and <strong>12</strong>9/Sv chimera, containing aheterozygous or homozygous p53 mutation. (NCI)C76182 B6C3F1 Derived from a cross between a C57BL/6 female and aC3H male, this hybrid strain is commonly used in theproduction of transgenic mice.C37357 BALB/C Derived from albino mice stocks originallydisseminated by Bagg (1913) to Snell in 1932 that hasan albino coat with genotype A,b,c.C53897 BEAGLE The Beagle is a hardy, sturdy squarely-built, smallhound, with a short coat in tri-color, red and white,orange and white, or lemon and white. The ears arelong, wide and pendant. There are two height classes,13-15 inches (33-38 cm) and under 13 inches (33cm). Weight: 20-25 pounds (9-11 kg).C76362 BROILER BREEDS Derived from a cross between a white cornish maleand a barred rock female in the 1930s, modern broilerchickens are an F2 hybrid of broiler breedscharacterized by high fecundity and fast growth.AKR/J MouseB6.<strong>12</strong>9-Trp53tm1Brd N5B6C3 MouseBALB/c MouseBeagleBroiler ChickenC14395 BROWN NORWAY The common rat, Rattus norvegicus. BN, Rat StrainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 365 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77530 - STRAIN - Strain/SubstrainCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC14396 BUFFALO Derived from Buffalo stock of H. Morris to the NIH in1950 and disseminated from Charles River since1998, the Buffalo is a white albino rat, genotype c.C37376 C57BL/10 Derived by Little (1921) from A Lathrop stocks andseparated out before 1937, the C57BL/10 mouse has ablack coat and carries a Y chromosome of Asian Musmusculus origin and a LINE-1 element from Musspretus. Substrain C57BL/10 differs from othersubstrains at multiple loci, including H9, Igh2 and Lv,on chromosome 4 and has a high incidence ofspontaneous mutations.C14424 C57BL/6 Derived by Little (1921) from A Lathrop stocks andseparated out before 1937, the C57BL/6 mouse has ablack coat and carries a Y chromosome of Asian Musmusculus origin and a LINE-1 element from Musspretus. Substrain C57BL/6 differs from othersubstrains at multiple loci, including H9, Igh2 and Lv,on chromosome 4. This mouse model is prone to thedevelopment of fatty lesions in the aorta similar toatheromatous plaque in humans, as well ashyperglycemia, hyperinsulinemia,hypercholesterolemia and non-insulin-dependentdiabetes mellitus in response to a high fat diet.C76364 CALIFORNIA One of the larger rabbit breeds, the Californian has arounded, medium-length body with a short coat that iswhite with black on the nose, ears, feet, legs, and tail.This lagomorph also has pink eye color with genotypenp.C98711 CB6F1-TgN (RasH2) A transgenic mouse at F1 generation with backgroundstrain C57BL/6 crossed with BALB/cAn, containingthree copies of the human c-Ha-Ras gene introduced intandem. (NCI)C37396 CBA/CA The CBA mouse from Strong (1920) was disseminatedto Jackson Laboratory and then onto Haldane andGruneberg (1932) and finally onto Carter (1947). TheCBA/Ca female mice have long life spans whilst maleshave short life spans. Both males and females havehigh ceruloplasmin levels.C37399 CBA/J Developed by Strong (1920), the CBA/J mouse wasdisseminated to Andervont (1947) and then to JacksonLaboratory (1948). The CBA/J strain carries the genefor retinal degeneration (rd).C76183 CD1 (ICR) BR Derived from Rockefeller Swiss mice that weredisseminated to the Institute of Cancer Research inPhiladelphia (1948).C37369 CEH/HEJ Derived from the C3H progenitor strain that waspassed to Heston in 1941 and to Jackson Lab in 1947.The C3H/HeJ mouse has an agouti coat color andgenotype +, rd.BUF, Rat StrainC57BL/10 MouseC57BL/6 MouseCalifornia RabbitCB6F1-TgN (RasH2)CBA/Ca MouseCBA/J MouseICR BR MouseC3H/HeJ MouseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 366 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77530 - STRAIN - Strain/SubstrainCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77116 CF-1 Thought to be wild albino in origin, this strain wasobtained by Carworth farms from a Missourilaboratory. It was intensely inbred by N. Goto in 1978from a single Carworth pair, the progeny of which isused today. The CF-1 mouse has an albino coat withgenotype c.C77091 CHINESE Originating in the deserts of northern China andMongolia and kept in captivity since 1919, thesehamsters exhibit a whitish/grey/brown coat color witha black stripe down the spine.C77092 CHINESE SYRIAN A hamster derived from a cross between a Chinesehamster and Syrian hamster.C77093 CHINESE SYRIAN GOLD A hamster derived from a cross between a Chinesehamster and a Syrian hamster, with a golden-coloredcoat.CF-1 MouseChinese HamsterChinese Syrian HamsterChinese Syrian Gold HamsterC77117 COTTON The rat of the genus and species Sigmodon hispidus. Cotton RatC14232 CYNOMOLGUS The macaque, Macaca fascicularis. Cynomolgus MonkeyC77113 CYNOMOLGUS MAURITIUS The macaque, Macaca fascicularis, which originatesfrom Mauritius.C76186 DAHL-S Derived by Rapp from a colony of Sprague-Dawleyrats that were initially derived by LK Dahl atBrookhaven National Laboratories. The SS rat strainhas been selected for its acute salt sensitivity.C14606 DBA/1 Derived from crosses made by Little in 1929-1930between DBA progenitors. The DBA/1 mouse has a qH2 haplotype and carries the Cdh23 hl mutation thatresults in progressive hearing loss after 10 months ofage. The DBA/1 and DBA/2 mice differ at loci Car2,Ce2, Hc, H2, If1, Lsh, Tla, and Qa3. The strain iscommonly used as a model for rheumatoid arthritis asit mimics hallmarks of the human disease whenimmunized with type II collagen. (NCI)C14604 DBA/2 Derived from crosses made by Little in 1929-1930between DBA progenitors. The DBA/2 mouse has a dH2 haplotype and carries the Cdh23ahl mutation thatresults in a progressive hearing loss beginning at 3-4weeks of age and severe hearing loss after 3 months ofage. Alleles GpnmbR150X and Tyrp1isa contribute toan eye phenotype that closely resembles humanhereditary glaucoma. The DBA/2 mouse strain showssevere intolerance to alcohol and morphine and isnaturally CD94 deficient. (NCI)C77<strong>12</strong>4 DOMESTIC SHORT HAIR The name for a short-haired cat that does not have apedigree nor belong to a specific cat breed.C77088 DUNKIN-HARTLEY Albino outbred guinea pig belonging to the English(short-haired) breed. The Dunkin Hartley guinea pighas a white coat color (acromelanic albino) and redeyes and requires a nutritional source of vitamin C tosustain normal function.Cynomolgus Maritius MonkeySS, Rat StrainDBA/1 MouseDBA/2 MouseDomestic Short Hair CatDunkin Hartley Guinea PigSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 367 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77530 - STRAIN - Strain/SubstrainCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77101 DUROC-CROSS An older breed of American domestic pig, the Durocbreed is of medium length with a muscular,large-framed body. This pig breed is red-colored withpartially drooping ears and is one of the mostaggressive breeds of swine.C76365 DUTCH BELTED A smaller sized lagomorph, the Dutch belted rabbit hasblack hindquarters, ears and black patches around theeyes while the feet, face, and front half of the body arewhite. The Dutch belted rabbit is commonly utilized inbiomedical research for toxicology studies,ophthalmological research, and developmental toxicitystudies. This lagomorphs' small size lends itself tostudies in which the study drug is available in smallerquantities or if housing space is a consideration.C14401 FISCHER 344 Derived by Curtiss and Dunning (1920) at ColumbiaUniversity and disseminated to Heston (1949) and thento the NIH (1951). The Fischer 344 rat has an albinocoat and genotype c.C14474 FVB/N NIH general purpose Swiss mice were selected forresistance or sensitivity to histamine challengefollowing pertussis vaccination. The sensitive strain,HFSF/N, was subsequently found to have the Fv1ballele, which sensitizes the mice to B strain FriendLeukemia virus. The FVB/N strain has an albino coatwith genotype A,B,c,D,P, and has a vigorousreproductive performance.Duroc PigDutch Belted RabbitF344, Rat StrainFVB/N MouseC98733FVB/NTac-Tg(Hba-x-v-Ha-ras)TG.ACLedA transgenic mouse with background strain FVB/Ncontaining a mouse Hba-x promoter coupled to anHras1 coding sequence with activating mutations atG<strong>12</strong> (G<strong>12</strong>R) and A59 (A59T) followed by a SV40polyadenylation signal. (NCI)FVB/NTac-Tg(Hba-x-v-Ha-ras)TG.ACLedC77102 GOTTINGEN The smallest of the common miniature breeds, thisbreed is 10-14kg at sexual maturity with a shortenedsnout and rounded appearance. The Gottingen pig haswhite skin and hair. It is used in a variety ofapplications in biomedical research includingcardiovascular studies, and its small size makes it anideal animal model due to its relative ease of handlingand smaller housing requirements. (NCI)C77103 HAMPSHIRE One of the oldest original early American pig strains,the Hampshire pig originated from the Old Englishbreed and was imported to North American in themid-1800s. The Hampshire pig has black skin and haircovering most of its body with a white portion of skincovering its front limbs and back. The Hampshire pig isone of the larger pig breeds used in biomedicalresearch. (NCI)C77104 HANFORD The largest of the miniature breeds, it reaches 25-40kgat sexual maturity. The Hanford pig is white with anelongated snout and has the largest heart and bloodvessels of all pig breeds. It is used in biomedicalresearch, among other things, in the testing ofimplantable devices in human cardiovascular research.(NCI)Gottingen PigHampshire PigHanford PigSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 368 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77530 - STRAIN - Strain/SubstrainCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77090 HARTLEY Albino outbred guinea pig belonging to the English(short-haired) breed. The Hartley guinea pig wasimported from the Medical Research Council,Millhill, England, to Charles River in 1968 forpropagation. The Hartley Guinea Pig has a white coatcolor (acromelanic albino) and red eyes and requires anutritional source of vitamin C to sustain normalfunction.Hartley Guinea PigC77089HARTLEY ALBINOHAIRLESSDerived from inbred Hartley stocks at the EastmanKodak Company and Montreal's Institute ArmandFrappier, having undergone spontaneous mutation thatled to hairlessness and athymicity. The mutation thatspawned the Hartley Hairless Guinea Pig was thenre-derived at Charles River to restore thymus functionwhile maintaining hairlessness.Hartley Albino Hairless Guinea PigC76187 ICO:OFA (SD) A hairless Sprague-Dawley rat from the Charles Riveraffiliate IFFA Credo (Labresle, France). (NCI)C76366 JAPANESE WHITE A white colored rabbit characterized by efficientsuperovulation and spontaneous formation oflymphoma. It is used as an animal model forGuillan-Barre syndrome in humans, toxicology,virology. (NCI)C77094 LAKEVIEW LVG Derived from the original group of Syrian hamsterscaptured by Dr. Israel Aharoni in 1930 and importedinto the United States in 1938, followed by colonyderivation at Lakeview in 1949 and 1951 and toCharles River in 1969, where the strain is propagatedtoday.C77105 LANDRACE-CROSS Developed in Denmark by crossing native pigs with theLarge White pig breed. The Landrace pig was importedinto the UK in 1949 and disseminated worldwidebeginning in the 1950s. This breed is characterized bywhite skin and the absence of black hair as well as lopears and a long middle, light forequarter. The Landracebreed is susceptible to Porcine stress syndrome andmalignant hyperthermia under anesthetic. This is oneof the largest breeds in use in biomedical research.(NCI)C77098 LEGHORNS A small, commonly white-colored breed of poultrythat is renowned for its ability to produce up to 300chalk white eggs per year. The fully-grown leghornchicken averages 3-6 pounds in weight and ischaracterized by being noisy, flighty, and easilyexcited. The leghorn has a lifespan of 5-11 years in thewild. In pre-clinical research, the leghorn is aconsistent provider of eggs for embryonic, angiogenic,and vasculogenic research. (NCI)C76188 LONG EVANS Derived by Long and Evans (1915) by crossing femaleWistar rats with a wild gray male, the Long-Evans ratwas disseminated to Charles River from CanadianBreeding Farm and Laboratories (1978). This outbredrat breed is white with a black or brown hood.OFA SD, Rat StrainJapanese White RabbitLVG HamsterLandrace PigLeghorn ChickenLE, Rat StrainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 369 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77530 - STRAIN - Strain/SubstrainCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC77106 MICROPIG A Yucatan or other pig breed that is bred specificallyfor its small size. The micropig weighs between14-20kg at sexual maturity. (NCI)C77107 MINIPIG A Yucatan, Gottingen, or other pig breed that is bredspecifically for its small size. The Minipig weighsbetween 20-30kg at sexual maturity. (NCI)C77100 MONGOLIAN A rodent belonging to subfamily Gerbillinae, Merionesunguiculatus.MicropigMinipigMongolian GerbilC53951 MONGREL A dog that is not purebred. Mix BreedC77109 NEW ZEALAND BLACK A New Zealand rabbit with black coat color and coatgenotype Si, VV, E, D, C, B.C77110 NEW ZEALAND HYBRID A rabbit that is derived from a cross between twodifferent New Zealand strains of determinate orindeterminate coat genotype.C77111 NEW ZEALAND RED A New Zealand rabbit with a red or agouti coat and coatgenotype w, VV, en, e.C771<strong>12</strong> NEW ZEALAND WHITE An albino New Zealand rabbit with coat genotype c, VVand eye color genotype np.New Zealand Black RabbitNew Zealand Hybrid RabbitNew Zealand Red RabbitNew Zealand White RabbitC14233 RHESUS A pale brown macaque, Macaca mulatta. Rhesus MonkeyC77099 ROSS A small white-colored broiler breed that averages4-5.5 pounds when fully grown, with females averaging<strong>12</strong>0 eggs laid per year. Two substrains exist of Rosschickens; the Ross 308 and the Ross 708. The Ross308 weighs between 3.8-5.5 pounds and is slightlysmaller than the Ross 708 which can grow to be largerthan 5.5 pounds. The Ross 708 is bred specifically forhigh meat yield and ease in deboning. (NCI)C144<strong>12</strong> SHR The spontaneous hypertensive rat was derived byOkamoto at the Kyoto school of medicine (1963)from a cross between an outbred Wistar Kyoto malewith a significant elevation of blood pressure and afemale Wistar Kyoto with elevated blood pressure.SHR rats develop hypertension spontaneously withoutexception at the age of 7-15 weeks with a systolicblood pressure plateau of about 200 mmHg. Thegenetic basis is polygenic, with at least three majorgenes involved (Tanase and Suzuki 1971, Yen et al1974).Ross ChickenSHR, Rat StrainC91819 SINCLAIR MINIPIG SinclairMiniature Swine;Sinclair S-1MinipigA strain of pig developed by the Hormel Institute at theUniversity of Minnesota in 1949, acquired by theUniversity of Missouri in 1965 and now exclusivelybred at the Sinclair Research Center. This strain of piggrows to be no larger than 70 kg and exhibits multiplecoat colors and patterns. The Sinclair minipig is usedin biomedical research for a variety of applications.Sinclair MinipigC98782 SKH1-Hr hr An uncharacterized and non-pedigreed hairless albinomouse strain that is immunocompetent and euthymic.(NCI)SKH1-Hr hrSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 370 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77530 - STRAIN - Strain/SubstrainCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC76189 SPRAGUE-DAWLEY Derived from Wistar rats at Sprague-Dawley farms,this rat strain is characterized by a calm temperamentwhich lends itself to ease of handling. This rat strainhas the following anatomical features: absentgallbladder, a one-lobed left lung and a four-lobedright lunch, the inability to vomit, and the productionof dark colored eye secretions during periods ofstress.C77114 SQUIRREL A small diurnal primate with nails instead of claws,Saimiri sciureus.C77095 SYRIAN A captive hamster strain derived from a mother andeight pups that were captured in the wild in Aleppo,Syria by Dr. Israel Aharoni in 1930.C76190 WISTAR An outbred strain of albino brown rat, this strain wasdeveloped at the Wistar Institute by Donaldson,Greenman, and King (1906). The Wistar rat has a widehead, long ears, and its tail length is always less than itsbody length. A wide variety of rat inbred strains havebeen derived from the Wistar.C14390 WISTAR FURTH A Wistar substrain derived by Furth (1945), this inbredrat strain is a white albino with pink eyes, genotype c.The Wistar Furth rat carries a heteropyenotic Ychromosome that is used as a cellular marker.C76191 WISTAR HAN A Wistar substrain established in Hanover, Germany(1964), this rat breed is a white albino with pink eyes,genotype c.C76192 WISTAR KYOTO An outbred Wistar substrain derived at Kyoto schoolof medicine and disseminated to the NIH in 1971 andfinally to Charles River in 1974. The Wistar Kyoto is awhite albino with pink eyes, genotype c.C76193 WISTAR WU A Wistar substrain that was disseminated to GlaxoLaboratory (UK) from the Wistar Institute inPhiladelphia in 1933, then to the Dutch Institution forNutrition (Amsterdam, The Netherlands) andmaintained by Unilever Company (Vlaardingen, TheNetherlands) from 1941. This strain is nowdisseminated by Harland Nederland. The WistarUnilever rat is an albino, genotype c and pink eyes.C77108 YUCATAN Originating from Mexico and Central America, thisbreed has a straight back and no potbelly, short snout,sparse hair coat and medium size ears. The Yucatan pigis slate gray to black in color. Its uses in biomedicalresearch are varied and include diabetes research,cardiovascular research, angiogenesis, andophthalmological research among others. (NCI)C91817 YUCATAN MICROPIG A strain of Yucatan pig that weighs less than 55 kgwhen full grown. It was developed at Colorado StateUniversity in 1978 and is used extensively inbiomedical research.SD, Rat StrainSquirrel MonkeySyrian HamsterWIST, Rat StrainWF, Rat StrainWH, Rat StrainWKY, Rat StrainWistar Unilever, Rat StrainYucatan PigYucatan MicropigSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 371 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C77530 - STRAIN - Strain/SubstrainCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC91818 YUCATAN MINIPIG YucatanMiniature SwineA strain of Yucatan pig that is found in the wild inCosta Rica and Mexico. It is a hairless, black or greycolored swine and weighs less than 70 kilograms atadulthood. It is used extensively in biomedicalresearch.Yucatan MinipigC76194 ZUCKER Derived from a spontaneous mutation in the leptinreceptor that appeared in a 13M rat colony bred at theZucker Laboratory of Comparative Pathology (Stow,MA), genotype leprfa.Z, Rat StrainSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 372 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90007 - STSPRM - <strong>SEND</strong> Trial Summary Parameter Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC25150 Age Age How long something has existed; elapsed time sincebirth. (NCI)AgeC90352 Age Text Age Text A textual representation of a chronological age. (NCI) Age TextC50400 Age Unit Age Unit Those units of time that are routinely used to expressthe age of a person. (NCI)Age UnitC90354 Alternate Study ID Alternate StudyIDA backup sequence of characters used to identify astudy. (NCI)Alternate Study IdentifierC83216 Arm Code Arm Code A character or string that represents a planned arm of atrial or study.C90359 Associated Study Associated Study An indication that one study is related to another.(NCI)C90364 Basal Diet Basal Diet The fundamental nutritional components thatconstitute an organism's daily intake of foodstuffs.(NCI)C90366 Bedding Bedding That which comprises the place where a subject sleeps.(NCI)C90365 Bedding Change Bedding Change A replacement of the existing materials that make upthe sleeping area of a subject. (NCI)Planned Arm CodeAssociated StudyBasal DietBedding MaterialBedding ChangeC90372 Class Of Compound Class OfCompoundA classification of the type of trial or study agent.Class of Trial AgentC49647 Control Type Control Type Comparator against which the study treatment isevaluated (e.g., concurrent (placebo, no treatment,dose-response, active), external (historical, publishedliterature).Control TypeC25488 Dose Level Dose Level;Dose perAdministrationThe amount of drug administered to a patient or testsubject at one time or the total quantity administered.[AMA Manual of Style] (CDISC Glossary)DoseC73558 Dose Units Dose Units The unit of measure for the dosage form. Dosage Form UnitC90378 Dosing Duration Dosing Duration The interval of time over which a course of dosesoccurs. (NCI)C90377 Drinking Water Drinking Water The type of drinking water that is planned to beprovided to the subjects in a set (e.g., tap water,acidified, reverse osmosis, etc.).Duration of DosingDrinking WaterC90379End Date/Time Of DoseIntervalEnd Date/TimeOf Dose IntervalThe date and time at which the dosing intervalconcludes. (NCI)End Date Time Of Dose IntervalC90380 Environmental Temperature EnvironmentalTemperatureThe temperature of the surroundings. (NCI)Environmental TemperatureC90381Environmental TemperatureUnitsEnvironmentalTemperatureUnitsThe units of measure that are used to express thetemperature of the surroundings. (NCI)Environmental Temperature UnitsC90382 Experimental End Date ExperimentalEnd DateC90487 Experimental Start Date ExperimentalStart DateExperimental completion date means the last date onwhich data are collected from the study. (OECD)Experimental starting date means the date on which thefirst study specific data are collected. (OECD)Experiment End DateExperiment Start DateSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 373 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90007 - STSPRM - <strong>SEND</strong> Trial Summary Parameter Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90383 Feeding Regimen Feeding Regimen A plan that specifies a diet, amount and schedule ofnutritional intake .Feeding RegimenC90389Good Laboratory PracticeTypeGood LaboratoryPractice TypeA quality system concerned with the organizationalprocess and the conditions under which non-clinicalhealth and environmental safety studies are planned,performed, monitored, recorded, archived andreported. (OECD)Good Laboratory Practice TypeC90391 Group Label Group Label Alpha-numeric character(s) assigned to identify aparticular collection of subjects possessing commoncharacteristic(s).C90394 Housing Group Housing Group A classification of a group of animals based upon theirshared living space.Group LabelHousing GroupC90395 Housing Humidity HousingHumidityC90396 Housing Humidity Units HousingHumidity UnitsThe amount of water vapor in the air of a living space.The units of measure that are used to express thehumidity of a living space.Housing HumidityHousing Humidity UnitsC90397 Housing Type Housing Type The classification of a living space. Housing TypeC90398 IACUC Number IACUC Number The animal welfare assurance number issued by theNIH Office of Laboratory Animal Welfare (OLAW)after research protocols and evaluations are reviewedby the Institutional Animal Care and Use Committee(IACUC). (NCI)IACUC NumberC41161Investigational Therapy orTreatmentInvestigationalTherapy orTreatmentThe investigational product under study.Protocol AgentC90419 Light Cycle Light Cycle The period of light that a subject is exposed to in aperiod of time, usually expressed as the amount oftime in a 24 hour cycle.Light CycleC90422 Method Of Identification Method OfIdentificationC90423 Method Of Termination Method OfTerminationThe mechanism by which the test subject is identified.The mechanism or means by which a life is ended.Method Of IdentificationMethod of Termination of LifeC90437Planned Number of FemaleSubjectsPlanned Numberof FemaleSubjectsThe intended quantity of female subjects.Planned Number of Female SubjectsC90438Planned Number of MaleSubjectsPlanned Numberof Male SubjectsThe intended quantity of male subjects.Planned Number of Male SubjectsC95106 Planned Number of Subjects Planned Numberof SubjectsThe planned number of subjects to be entered in anonclinical study.Planned Number of NonclinicalSubjectsC92645Primary Treatment CASRegistry NumberPrimaryTreatment CASRegistry NumberThe Chemical Abstract Service registry number of theinvestigational product (test article).Study Agent CAS Registry NumberC92646Primary Treatment UniqueIngredient IDPrimaryTreatmentUniqueIngredient IDThe Unique Ingredient Identifier of the investigationalproduct (test article).Study Agent Unique IngredientIdentifierC19924 Principal Investigator PrincipalInvestigatorAn investigator who is responsible for defined aspectsof a study, as specified in the study protocol.Principal InvestigatorSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 374 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90007 - STSPRM - <strong>SEND</strong> Trial Summary Parameter Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90439 Project License Number Project LicenseNumberC90446 Recovery Period Recovery Period;RecoverySacrifice PeriodC38114 Route of Administration Route ofAdministrationThe identifier assigned to a project that conveys aparticular authorization. (NCI)The duration from the end of dosing to the finalsacrifice of the subject. (NCI)The course by which a substance was administered inorder to reach the site of action in the body.Project License NumberRecovery Sacrifice PeriodRoute of AdministrationC96370<strong>SEND</strong> Controlled<strong>Terminology</strong> Version<strong>SEND</strong>Controlled<strong>Terminology</strong>VersionThe version of the Standard for the Exchange ofNonclinical Data Controlled <strong>Terminology</strong> that is beingused in the study.Standard for the Exchange ofNonclinical Data Controlled<strong>Terminology</strong> VersionC90458<strong>SEND</strong> Implementation GuideVersion<strong>SEND</strong> IGVersionThe version of the Standard for the Exchange ofNonclinical Data Implementation Guide that is beingused in the study.Standard for the Exchange ofNonclinical Data ImplementationGuide VersionC90455 Set Label Set Label Character(s) assigned to identify a particular set ofsubjects or ideas. (NCI)Set LabelC49696 Sex Of Participants Sex OfParticipantsThe specific sex, either male, female, or mixed of thesubject group being studied. (NCI)Sex of Study GroupC83081 Site Identifier Site Identifier A sequence of characters used to identify, name, orcharacterize the study site. (NCI)C96433 Species Species The common name for an animal used as the testsystem on a study (e.g., dog, monkey, mouse, rabbit,rat).Study Site Identifier<strong>SEND</strong> Test System Common NameC90463 Sponsor's Monitor Sponsor'sMonitorC90457 Sponsor's Reference ID Sponsor'sReference IDC90456 Sponsor-Defined Group Code Sponsor-DefinedGroup CodeC95107 Sponsoring Organization SponsoringOrganizationThe individual working for the sponsor responsible foroverseeing the activities of the study.Character(s) used to identify a specific sponsor.Alpha-numeric character(s) assigned by the sponsor toidentify a particular collection of subjects possessingcommon characteristic(s)An entity that is responsible for the initiation,management, and/or financing of a nonclinical study.(NCI)Study Sponsor MonitorSponsor Reference IdentifierSponsor Defined Group CodeNonclinical Study SponsorC90459Start Date/Time Of DoseIntervalStart Date/TimeOf Dose IntervalThe date and time of the beginning of a dosing interval.(NCI)Start Date Time Of Dose IntervalC96373 Strain/Substrain Strain/Substrain The vendor-supplied species/strain/substraindesignation for the test system under study. It maycombine the species, background strain, substrain, andassociated genetic modifications as supplied by thevendor (e.g. FISCHER 344, SPRAGUE-DAWLEYIGS, WISTAR Kyoto, BEAGLE, CYNOMOLGUS,RHESUS and CHIMPANZEE).<strong>SEND</strong> Test System StrainC90460 Strain/Substrain Details Strain/SubstrainDetailsAdditional clarifying details regarding the test systemunder study, such as a description of a phenotypicalteration associated with the specific geneticmodification captured or collected in theSTRAIN/SUBSTRAIN variable.Strain Substrain DetailsC90461 Study Category Study Category The classification of the study. (NCI) Study CategorySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 375 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90007 - STSPRM - <strong>SEND</strong> Trial Summary Parameter Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC95082 Study Design Study Design A plan detailing how a trial or study will be performedin order to represent the phenomenon underexamination, to answer the research questions thathave been asked, and defining the methods of dataanalysis. Study design is driven by the researchhypothesis being posed, studysubject/population/sample available,logistics/resources: technology, support, networking,collaborative support, etc.C51878 Study Director Study Director A person who has overall responsibility for thetechnical conduct of a study, as well as for theinterpretation, analysis, documentation and reportingof results, and represents the single point of studycontrol. (FDA)Nonclinical Study DesignStudy ChairC99156 Study End Date Study End Date;StudyCompletion DateC95104 Study Is Randomized Study IsRandomizedThe date on which the final report is signed by thestudy director. Also known as Study Completion Date.(FDA)The process of assigning nonclinical study subjects totreatment or control groups using an element ofchance to determine the assignments in order toreduce bias. (NCI)Study End DateNonclinical RandomizationC95105 Study Length Study Length The anticipated length of a nonclinical study measuredas a unit of time. (NCI)Nonclinical Study LengthC99157 Study Start Date Study Start Date;Study InitiationDateThe date on which the protocol is signed by the studydirector. Also known as Study Initiation Date. (FDA)Study Start DateC95108 Study Title Study Title The name of a nonclinical study. Nonclinical Study TitleC92644 Study Type Study Type The type of nonclinical study performed e.g. SingleDose, Repeat Dose. (NCI)Nonclinical Study TypeC90467 Test Facility Country Test FacilityCountryC90468 Test Facility Location Test FacilityLocationThe country of the place in which a nonclinicallaboratory study takes place, i.e., actually uses the testarticle in a test system. Testing facility includes anyestablishment required to register under section 510of the act that conducts nonclinical laboratory studiesand any consulting laboratory described in section 704of the act that conducts such studies. Testing facilityencompasses only those operational units that arebeing or have been used to conduct nonclinicallaboratory studies. (FDA)The geographic area of the place in which a nonclinicallaboratory study takes place, i.e., actually uses the testarticle in a test system. Testing facility includes anyestablishment required to register under section 510of the act that conducts nonclinical laboratory studiesand any consulting laboratory described in section 704of the act that conducts such studies. Testing facilityencompasses only those operational units that arebeing or have been used to conduct nonclinicallaboratory studies. (FDA)Test Facility CountryTest Facility LocationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 376 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90007 - STSPRM - <strong>SEND</strong> Trial Summary Parameter Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90469 Test Facility Name Test FacilityNameThe name of the place in which a nonclinicallaboratory study takes place, i.e., actually uses the testarticle in a test system. Testing facility includes anyestablishment required to register under section 510of the act that conducts nonclinical laboratory studiesand any consulting laboratory described in section 704of the act that conducts such studies. Testing facilityencompasses only those operational units that arebeing or have been used to conduct nonclinicallaboratory studies. (FDA)Test Facility NameC90470 Test Site Country Test Site Country The country in which a phase(s) of a study isconducted. (OECD)Test Site CountryC90471 Test Site Location Test SiteLocationThe geographic location(s) at which a phase(s) of astudy is conducted. (OECD)Test Site LocationC90472 Test Site Name Test Site Name The name of the location(s) at which a phase(s) of astudy is conducted. (OECD)Test Site NameC90473 Test Subject Supplier Test SubjectSupplier; TestSubject SupplierNameC90474 Test Subject Supplier Site Test SubjectSupplier SiteC90399 Time to Interim Sacrifice Time to InterimSacrificeC90466 Time to Terminal Sacrifice Time to TerminalSacrificeC90477 Toxicokinetic Description ToxicokineticDescriptionC927 Treatment Vehicle TreatmentVehicleThe name of the organization that supplied the testsubjects.The geographic location of the organization thatsupplied the test subjects.The planned duration from the start of dosing to theinterim sacrifice of the subject. (NCI)The duration from the start of dosing to the finalsacrifice of the subject. (NCI)Designation that a set will have samples drawn fortoxicokinetic analysis.A carrier or inert medium used as a solvent (or diluent)in which a medicinally active agent is formulated andor administered. (NCI)Test Subject SupplierTest Subject Supplier SiteInterim Sacrifice PeriodTerminal Sacrifice PeriodToxicokinetic DescriptionDrug VehicleC90486 Water Delivery Water Delivery The mechanism by which water is made available.(NCI)Water DeliverySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 377 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90009 - STSPRMCD - <strong>SEND</strong> Trial Summary Parameter Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC25150 AGE Age How long something has existed; elapsed time sincebirth. (NCI)AgeC90352 AGETXT Age Text A textual representation of a chronological age. (NCI) Age TextC50400 AGEU Age Unit Those units of time that are routinely used to expressthe age of a person. (NCI)Age UnitC90354 ALTSTDID Alternate StudyIDA backup sequence of characters used to identify astudy. (NCI)Alternate Study IdentifierC83216 ARMCD Arm Code A character or string that represents a planned arm of atrial or study.C90359 ASOCSTDY Associated Study An indication that one study is related to another.(NCI)C90365 BEDCHNG Bedding Change A replacement of the existing materials that make upthe sleeping area of a subject. (NCI)C90366 BEDDING Bedding That which comprises the place where a subject sleeps.(NCI)Planned Arm CodeAssociated StudyBedding ChangeBedding MaterialC90372 CLASS Class OfCompoundA classification of the type of trial or study agent.Class of Trial AgentC90364 DIET Basal Diet The fundamental nutritional components thatconstitute an organism's daily intake of foodstuffs.(NCI)C90378 DOSDUR Dosing Duration The interval of time over which a course of dosesoccurs. (NCI)Basal DietDuration of DosingC90379 DO<strong>SEND</strong>TC End Date/TimeOf Dose IntervalC90459 DOSSTDTC Start Date/TimeOf Dose IntervalC90380 ENVTEMP EnvironmentalTemperatureC90381 ENVTEMPU EnvironmentalTemperatureUnitsC90382 EXPENDTC ExperimentalEnd DateC90487 EXPSTDTC ExperimentalStart DateThe date and time at which the dosing intervalconcludes. (NCI)The date and time of the beginning of a dosing interval.(NCI)The temperature of the surroundings. (NCI)The units of measure that are used to express thetemperature of the surroundings. (NCI)Experimental completion date means the last date onwhich data are collected from the study. (OECD)Experimental starting date means the date on which thefirst study specific data are collected. (OECD)End Date Time Of Dose IntervalStart Date Time Of Dose IntervalEnvironmental TemperatureEnvironmental Temperature UnitsExperiment End DateExperiment Start DateC90383 FEEDREG Feeding Regimen A plan that specifies a diet, amount and schedule ofnutritional intake .Feeding RegimenC90389 GLPTYP Good LaboratoryPractice TypeA quality system concerned with the organizationalprocess and the conditions under which non-clinicalhealth and environmental safety studies are planned,performed, monitored, recorded, archived andreported. (OECD)Good Laboratory Practice TypeC90391 GRPLBL Group Label Alpha-numeric character(s) assigned to identify aparticular collection of subjects possessing commoncharacteristic(s).Group LabelSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 378 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90009 - STSPRMCD - <strong>SEND</strong> Trial Summary Parameter Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90394 HOUSEGRP Housing Group A classification of a group of animals based upon theirshared living space.Housing GroupC90397 HOUSETYP Housing Type The classification of a living space. Housing TypeC90395 HUMIDT HousingHumidityC90396 HUMIDTU HousingHumidity UnitsThe amount of water vapor in the air of a living space.The units of measure that are used to express thehumidity of a living space.Housing HumidityHousing Humidity UnitsC90398 IACUC IACUC Number The animal welfare assurance number issued by theNIH Office of Laboratory Animal Welfare (OLAW)after research protocols and evaluations are reviewedby the Institutional Animal Care and Use Committee(IACUC). (NCI)IACUC NumberC90422 IDMETH Method OfIdentificationC90399 INTSAC Time to InterimSacrificeThe mechanism by which the test subject is identified.The planned duration from the start of dosing to theinterim sacrifice of the subject. (NCI)Method Of IdentificationInterim Sacrifice PeriodC90419 LIGHT Light Cycle The period of light that a subject is exposed to in aperiod of time, usually expressed as the amount oftime in a 24 hour cycle.Light CycleC90423 MTHTRM Method OfTerminationC19924 PINV PrincipalInvestigatorC90437 PLANFSUB Planned Numberof FemaleSubjectsC90438 PLANMSUB Planned Numberof Male SubjectsC90439 PPL Project LicenseNumberC90446 RECSAC Recovery Period;RecoverySacrifice PeriodC38114 ROUTE Route ofAdministrationC90460 SBSTRAIN Strain/SubstrainDetailsThe mechanism or means by which a life is ended.An investigator who is responsible for defined aspectsof a study, as specified in the study protocol.The intended quantity of female subjects.The intended quantity of male subjects.The identifier assigned to a project that conveys aparticular authorization. (NCI)The duration from the end of dosing to the finalsacrifice of the subject. (NCI)The course by which a substance was administered inorder to reach the site of action in the body.Additional clarifying details regarding the test systemunder study, such as a description of a phenotypicalteration associated with the specific geneticmodification captured or collected in theSTRAIN/SUBSTRAIN variable.Method of Termination of LifePrincipal InvestigatorPlanned Number of Female SubjectsPlanned Number of Male SubjectsProject License NumberRecovery Sacrifice PeriodRoute of AdministrationStrain Substrain DetailsSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 379 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90009 - STSPRMCD - <strong>SEND</strong> Trial Summary Parameter Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC95082 SDESIGN Study Design A plan detailing how a trial or study will be performedin order to represent the phenomenon underexamination, to answer the research questions thathave been asked, and defining the methods of dataanalysis. Study design is driven by the researchhypothesis being posed, studysubject/population/sample available,logistics/resources: technology, support, networking,collaborative support, etc.C90455 SETLBL Set Label Character(s) assigned to identify a particular set ofsubjects or ideas. (NCI)Nonclinical Study DesignSet LabelC49696 SEXPOP Sex OfParticipantsThe specific sex, either male, female, or mixed of thesubject group being studied. (NCI)Sex of Study GroupC83081 SITEID Site Identifier A sequence of characters used to identify, name, orcharacterize the study site. (NCI)C95105 SLENGTH Study Length The anticipated length of a nonclinical study measuredas a unit of time. (NCI)Study Site IdentifierNonclinical Study LengthC96370 SNDCTVER <strong>SEND</strong>Controlled<strong>Terminology</strong>VersionC90458 SNDIGVER <strong>SEND</strong> IGVersionThe version of the Standard for the Exchange ofNonclinical Data Controlled <strong>Terminology</strong> that is beingused in the study.The version of the Standard for the Exchange ofNonclinical Data Implementation Guide that is beingused in the study.Standard for the Exchange ofNonclinical Data Controlled<strong>Terminology</strong> VersionStandard for the Exchange ofNonclinical Data ImplementationGuide VersionC96433 SPECIES Species The common name for an animal used as the testsystem on a study (e.g., dog, monkey, mouse, rabbit,rat).<strong>SEND</strong> Test System Common NameC90456 SPGRPCD Sponsor-DefinedGroup CodeC95106 SPLANSUB Planned Numberof SubjectsC90474 SPLRLOC Test SubjectSupplier SiteC90473 SPLRNAM Test SubjectSupplier; TestSubject SupplierNameC90457 SPREFID Sponsor'sReference IDC95104 SRANDOM Study IsRandomizedC95107 SSPONSOR SponsoringOrganizationAlpha-numeric character(s) assigned by the sponsor toidentify a particular collection of subjects possessingcommon characteristic(s)The planned number of subjects to be entered in anonclinical study.The geographic location of the organization thatsupplied the test subjects.The name of the organization that supplied the testsubjects. (NCI)Character(s) used to identify a specific sponsor.The process of assigning nonclinical study subjects totreatment or control groups using an element ofchance to determine the assignments in order toreduce bias. (NCI)An entity that is responsible for the initiation,management, and/or financing of a nonclinical study.(NCI)Sponsor Defined Group CodePlanned Number of NonclinicalSubjectsTest Subject Supplier SiteTest Subject SupplierSponsor Reference IdentifierNonclinical RandomizationNonclinical Study SponsorC92644 SSTYP Study Type The type of nonclinical study performed e.g. SingleDose, Repeat Dose. (NCI)Nonclinical Study TypeSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 380 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90009 - STSPRMCD - <strong>SEND</strong> Trial Summary Parameter Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90461 STCAT Study Category The classification of the study. (NCI) Study CategoryC51878 STDIR Study Director A person who has overall responsibility for thetechnical conduct of a study, as well as for theinterpretation, analysis, documentation and reportingof results, and represents the single point of studycontrol. (FDA)Study ChairC99156 STENDTC Study End Date;StudyCompletion DateThe date on which the final report is signed by thestudy director. Also known as Study Completion Date.(FDA)Study End DateC95108 STITLE Study Title The name of a nonclinical study. Nonclinical Study TitleC90463 STMON Sponsor'sMonitorThe individual working for the sponsor responsible foroverseeing the activities of the study.Study Sponsor MonitorC96373 STRAIN Strain/Substrain The vendor-supplied species/strain/substraindesignation for the test system under study. It maycombine the species, background strain, substrain, andassociated genetic modifications as supplied by thevendor (e.g. FISCHER 344, SPRAGUE-DAWLEYIGS, WISTAR Kyoto, BEAGLE, CYNOMOLGUS,RHESUS and CHIMPANZEE).<strong>SEND</strong> Test System StrainC99157 STSTDTC Study Start Date;Study InitiationDateThe date on which the protocol is signed by the studydirector. Also known as Study Initiation Date. (FDA)Study Start DateC49647 TCNTRL Control Type Comparator against which the study treatment isevaluated (e.g., concurrent (placebo, no treatment,dose-response, active), external (historical, publishedliterature).Control TypeC90467 TFCNTRY Test FacilityCountryC90477 TKDESC ToxicokineticDescriptionC90466 TRMSAC Time to TerminalSacrificeC41161 TRT InvestigationalTherapy orTreatmentC92645 TRTCAS PrimaryTreatment CASRegistry NumberC25488 TRTDOS Dose Level;Dose perAdministrationThe country of the place in which a nonclinicallaboratory study takes place, i.e., actually uses the testarticle in a test system. Testing facility includes anyestablishment required to register under section 510of the act that conducts nonclinical laboratory studiesand any consulting laboratory described in section 704of the act that conducts such studies. Testing facilityencompasses only those operational units that arebeing or have been used to conduct nonclinicallaboratory studies. (FDA)Designation that a set will have samples drawn fortoxicokinetic analysis.The duration from the start of dosing to the finalsacrifice of the subject. (NCI)The investigational product under study.The Chemical Abstract Service registry number of theinvestigational product (test article).The amount of drug administered to a patient or testsubject at one time or the total quantity administered.[AMA Manual of Style] (CDISC Glossary)Test Facility CountryToxicokinetic DescriptionTerminal Sacrifice PeriodProtocol AgentStudy Agent CAS Registry NumberDoseC73558 TRTDOSU Dose Units The unit of measure for the dosage form. Dosage Form UnitSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 381 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90009 - STSPRMCD - <strong>SEND</strong> Trial Summary Parameter Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92646 TRTUNII PrimaryTreatmentUniqueIngredient IDC927 TRTV TreatmentVehicleThe Unique Ingredient Identifier of the investigationalproduct (test article).A carrier or inert medium used as a solvent (or diluent)in which a medicinally active agent is formulated andor administered. (NCI)Study Agent Unique IngredientIdentifierDrug VehicleC90470 TSCNTRY Test Site Country The country in which a phase(s) of a study isconducted. (OECD)Test Site CountryC90471 TSLOC Test SiteLocationThe geographic location(s) at which a phase(s) of astudy is conducted. (OECD)Test Site LocationC90472 TSNAM Test Site Name The name of the location(s) at which a phase(s) of astudy is conducted. (OECD)Test Site NameC90468 TSTFLOC Test FacilityLocationC90469 TSTFNAM Test FacilityNameThe geographic area of the place in which a nonclinicallaboratory study takes place, i.e., actually uses the testarticle in a test system. Testing facility includes anyestablishment required to register under section 510of the act that conducts nonclinical laboratory studiesand any consulting laboratory described in section 704of the act that conducts such studies. Testing facilityencompasses only those operational units that arebeing or have been used to conduct nonclinicallaboratory studies. (FDA)The name of the place in which a nonclinicallaboratory study takes place, i.e., actually uses the testarticle in a test system. Testing facility includes anyestablishment required to register under section 510of the act that conducts nonclinical laboratory studiesand any consulting laboratory described in section 704of the act that conducts such studies. Testing facilityencompasses only those operational units that arebeing or have been used to conduct nonclinicallaboratory studies. (FDA)Test Facility LocationTest Facility NameC90377 WATER Drinking Water The type of drinking water that is planned to beprovided to the subjects in a set (e.g., tap water,acidified, reverse osmosis, etc.).C90486 WTRDLVRY Water Delivery The mechanism by which water is made available.(NCI)Drinking WaterWater DeliverySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 382 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90005 - TFTEST - Tumor Findings Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90479 Tumor Examination TumorExaminationAn assessment or evaluation of a neoplastic mass.(NCI)Tumor ExaminationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 383 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C90006 - TFTESTCD - Tumor Findings Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC90479 TUMEX TumorExaminationAn assessment or evaluation of a neoplastic mass.(NCI)Tumor ExaminationSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 384 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC25613 % Percentage A fraction or ratio with 100 understood as thedenominator. (NCI)PercentageC48571 %(v/v) Percent Volumeper VolumeC48527 %(w/v) Percent Weightper VolumeC48528 %(w/w) Percent Weightper WeightC102695 /100 HPFs per 100 HighPowered FieldsC96619 /HPF per HighPowered FieldC96620 /LPF per LowPowered FieldA percent ratio of volume to volume, defined by theequation: [volume of solute (in ml)/ volume ofsolution (in ml)](100), typically used for admixturesof solutions.(NCI)A percent ratio of weight to volume, defined by theequation: [weight of solute (in gm)/volume of solution(in dl)](100). Since the numerator and denominator ofthis ratio have different units, it is not a truepercentage. A 1% w/v solution is defined as being 1gram of solute dissolved in 100 milliliters ofsolvent.(NCI)A percent ratio of weight to weight, defined by theequation: [weight of solute (in gm)/weight of solution(in gm)](100).(NCI)A unit of measurement of the number of entities perunit of area equal to one hundred high powered fields.A unit of measure equal to the instances of an entityper visual field of a microscope set to its maximummagnification power.A unit of measure equal to the instances of an entityper visual field of a microscope set to its minimummagnification power.Percent Volume per VolumePercent Mass per VolumePercent Mass per MassPer 100 High Powered FieldsPer High Powered FieldPer Low Powered FieldC25473 /day Per Day A frequency rate of occurrences of something within aperiod of time equal to twenty-four hours. (NCI)C66966 /h Per Hour A rate of occurrences of something within a period oftime equal to sixty minutes.C103452 /mL Per Milliliter A volume unit equal to one milliliter used as adenominator to build a derived unit expressed as aratio. (NCI)/day/hPer MilliliterC64498 /month Every Month;Per MonthEvery month. (NCI)MonthlyC67254 /uL Per Microliter A volume unit equal to one microliter used as adenominator to build a derived unit expressed as aratio. (NCI)/uLC67069 /wk Every week; PerWeekC71185 100 IU/mL 100 Internationalunits/MilliliterEvery week. (NCI)A unit of arbitrary substance concentration (biologicactivity concentration) defined as the concentration ofone hundred international units per one milliliter ofsystem volume.(NCI)Weekly100 International Units per MilliliterC67308 10^<strong>12</strong>/L 10^6/uL A unit of measurement equal to 10 to the twelfthpower of the number of entities per unit of volumeequal to one liter.Million per MicroliterC68895 10^3 CFU Thousand ColonyForming Units;Thousand CFUA unit of measurement of viable bacterial numbersequal to 10 to the third power colony forming units.Thousand Colony Forming UnitsSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 385 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC68899 10^3 CFU/g Thousand ColonyForming Unitsper Gram;Thousand CFU/gC68903 10^3 CFU/mL Thousand ColonyForming Unitsper Milliliter;ThousandCFU/mLA unit of measurement of viable bacterial organisms ina unit mass of substance of interest defined as thenumber of 10 to the third power colony forming unitsin one gram of substance.A unit of measurement of viable bacterial organisms ina unit volume of substance of interest defined as thenumber of 10 to the third power colony forming unitsin one milliliter of substance.Thousand Colony Forming Units perGramThousand Colony Forming Units perMilliliterC98788 10^3 DNA copies/mL A unit of measurement equal to 10 to the third powerof the number of deoxyribonucleic acid (DNA) copiesper unit of volume equal to one milliliter.C98790 10^3 RNA copies/mL A unit of measurement equal to 10 to the third powerof the number of ribonucleic acid (RNA) copies perunit of volume equal to one milliliter.C100897 10^3 copies/mL The unit of concentration expressed as the number of10 to the third power copies in unit volume equal toone milliliter. (NCI)Thousand DNA Copies per MilliliterThousand RNA Copies per MilliliterThousand Copies per MilliliterC71187 10^3 organisms ThousandOrganismsC71190 10^3 organisms/g ThousandOrganisms perGram; ThousandOrganisms/gC71195 10^3 organisms/mL ThousandOrganisms perMilliliter;ThousandOrganisms/mLA unit of measure of quantity of organisms expressedin 10 to the third power of organisms.A unit of measure of organism content expressed in 10to the third power of organisms per unit of mass equalto one gram.A unit of measure of organism concentrationexpressed in 10 to the third power of organisms perunit of volume equal to one milliliter.Thousand OrganismsThousand Organisms per GramThousand Organisms per MilliliterC98789 10^3/hpf A unit of measurement equal to 10 to the third powerof the number of entities per unit of area equal to onehigh powered field.C98787 10^4/hpf A unit of measurement equal to 10 to the fourth powerof the number of entities per unit of area equal to onehigh powered field.C98743 10^5/hpf A unit of measurement equal to 10 to the fifth powerof the number of entities per unit of area equal to onehigh powered field.Thousand per High Powered FieldTen Thousand per High PoweredFieldHundred Thousand per High PoweredFieldC68896 10^6 CFU Million ColonyForming Units;Million CFUC68900 10^6 CFU/g Million ColonyForming Unitsper Gram;Million CFU/gC68904 10^6 CFU/mL Million ColonyForming Unitsper Milliliter;Million CFU/mLA unit of measurement of viable bacterial numbersequal to 10 to the sixth power colony forming units.A unit of measurement of viable bacterial organisms ina unit mass of substance of interest defined as thenumber of 10 to the sixth power colony forming unitsin one gram of substance.A unit of measurement of viable bacterial organisms ina unit volume of substance of interest defined as thenumber of 10 to the sixth power colony forming unitsin one milliliter of substance.Million Colony Forming UnitsMillion Colony Forming Units perGramMillion Colony Forming Units perMilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 386 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC98756 10^6 DNA copies/mL A unit of measurement equal to 10 to the sixth powerof the number of deoxyribonucleic acid (DNA) copiesper unit of volume equal to one milliliter.Million DNA Copies per MilliliterC67335 10^6 IU MillionInternationalUnits; Million IUA unit of biological activity equal to 10 to the sixthpower international units.Million International UnitsC98757 10^6 IU/mL A unit of measurement equal to 10 to the sixth powerof the number of international units of an entity perunit of volume equal to one milliliter.C98760 10^6 RNA copies/mL A unit of measurement equal to 10 to the sixth powerof the number of ribonucleic acid (RNA) copies perunit of volume equal to one milliliter.C100898 10^6 copies/mL The unit of concentration expressed as the number of10 to the sixth power copies in unit volume equal toone milliliter. (NCI)Million International Units perMilliliterMillion RNA Copies per MilliliterMillion Copies per MilliliterC71188 10^6 organisms MillionOrganismsC71191 10^6 organisms/g MillionOrganisms perGram; MillionOrganisms/gC71196 10^6 organisms/mL MillionOrganisms perMilliliter;MillionOrganisms/mLC71193 10^6 organisms/mg MillionOrganisms perMilligram;MillionOrganisms/mgA unit of measure of quantity of organisms expressedin 10 to the sixth power of organisms.A unit of measure of organism content expressed in 10to the sixth power of organisms per unit of mass equalto one gram.A unit of measure of organism concentrationexpressed in 10 to the sixth power of organisms perunit of volume equal to one milliliter.A unit of measure of organism content expressed in 10to the sixth power of organisms per unit of mass equalto one milligram.Million OrganismsMillion Organisms per GramMillion Organisms per MilliliterMillion Organisms per MilligramC67452 10^6/L 10^3/mL A unit of measurement equal to 10 to the sixth powerof the number of entities per unit of volume equal toone liter.C98758 10^6/g A unit of measurement equal to 10 to the sixth powerof the number of entities per unit of mass equal to onegram.C98759 10^6/hpf A unit of measurement equal to 10 to the sixth powerof the number of entities per unit of area equal to onehigh powered field.C98786 10^7/L 10^6/dL A unit of measurement equal to 10 to the seventhpower of the number of entities per unit of volumeequal to one liter.Thousand per MilliliterMillion per GramMillion per High Powered FieldTen Million per LiterC68897 10^9 CFU Billion ColonyForming Units;Billion CFUC68901 10^9 CFU/g Billion ColonyForming Unitsper Gram;Billion CFU/gA unit of measurement of viable bacterial numbersequal to 10 to the ninth power colony forming units.A unit of measurement of viable bacterial organisms ina unit mass of substance of interest defined as thenumber of 10 to the ninth power colony forming unitsin one gram of substance.Billion Colony Forming UnitsBillion Colony Forming Units perGramSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 387 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC68905 10^9 CFU/mL Billion ColonyForming Unitsper Milliliter;Billion CFU/mLC71189 10^9 organisms BillionOrganismsC71192 10^9 organisms/g BillionOrganisms perGram; BillionOrganisms/gC71197 10^9 organisms/mL BillionOrganisms perMilliliter;BillionOrganisms/mLC71194 10^9 organisms/mg BillionOrganisms perMilligram;BillionOrganisms/mgC67255 10^9/L 10^3/uL;10^3/mm3C77534 AFU ArbitraryFluorescenceUnitC48473 AMPULE Ampule DosingUnitC102404 APPLICATION ApplicationDosing UnitA unit of measurement of viable bacterial organisms ina unit volume of substance of interest defined as thenumber of 10 to the ninth power colony forming unitsin one milliliter of substance.A unit of measure of quantity of organisms expressedin 10 to the ninth power of organisms.A unit of measure of organism content expressed in 10to the ninth power of organisms per unit of mass equalto one gram.A unit of measure of organism concentrationexpressed in 10 to the ninth power of organisms perunit of volume equal to one milliliter.A unit of measure of organism content expressed in 10to the ninth power of organisms per unit of mass equalto one milligram.A unit of measurement equal to 10 to the ninth powerof the number of entities per unit of volume equal toone liter.Arbitrary unit(s) of fluorescent luminescence. (NCI)A dosing measurement based on the ampule unit.(NCI)A dosing measurement based on the amount ofsubstance applied.Billion Colony Forming Units perMilliliterBillion OrganismsBillion Organisms per GramBillion Organisms per MilliliterBillion Organisms per MilligramBillion per LiterArbitrary Fluorescence UnitsAmpule Dosing UnitApplication Dosing UnitC73686 AU Absorbance Unit A unit of optical density expressed as the absorbanceof light transmitted through the medium on thelogarithmic scale. (NCI)AUC70504 AU/mL Allergy Unit perMilliliterC73687 AU/min Absorbance Unitper MinuteC70500 AgU/mL Antigen Unit perMilliliterUnit of measure of potency of allergenic productexpressed as a number of allergy units per onemilliliter of formulation.(NCI)A unit of a speed of optical density change expressedas a logarithm of absorbance of light transmittedthrough a partially absorbing substance per minute.(NCI)A measure of an antigen potency defined as a numberof antigen units per one milliliter of product.(NCI)Allergy Unit per MilliliterAU/minAntigen Unit per MilliliterC48474 BAG Bag Dosing Unit A dosing measurement based on the bag unit.(NCI) Bag Dosing UnitC48475 BAR Bar Dosing Unit A dosing measurement based on the bar unit.(NCI) Bar Dosing UnitC70505 BAU BioequivalentAllergy UnitA unit used for standardization of an allergenic productbased on evaluation of product potency againstreference standard in combined in vivo (skin test) andin vitro (IgE-based ELISA) testing.(NCI)Bioequivalent Allergy UnitSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 388 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49673 BEATS/MIN Beats per Minute The number of heartbeats measured per minute time.(NCI)Beats per MinuteC48476 BOLUS Bolus DosingUnitC48477 BOTTLE Bottle DosingUnitA dosing measurement based on the bolus unit.(NCI)A dosing measurement based on the bottle unit.(NCI)Bolus Dosing UnitBottle Dosing UnitC48478 BOX Box Dosing Unit A dosing measurement based on the box unit.(NCI) Box Dosing UnitC49674 BREATHS/MIN Breaths perMinuteThe number of breaths (inhalation and exhalation)taken per minute time. (NCI)Breaths per MinuteC42562 Bq Becquerel A unit of activity of a radionuclide, equal to onenuclear disintegration or other nuclear transition froma particular energy state occurring in an amount of aradionuclide during one second-long timeinterval.(NCI)BecquerelC71165 Bq/L Becquerel perLiterC70522 Bq/g Becquerel perGramC70521 Bq/kg Becquerel perKilogramC71167 Bq/mL Becquerel perMilliliter; kBq/L;Kilobecquerelper LiterC70524 Bq/mg Becquerel perMilligram;kBq/g;Kilobecquerelper GramC71166 Bq/uL Becquerel perMicroliter;kBq/mL;Kilobecquerelper Milliliter;MBq/L;Megabecquerelper LiterC70523 Bq/ug Becquerel perMicrogram;kBq/mg;Kilobecquerelper Milligram;MBq/g;Megabecquerelper Gram;Bq/mcgA unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one Becquerel per unit volume equalto one liter.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one Becquerel of the sample with totalmass of one gram.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one Becquerel of the sample with totalmass of one kilogram.(NCI)A unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one Becquerel per unit volume equalto one milliliter or one kilobecquerel per liter.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one Becquerel of the sample with totalmass of one milligram.(NCI)A unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one Becquerel per unit volume equalto one millionth of a liter.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one Becquerel of the sample with totalmass of one microgram.(NCI)Becquerel per LiterBecquerel per GramBecquerel per KilogramBecquerel per MilliliterBecquerel per MilligramBecquerel per MicroliterBecquerel per MicrogramSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 389 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42559 C Degree Celsius A unit of temperature, named after the Swedishastronomer Anders Celsius, who first proposed thetemperature scale designed so that the freezing pointof water is 0 degrees and the boiling point is 100degrees at standard atmospheric pressure. The currentofficial definition of the Celsius sets 0.01 C to be atthe triple point of water and a degree to be 1/273.16 ofthe difference in temperature between the triple pointof water and absolute zero. One degree Celsiusrepresents the same temperature difference as oneKelvin.(NCI)Degree CelsiusC48479 CAN Can Dosing Unit A dosing measurement based on the can unit.(NCI) Can Dosing UnitC102405 CAPFUL Capful DosingUnitC48480 CAPSULE Capsule DosingUnit; capC48481 CARTRIDGE Cartridge DosingUnitC70534 CCID 50 50 Percent CellCulture InfectiveDoseC70535 CCID 50/dose 50 Percent CellCulture InfectiveDose per DoseC68898 CFU/g Colony FormingUnit per GramC68902 CFU/mL Colony FormingUnit perMilliliterA unit of measure equal to the amount that the cap onthe bottle can contain.A dosing measurement based on the capsule unit.(NCI)A dosing measurement based on the cartridgeunit.(NCI)A potency unit defined as a minimal dose of infectiousmaterial at which preparation causes cytopathic effects(changes in the morphology and metabolism of cellculture cells, indicating cell death, due to suspectedinfection) in the 50% of the cell culture-containingvessels inoculated with that dilution of infectiousmaterial.(NCI)A potency unit equal to the potency at which one doseof preparation contains one 50 percent cell cultureinfective dose.(NCI)A unit of measurement of viable bacterial organisms ina unit mass of substance of interest defined as thenumber of colony forming units in one gram ofsubstance.(NCI)A unit of measurement of viable bacterial organisms ina unit volume of substance of interest defined as thenumber of colony forming units in one milliliter ofsubstance.(NCI)Capful Dosing UnitCapsule Dosing UnitCartridge Dosing Unit50 Percent Cell Culture InfectiveDose50 Percent Cell Culture InfectiveDose per DoseColony Forming Unit per GramColony Forming Unit per MilliliterC48483 COAT Coat Dosing Unit A dosing measurement based on the coat unit.(NCI) Coat Dosing UnitC48484 CONTAINER ContainerDosing UnitA dosing measurement based on the containerunit.(NCI)Container Dosing UnitC54703 CUP Cup Dosing Unit A dosing measurement based on the cup unit.(NCI) Cup Dosing UnitC48489 CYLINDER Cylinder DosingUnitA dosing measurement based on the cylinderunit.(NCI)Cylinder Dosing UnitC48466 Ci Curie A unit of radioactivity defined as 3.7 E10 atomicdisintegrations or other nuclear transformations persecond. One Curie is equal to 37 gigabecquerels.(NCI)CurieC71170 Ci/L Curie per Liter;uCi/uL;Microcurie perMicroliterA unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one Curie per unit volume equal toone liter.(NCI)Curie per LiterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 390 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC70528 Ci/g Curie per Gram;uCi/ug;Microcurie perMicrogram;mCi/mg;Millicurie perMilligramC70529 Ci/kg Curie perKilogram;uCi/mg;Microcurie perMilligram;mCi/g;Millicurie perGramC71172 Ci/mL Curie perMilliliterC70531 Ci/mg Curie perMilligram;mCi/ug;Millicurie perMicrogramC71171 Ci/uL Curie perMicroliter;Ci/mcLC70530 Ci/ug Curie perMicrogram;Ci/mcgA unit of specific radioactivity (massic activity) equalto activity of one Curie of the sample with total massof one gram.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one Curie of the sample with total massof one kilogram.(NCI)A unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one Curie per unit volume equal toone milliliter.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one Curie of the sample with total massof one milligram.(NCI)A unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one Curie per unit volume equal toone millionth of a liter.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one Curie of the sample with total massof one microgram.(NCI)Curie per GramCurie per KilogramCurie per MilliliterCurie per MilligramCurie per MicroliterCurie per MicrogramC42550 Coulomb Coulomb A unit of quantity of electricity, equal to the quantityof charge transferred in one second across a conductorin which there is a constant current of oneAmpere.(NCI)C25301 DAYS Day The time for Earth to make a complete rotation on itsaxis; ordinarily divided into twenty-four hours. Thisalso refers to a specific day. (NCI)C70501 DAgU D Antigen Unit A unit of potency of poliovirus vaccine used forpoliomyelitis prevention. The unit is poliovirustype-specific.(NCI)CoulombDayD Antigen UnitC70502 DAgU/mL D Antigen Unitper MilliliterA unit of potency of poliovirus vaccine expressed as anumber of D antigen units per one milliliter of vaccineformulation.(NCI)D Antigen Unit per MilliliterC100899 DIOPTER Diopter A unit of measurement of the optical power of acurved mirror or lens represented by the inverse of thefocal length in meters.DiopterC48490 DISK Disk Dosing Unit A dosing measurement based on the disk unit.(NCI) Disk Dosing UnitC98719 DNA copies/mL DNA Copies perMilliliterThe unit of concentration of deoxyribonucleic acid(DNA) copies expressed as a number of copies in unitvolume equal to one milliliter.DNA Copies per MilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 391 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC48492 DRUM Drum DosingUnitC70532 EID 50 50 PercentEmbryo InfectiveDoseC70533 EID 50/dose 50 PercentEmbryo InfectiveDose per DoseC68875 ELISA unit Enzyme-LinkedImmunosorbentAssay UnitC68876 ELISA unit/dose Enzyme-LinkedImmunosorbentAssay Unit perDoseC68877 ELISA unit/mL Enzyme-LinkedImmunosorbentAssay Unit perMilliliterC96599 EU Ehrlich Units;EU/dLC44277 F DegreeFahrenheitC96649 FEU FibrinogenEquivalent UnitsA dosing measurement based on the drum unit.(NCI)Amount of infectious agent or biological product thatcauses infection in the 50% of embryos such aschicken embryos used in the product potency assay orpathogen activity assay.(NCI)A potency unit for measuring infectious activity of abiologic product or an infectious agent preparationequal to the potency at which one dose of infectiousmaterial contains one 50 percent embryo infectivedose.(NCI)A unit for measuring concentration or/and reactivity ofa test substance (an antigen or antibody of interest) asdefined in the literature reference standard for theparticular quantitative enzyme-linked immunosorbentassay method. The enzyme-linked immunosorbentassay unit is used to express potency ofimmunologically active substances and products, e.g.vaccines.(NCI)A unit for measuring potency of immunologicallyactive substance in a product determined as reactivityin a quantitative immunoassay for particular antigen orantibody and expressed per quantity of preparationused as a single dose.(NCI)A unit for measuring potency of immunologicallyactive substance in a product determined as reactivityin a quantitative immunoassay for particular antigen orantibody and expressed per unit volume equal to onemilliliter.(NCI)A unit of measure equal to one milligram ofurobilinogen per deciliter.The Fahrenheit temperature scale is named after theGerman physicist Gabriel Fahrenheit (1686-1736),who proposed it in 1724. In this scale, the freezingpoint of water is 32 degrees Fahrenheit and the boilingpoint is 2<strong>12</strong> degrees, placing the boiling and meltingpoints of water 180 degrees apart. In this scale adegree Fahrenheit is 5/9ths of a Kelvin (or of a degreeCelsius), and minus 40 degrees Fahrenheit is equal tominus 40 degrees Celsius. (NCI)A unit of measure for the concentration of fibrindegradation products in a sample, calculated basedupon the mass of fibrinogen contained within thatsample.Drum Dosing Unit50 Percent Embryo Infective Dose50 Percent Embryo Infective Doseper DoseEnzyme-Linked ImmunosorbentAssay UnitEnzyme-Linked ImmunosorbentAssay Unit per DoseEnzyme-Linked ImmunosorbentAssay Unit per MilliliterEhrlich UnitDegree FahrenheitFibrinogen Equivalent UnitSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 392 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71321 FINGERTIP UNIT Fingertip Unit An arbitrary dosing unit used predominantly forsemisolid topical formulations such as cream,ointment, paste, etc. One fingertip unit is the amountof a product that is squeezed out from a standard tubewith 5-millimeter diameter nozzle along an adult'sfingertip. A fingertip length is defined from the tip ofthe index finger to the first finger crease. A fingertipdosing unit varies with age and size of the body. Theaverage fingertip unit is equal to approximately 0.5gram for an adult male and 0.4 gram for an adultfemale.(NCI)C42552 Farad Farad A unit of capacitance equal to the capacitance of acapacitor having an equal and opposite charge of onecoulomb on each plate and a potential difference ofone volt between the plates.(NCI)C70513 GBq Gigabecquerel A unit of radioactivity equal to one billion nucleardisintegrations or other nuclear transformations persecond, or to 10E9 Becquerels.(NCI)Fingertip Dosing UnitFaradGigabecquerelC70525 GBq/g Gigabecquerelper Gram;kBq/ug;Kilobecquerelper MicrogramC70527 GBq/mg Gigabecquerelper Milligram;Megabecquerelper Microgram;MBq/mcg;MBq/ugC70526 GBq/ug Gigabecquerelper microgram;MBq/ng;Megabecquerelper nanogram;GBq/mcgA unit of specific radioactivity (massic activity) equalto activity of one gigabecquerel of the sample withtotal mass of one gram.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one gigabecquerel of the sample withtotal mass of one milligram.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one gigabecquerel of the sample withtotal mass of one microgram.(NCI)Gigabecquerel per GramGigabecquerel per MilligramGigabecquerel per MicrogramC68915 Gauss Gauss The unit of magnetic flux density. A field of one Gaussexerts a force on a conductor, placed in the field of 0.1dyne per Ampere of current per centimeter ofconductor. One Gauss represents a magnetic flux ofone Maxwell per square centimeter of cross-sectionperpendicular to the field. One Gauss equals 10-4Tesla.(NCI)C18063 Gy Gray A unit of absorbed radiation dose. One gray is equal toan absorbed dose of one joule per kilogram of matter,or to 100 rads.(NCI)GaussGrayC48498 HOMEOPATHIC DILUTION HomeopathicDilution UnitA dosing measurement based on the homeopathicdilution unit.(NCI)Homeopathic Dilution UnitC25529 HOURS Hour; hr A unit measure of time equal to 3,600 seconds or 60minutes. It is approximately 1/24 of a median day.(NCI)HourC67274 HPF High PowerFieldThe area visible under the maximum magnificationpower of the objective being used inmicroscopy.(NCI)High Power FieldSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 393 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC42558 Henry Henry A unit of electric inductance. A coil with an inductanceof one Henry requires a flux of one Weber for eachAmpere of induced current. If it is the current whichchanges, then the induced field will generate apotential difference within the coil: if the inductanceis one Henry a current change of one Ampere persecond generates a potential difference of one volt.The Henry is a large unit; inductances in practicalcircuits are measured in millihenrys ormicrohenrys.(NCI)C42545 Hz Hertz A unit of frequency equal to one cycle persecond.(NCI)HenryHertzC48499 IMPLANT Implant DosingUnitA dosing measurement based on the implant unit.(NCI)Implant Dosing UnitC48500 IN Inch A traditional unit of length equal to 2.54 centimeters.(NCI)InchC48501 INHALATION InhalationDosing UnitC48579 IU InternationalUnitC67376 IU/L InternationalUnit per Liter;IU/L; mIU/mLC70493 IU/g InternationalUnit per GramC67379 IU/kg InternationalUnit perKilogramC7<strong>12</strong>09 IU/kg/h Internationalunits perKilogram perHourC67377 IU/mL InternationalUnit perMilliliter; kIU/L;KiloInternationalUnit per LiterA dosing measurement based on the inhalationunit.(NCI)The unitage assigned by the WHO (World HealthOrganization) to International BiologicalStandards - substances, classed as biological accordingto the criteria provided by WHO Expert Committee onBiological Standardization (e.g. hormones, enzymes,and vaccines), to enable the results of biological andimmunological assay procedures to be expressed inthe same way throughout the world. The definition ofan international unit is generally arbitrary andtechnical, and has to be officially approved by theInternational Conference for Unification ofFormulae.(NCI)Unit of arbitrary substance concentration (biologicactivity concentration) defined as the concentration ofone international unit per one liter of systemvolume.(NCI)A unit of measure of quantity of substance per unitmass, expressed in terms of the International Unit pergrams.(NCI)An arbitrary unit of substance content expressed ininternational units of biological activity per onekilogram of mass of the system. It is also used as adose calculation unit expressed in international unitsof biological activity per one kilogram of bodymass.(NCI)A dose calculation unit equal to one international unit(an arbitrary unit of biological activity) of a productper one kilogram of body mass administered per unitof time equal to one hour.(NCI)Unit of arbitrary substance concentration (biologicactivity concentration) defined as the concentration ofone international unit per one milliliter of systemvolume.(NCI)Inhalation Dosing UnitInternational UnitInternational Unit per LiterInternational Unit per GramInternational Unit per KilogramInternational Unit per Kilogram perHourInternational Unit per MilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 394 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC67380 IU/mg InternationalUnit perMilligramUnit of arbitrary substance concentration (biologicactivity concentration) defined as the concentration ofone international unit per one milligram ofsubstance.(NCI)International Unit per MilligramC48502 JAR Jar Dosing Unit A dosing measurement based on the jar unit.(NCI) Jar Dosing UnitC42548 Joule Joule A unit of electrical, mechanical, and thermal energy(as well as work and quantity of heat), equal to thework done when the point of application of a force ofone Newton is displaced through a distance of onemeter in the direction of the force or the work donewhen a current of one Ampere passes through aresistance of one ohm for one second. One joule isequal to 0.23889 gram-calorie (mean).(NCI)JouleC48503KALLIKREIN INHIBITORUNITKallikreinInhibitor UnitA dosing measurement based on the Kallikreininhibitor unit.(NCI)Kallikrein Inhibitor UnitC48504 KIT Kit Dosing Unit A dosing measurement based on the kit unit.(NCI) Kit Dosing UnitC48505 L Liter A unit of volume equal to a cubic decimeter, or onethousandth of cubic meter, or 1000 cubic centimeters,or approximately 61.023 744 cubic inches.(NCI)LiterC69160 L/h Liters per hour. Liter per HourC73725 L/kg Liter perKilogramC67388 L/min Liters perMinuteA dose calculation unit expressed in liter(s) perkilogram. (NCI)A unit of volumetric flow rate defined as the rate atwhich one liter of matter travels during the period oftime equal to one minute.(NCI)L/kgLiter per MinuteC67390 L/s L/sec Liters per second. Liter per secondC48531 LB Pound A traditional unit of mass. By international agreement,one avoirdupois pound is equal to exactly 0.453 59237 kilogram, 16 ounces, or 1.215 28 troy pounds.(NCI)PoundC48506 LOZENGE Lozenge DosingUnitA dosing measurement based on the lozenge unit.(NCI)Lozenge Dosing UnitC67307 LPF Low Power Field The area visible in one view field of the microscopeunder the low magnification of the objective beingused in microscopy.(NCI)Low Power FieldC68878 Log10 ELISA unit Log10Enzyme-LinkedImmunosorbentAssay UnitC68879 Log10 ELISA unit/dose Log10Enzyme-LinkedImmunosorbentAssay Unit perDoseA logarithmic-scale (base 10) unit for measuringconcentration and/or reactivity of a test substance (anantigen or antibody of interest) as defined in theliterature reference for the particular quantitativeenzyme-linked immunosorbent assay method.(NCI)A logarithmic-scale (base 10) unit for measuringpotency of immunologically active substance in aproduct determined as reactivity in a quantitativeimmunoassay for particular antigen or antibody andexpressed per quantity of preparation used as a singledose.(NCI)Log10 Enzyme-LinkedImmunosorbent Assay UnitLog10 Enzyme-LinkedImmunosorbent Assay Unit per DoseC705<strong>12</strong> MBq Megabecquerel A unit of radioactivity equal to one million nucleardisintegrations or other nuclear transformations persecond, or to 10E6 Becquerels.(NCI)MegabecquerelSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 395 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71169 MBq/uL Megabecquerelper Microliter;GBq/mL;Gigabecquerelper MilliliterC96691 MESF Molecules ofEquivalentSolubleFluorochromesC96687 MFI MedianFluorescenceIntensityA unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one million Becquerels per unitvolume equal to one millionth of a liter.(NCI)A unit of measure of the fluorescence intensity of afluorochrome-labeled sample, which is equivalent tothe fluorescence intensity of a solution containing anequivalent number of molecules of free fluorochromein solution, under identical experimental conditions.A unit of measure equal to the geometric meanfluorescence intensity of a log-normal distribution offluorescence signals.Megabecquerel per MicroliterMolecule of Equivalent SolubleFluorochromeMedian Fluorescence IntensityC67314 MHz Megahertz The SI derived unit of frequency; equal to one millionoscillations per second or to 10E6 hertz. (NCI)C29846 MONTHS Month One of the <strong>12</strong> divisions of a year as determined by acalendar. It corresponds to the unit of time ofapproximately to one cycle of the moon's phases,about 30 days or 4 weeks. (NCI)MHzMonthC71183 Mile InternationalMileA unit of distance equal to 5280 international feet,1760 international yards, or 1609.344 meters.(NCI)MileC7<strong>12</strong>04 NEBULE Nebule A unit of measurement based on the nebule dosingunit.(NCI)C42546 Newton Newton A unit of force which, when applied in a vacuum to abody having a mass of one kilogram, causes anacceleration of one meter per second squared. It isequal to 10E5 dynes.(NCI)Nebule Dosing UnitNewtonC62653 PACK A number of individual items packaged as a unit. PackC48520 PACKAGE Package DosingUnit; PackDosing UnitC48521 PACKET Packet DosingUnitC48524 PATCH Patch DosingUnitC48525 PELLET Pellet DosingUnitC67264 PFU Plaque FormingUnitC71198 PFU/dose Plaque FormingUnit per DoseA dosing measurement based on the packageunit.(NCI)A dosing measurement based on the packet unit.(NCI)A dosing measurement based on the patch unit.(NCI)A dosing measurement based on the pellet unit.(NCI)A measure of viable infectious entities (e.g. viralparticle or group of particles) in the specimen orproduct defined as the smallest quantity that canproduce a cytopathic effect in the host cell culturechallenged with the defined inoculum, visible under themicroscope or/and to the naked eye as a plaque. Anumber of plaque forming units (PFU) per unit volumeis a conventional way to refer the titer of an infectiveentity in a specimen or preparation.(NCI)A unit of potency of a biological product expressed asa quantity of viable infectious entities capable toproduce a cytopathic effect in the appropriate cellculture per a single dose of the preparation.(NCI)Package Dosing UnitPacket Dosing UnitPatch Dosing UnitPellet Dosing UnitPlaque Forming UnitPlaque Forming Unit per DoseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 396 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71199 PFU/mL Plaque FormingUnit perMilliliterC48530 POUCH Pouch DosingUnitA unit of potency of a biological product expressed asa quantity of viable infectious entities capable toproduce a cytopathic effect in the appropriate cellculture per one milliliter of the preparation.(NCI)A dosing measurement based on the pouch unit.(NCI)Plaque Forming Unit per MilliliterPouch Dosing UnitC48532 PRESSOR UNITS Pressor Unit A dosing measurement based on the pressor unit.(NCI) Pressor UnitC65060 PUFF Puff A means of delivering a defined dose of a therapeuticaerolized solution into either the upper or lowerrespiratory tract. Metered-dose inhalers or spraypumps are devices that provide a puff dose for deliveryinto either the oral or the nasal cavity.(NCI)C42547 Pa Pascal A unit of pressure equivalent to one Newton per squaremeter or 10 bars or to 1.45x10(E-4) pounds per squareinch.(NCI)C73993 Pack Year A quantification of lifetime tobacco exposure definedas (number of cigarettes smoked per day x number ofyears smoked)/20. One pack-year is smoking 20cigarettes a day for one year.Puff Dosing UnitPascalPack YearC48590 QUANTITY SUFFICIENT QuantitySufficientA quantity of an ingredient or product needed to bringup a volume or weight of the preparation to a finalamount as it is indicated in the prescription; also refersto a determination of an adequate supply of medicineto fulfill either a prescribed amount or a sufficientquantity to provide treatment over a specified timeframe.(NCI)Quantity SufficientC44256 RATIO A unit of measurement for the quotient of the amountof one entity to another.RatioC62609 RING Ring Dosing Unit A dosing measurement based on the ring unit.(NCI) Ring Dosing UnitC67441 RNA copies/mL RNA Copies perMilliliterThe unit of concentration of Ribonucleic Acid (RNA)copies expressed as a number of copies in unit volumeequal to one milliliter.(NCI)RNA Copy per MilliliterC18064 Rad Rad The special unit for absorbed radiation dose, which isthe amount of energy from any type of ionizingradiation (e.g., alpha, beta, gamma, neutrons, etc.)deposited in any medium (e.g., water, tissue, air). Adose of one rad means the absorption of 100 ergs pergram of absorbing tissue. One rad is equal to 0.01gray.(NCI)C70575 Roentgen Roentgen A unit of exposure to ionizing radiation. One Roentgenis the amount of gamma or x-rays required to produceions resulting in a charge of 2.58E-4Coulombs/kilogram of air under standardconditions.(NCI)RadRoentgenC71324 SACHET Sachet dosingunitC48536 SCOOPFUL Scoopful DosingUnitA dosing unit that contains a solid pharmaceuticalpreparation in the form of a small packet or bag madefrom a flexible, often porous material.(NCI)A dosing measurement based on the scoopfulunit.(NCI)Sachet Dosing UnitScoopful Dosing UnitSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 397 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC48537 SPRAY Spray DosingUnitC48538 STRIP Strip DosingUnitC48539 SUPPOSITORY SuppositoryDosing UnitC48540 SYRINGE Syringe DosingUnitA dosing measurement based on the spray unit.(NCI)A dosing measurement based on the strip unit.(NCI)A dosing measurement based on the suppositoryunit.(NCI)A dosing measurement based on the syringe unit.(NCI)Spray Dosing UnitStrip Dosing UnitSuppository Dosing UnitSyringe Dosing UnitC42555 Siemens Siemens A unit of electrical conductance, admittance, andsusceptance. A conductor has a conductance of oneSiemens if an electrical potential difference of onevolt produces a one Ampere current in it. Theconductance in Siemens is the reciprocal of itsresistance in ohms.(NCI)C42553 Sv Sievert A unit of equivalent radiation dose. One Sv is receivedwhen the actual absorbed dose of ionizing radiation,after being multiplied by the dimensionless factors Q(the relative biological efficiency or quality factor)and N (the product of any other multiplying factorsthat takes into account the distribution of energythroughout the dose), is one joule per kilogram. In thisscheme, the relationship between the absorbed dose ofradiation D and the dose equivalent H is, therefore,given by H = QND. Both Q and N are stipulated by theInternational Commission on Radiological Protection.One Sv is equal to 100 rem.(NCI)SiemensSievertC48542 TABLET Tablet DosingUnit; tabC48543 TAMPON Tampon DosingUnitC70537 TCID 50/dose 50 PercentTissue CultureInfective Doseper DoseC48547 TRACE Trace DosingUnitC48548 TROCHE Troche DosingUnitC48549 TUBE Tube DosingUnitC48541 Tbsp TablespoonDosing UnitA dosing measurement based on the tablet unit.(NCI)A dosing measurement based on the tampon unit.(NCI)A potency unit equal to the potency at which one doseof preparation contains one 50 percent tissue cultureinfective dose.(NCI)An extremely small amount.(NCI)A dosing measurement based on the troche unit.(NCI)A dosing measurement based on the tube unit.(NCI)A unit of volume informally used in pharmacy. Thetablespoon has been standardized at 15 milliliters inUS, Britain, Canada, and New Zealand, and at 20milliliters in Australia and some Europeancountries.(NCI)Tablet Dosing UnitTampon Dosing Unit50 Percent Tissue Culture InfectiveDose per DoseTrace Dosing UnitTroche Dosing UnitTube Dosing UnitTablespoon Dosing UnitC42557 Tesla Tesla A unit of magnetic flux density equal to the magnitudeof the magnetic field vector necessary to produce aforce of one Newton on a charge of one coulombmoving perpendicular to the direction of the magneticfield vector with a velocity of one meter per second. Itis equivalent to one Weber per square meter.(NCI)TeslaSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 398 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC44278 U Unit A single undivided thing occurring in the compositionof something else.UC67456 U/L mU/mL; Unit perLiterAn arbitrary unit of substance concentration equal tothe concentration at which one liter of mixturecontains one unit of a substance. (NCI)Unit per LiterC73773 U/animal Unit per Animal An arbitrary dosing unit expressed in unit(s) peranimal. (NCI)C77606 U/g Unit per Gram An arbitrary unit of substance content expressed inunit(s) per gram. (NCI)U/animalUnit per GramC73774 U/g/day Unit per Gramper DayC73775 U/g/h Unit per Gramper HourC73776 U/g/min Unit per Gramper MinuteC67465 U/kg Unit perKilogramC73777 U/kg/day Unit perkilogram per DayC73778 U/kg/h Unit perKilogram perHourC73779 U/kg/min Unit perKilogram perMinuteC67467 U/m2 Unit per SquareMeterC73783 U/m2/day Unit per SquareMeter per DayC73784 U/m2/h Unit per SquareMeter per HourC73785 U/m2/min Unit per SquareMeter perMinuteC77607 U/mL Unit perMilliliterC73780 U/mg Unit perMilligramAn arbitrary unit of substance rate expressed in unit(s)per gram per period of time equal to twenty-fourhours. (NCI)An arbitrary unit of substance rate expressed in unit(s)per gram per period of time equal to sixty minutes.(NCI)An arbitrary unit of substance rate expressed in unit(s)per gram per period of time equal to sixty seconds.(NCI)An arbitrary unit of substance content expressed inunits of biological activity per unit of mass equal toone kilogram. Unit per kilogram is also used as a dosecalculation unit expressed in arbitrary units per onekilogram of body mass. (NCI)An arbitrary unit of substance rate expressed in unit(s)per gram per period of time equal to twenty-fourhours. (NCI)An arbitrary unit of substance rate expressed in unit(s)per gram per period of time equal to sixty minutes.(NCI)An arbitrary unit of substance rate expressed in unit(s)per gram per period of time equal to sixty seconds.(NCI)A unit expressed as a number of arbitrary units ofsubstance per one square meter of a body surface area.An arbitrary unit of substance rate expressed in unit(s)per square meter per period of time equal totwenty-four hours. (NCI)An arbitrary unit of substance rate expressed in unit(s)per square meter per period of time equal to sixtyminutes. (NCI)An arbitrary unit of substance rate expressed in unit(s)per square meter per period of time equal to sixtyseconds. (NCI)An arbitrary unit of substance content expressed inunit(s) per milliliter. (NCI)An arbitrary unit of substance content expressed inunit(s) per milligram. (NCI)U/g/dayU/g/hU/g/minUnit per KilogramU/kg/dayU/kg/hU/kg/minU/m2U/m2/dayU/m2/hU/m2/minUnit per MilliliterUnit per MilligramSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 399 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92618 U/mmol Unit perMillimoleAn arbitrary unit of substance concentration equal tothe concentration at which one millimole of a mixturecontains one unit of a substance. (NCI)U/mmolC42551 V Volt A unit of electric potential and electromotive force,equal to the difference of electric potential betweentwo points on a conducting wire carrying a constantcurrent of one Ampere when the power dissipatedbetween the points is one watt. This is equivalent to thepotential difference across a resistance of one ohmwhen one Ampere of current flows through it.(NCI)VoltC48551 VIAL Vial Dosing Unit A dosing measurement based on the vial unit.(NCI) Vial Dosing UnitC48552 WAFER Wafer DosingUnitA dosing measurement based on the wafer unit.(NCI)Wafer Dosing UnitC29844 WEEKS Week Any period of seven consecutive days. (NCI) WeekC42549 Watt Watt A unit of power equal to the power which in onesecond produces or transfers the energy of one joule.The unit is used in measurements of power emitted,transferred or received as radiation, sound waves, heatflow rate, and rate of energy transfer. Equal to 1/746of horsepower.(NCI)C42556 Weber Weber A unit of magnetic flux, equal to the flux that producesin a circuit of one turn an electromotive force of onevolt, when the flux is uniformly reduced to zero withinone second.(NCI)C29848 YEARS Year The period of time that it takes for earth to make acomplete revolution around the sun, approximately365 days; a specific one year period.(NCI)C64553 ag Attogram A unit of mass equal to one quintillionth of a gram(10E-18 gram).(NCI)C68855 amol Attomole A unit of amount of substance equal to onequintillionth of a mole (10E-18 mole).(NCI)C42536 amp Ampere A unit of electric current, named after the Frenchphysicist Andre Ampere. It is that constant currentwhich, if maintained in two straight parallel conductorsof infinite length and zero diameter separated by onemeter in a vacuum, would produce between theseconductors a force equal to 2(10E7) Newton permeter of length. This is dependent upon the definitionsof the meter, kilogram, and second. One Ampererepresents 6.24 x 10(E18) unit electric chargecarriers, such as electrons, passing a specified fixedpoint in one second.(NCI)WattWeberYearAttogramAttomoleAmpereC64559 amu Atomic MassUnitC70497 anti-Xa IU Anti-Xa ActivityInternationalUnitA small unit of mass used to express atomic andmolecular masses. (NCI)A unit of unfractionated or low molecular weightheparin anticoagulation potency determined as theamount that neutralizes one unit of coagulation factorXa preparation defined as an international biologicalstandard by WHO (World Health Organization) FirstInternational Low Molecular Weight HeparinReference Standard.(NCI)amuAnti-Xa Activity International UnitSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 400 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC70498 anti-Xa IU/mL Anti-Xa ActivityInternationalUnit perMilliliterA specific anticoagulation activity of unfractionated orlow molecular weight heparin on factor Xa, expressedas a number of international anti-Xa heparin units perone milliliter of plasma.(NCI)Anti-Xa Activity International Unitper MilliliterC54711 atm Atmosphere A unit of pressure, equal to a barometer reading of 760mm Hg. 1 atmosphere is 101325 Pascals and 1.01325bar. This unit of pressure is roughly equal to theaverage atmospheric pressure at sea level on theearth.(NCI)C7<strong>12</strong>00 bel Bel A logarithmic ratio unit (base-10 logarithms) used toexpress relative magnitude of a physical quantity(usually power or intensity) in comparison with aspecified or implied reference level. Particularly, Belis used as a unit of relative sound intensity. In the lattercontext it is equal to ten decibels or to approximately1.15<strong>12</strong>93 nepers.(NCI)C64693 cGy Centigray The metric unit of absorbed radiation dose equal to theabsorption of one hundredth of joule of radiationenergy per kilogram of matter.C67193 cal Calorie A measurement of nutritional energy. The quantity ofthermal energy required to raise one gram of water onedegree Centigrade under standard conditions. 1 calorieequals 4.186 joules. (NCI)C42538 cd Candela The candela is the basic unit of luminous intensity. It isthe luminous intensity in a given direction of a smallmonochromatic light source at 540 terahertz emitting1/683 watt per steradian in that direction. This isdependent upon the definitions of the meter, kilogram,and second.(NCI)C67242 cells/uL cells/mm3 A unit of cell concentration expressed in cells per unitof area equal to one cubic millimeter.AtmosphereBelCentigraycalorieCandelaCells per MicroliterC64554 cg Centigram A unit of mass equal to one hundredth of a gram.(NCI) CentigramC49668 cm Centimeter A basic unit of length equal to one hundredth of ameter or approximately 0.393 700 787 inch. (NCI)C91060 cm H2O A unit of pressure defined by a column of water with aheight of one centimeter, frequently used to measurecentral venous pressure, intracranial pressure, and forpressures during mechanical ventilation.C102406 cm/s cm/sec A unit of both speed (scalar) and velocity (vector),defined as the distance of one centimeter travelled perunit time equal to one second. (NCI)CentimeterCentimeters of WaterCentimeter per SecondC48460 cm2 SquareCentimeterA unit of area measurement equal to a squaremeasuring one centimeter on each side. One squarecentimeter is equal to 10E-4 square meter.(NCI)Square CentimeterC68687 cmol Centimole A unit of amount of substance equal to one hundredthof a mole (10E-2 mole).(NCI)C100900 copies/mL A unit of concentration expressed as a number ofcopies per unit volume equal to one milliliter.CentimoleCopies per MilliliterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 401 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71176 cycle/min Cycle per Minute A unit of frequency equal to the frequency at whichone complete execution of a periodically repeatedphenomenon, alternation, event, or sequence of eventsoccurs per unit of time equal to one minute.(NCI)Cycle per MinuteC71480 cycle/sec Cycle perSecondA unit of frequency equal to the frequency at whichone complete execution of a periodically repeatedphenomenon, alternation, event, or sequence of eventsoccurs per unit of time equal to one second.(NCI)Cycle per SecondC102407 dB Decibel A unit of measure representing the intensity of anelectrical signal or sound which is equal to ten timesthe logarithm of the ratio of two signals.C64697 dL Deciliter The unit of volume equal to one tenth of a liter.Accepted for use with the SI. (NCI)DecibeldLC68667 deg Degree Unit ofPlane AngleA unit of plane angle measurement equal to the lengthof the arc cut out by the angle, divided by thecircumference of the circle, and multiplied by 360.The symbol for degrees is a small superscript circle.One radian is about 57 degrees and one degree ispi/180 radians.(NCI)Degree Unit of Plane AngleC68685 dmol Decimole A unit of amount of substance equal to one tenth of amole (10E-1 mole).(NCI)C64564 dram Dram A unit of mass equal to 1/16 Avoirdupois ounce or1/256 Avoirdupois pound. One dram equalsapproximately 1.7718451953<strong>12</strong>5 grams.(NCI)C70470 dyn Dyne A unit of force defined as the force that accelerates amass of one gram at the rate of one centimeter persecond squared. One dyne is equal to 10E-5 Newtonand 2.248E-6 pounds of force.(NCI)DecimoleDram Mass UnitDyneC67273 eq EquivalentWeightA unit of relative amount of a substance that combineswith or displaces 8.0 grams of oxygen or 1.008 gramof hydrogen. The unit is usually expressed in grams andis equal to the amount of substance that gains or losesone mole of electrons in a redox reaction, or to theamount of substances that releases or accepts onemole of hydrogen ions in a neutralization reaction; orto the amount of electrolyte that carries one mole ofpositive or negative charge. This is a large unit andmeasurements are more often done in its derivatives,e.g. in milliequivalents.(NCI)Equivalent WeightC64780 fL Femtoliter The unit of volume equal 10E-15 liter. FemtoliterC64552 fg Femtogram A unit of mass equal to one quadrillionth of a gram(10E-15 gram).(NCI)C68854 fmol Femtomole A unit of amount of substance equal to onequadrillionth of a mole (10E-15 mole).(NCI)FemtogramFemtomoleC68887 fmol/L Femtomole perLiterC73711 fmol/g Femtomole perGramA unit of concentration (molarity unit) equal to onequadrillionth of a mole (10E-15 mole) of solute in oneliter of solution. (NCI)A molality unit that describes the amount of substance,expressed in femtomole(s) per gram. (NCI)fmol/Lfmol/gSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 402 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC7<strong>12</strong>53 ft Foot A unit of length defined by the U.S. National Bureau ofStandards as 30.48 centimeters. It is equal to 0.3048meter, <strong>12</strong> inches, or to approximately 0.999998survey foot.(NCI)C48461 ft2 Square Foot A unit of area equal to 144 square inches, 929.0304square centimeters, or 9.290304E-2 squaremeters.(NCI)International FootSquare FootC68859 ft3 Standard CubicFootA unit used in physical chemistry to express theamount of substance of an ideal gas in one cubic footat 60 degrees Fahrenheit and pressure of oneatmosphere.(NCI)Standard Cubic FootC48155 g Gram A metric unit of mass equal to one one thousandth of akilogram. (NCI)GramC42576 g/L g/L; Gram perLiter; kg/m3;Kilogram perCubic Metre;Microgram perMicroliter;Milligram perMilliliter; ug/uL;mg/mLA unit of mass concentration defined as theconcentration of one kilogram of a substance per unitvolume of the mixture equal to one cubic meter, or theconcentration of one milligram of a substance per unitvolume of the mixture equal to one milliliter, or onegram of a substance per one liter of the mixture. It isalso a unit of mass density (volumic mass) defined asthe density of substance which mass equal to onemilligram occupies the volume one milliliter.(NCI)Kilogram per Cubic MeterC73713 g/animal Gram per Animal A unit of measure expressed in gram(s) per animal. g/animalC73714 g/animal/day Gram per Animalper DayC73715 g/animal/wk Gram per Animalper WeekA unit of measure expressed in gram(s) per animal perperiod of time equal to twenty-four hours.A unit of measure expressed in gram(s) per animal perperiod of time equal to seven days.g/animal/dayGram per Animal per WeekC73716 g/cage Gram per Cage A unit of measure expressed in gram(s) per cage. g/cageC73717 g/cage/day Gram per Cageper DayC73718 g/cage/wk Gram per Cageper WeekC7<strong>12</strong>01 g/cm2 Gram per SquareCentimeterC64783 g/dL Gram perDeciliter; g%C67372 g/day Gram per Day;g/d; gram/day;g/24h; gram/24HoursA unit of measure expressed in gram(s) per cage perperiod of time equal to twenty-four hours.A unit of measure expressed in gram(s) per cage perperiod of time equal to seven days.A unit of area density defined as a spread rate at whichone gram of a substance is spread over the area of onesquare centimeter. The unit is also used as a dosecalculation unit.(NCI)A unit of mass concentration defined as theconcentration of one gram of a substance per unitvolume of the mixture equal to one deciliter (100milliliters). The concept also refers to the metric unitof mass density (volumic mass) defined as the densityof substance which mass equal to one gram occupiesthe volume one deciliter.(NCI)A dose calculation unit expressed in gram(s) perperiod of time equal to twenty-four hours.g/cage/dayGram per Cage per WeekGram per Square CentimeterGram per DeciliterGram per 24 HoursC70453 g/g Gram per Gram A unit of a mass fraction expressed as a number ofgrams of substance per gram of mixture.g/gSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 403 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC73720 g/g/day Gram per Gramper DayC69104 g/kg Gram perKilogram; mg/g;Milligram perGramC66975 g/kg/day Gram perKilogram perDay; mg/g/dayC67282 g/m2 Gram per SquareMeterC73722 g/m2/day Gram per SquareMeter per DayA unit of measure expressed in gram(s) per gram perperiod of time equal to twenty-four hours.A unit of a mass fraction expressed as a number ofgrams of substance per kilogram of mixture. The unitis also used as a dose calculation unit.(NCI)A dose administration rate unit equal to the rate atwhich one gram of a product per kilogram of bodymass is delivered or administered over the period ofone day. (NCI)A unit of area density defined as a spread rate at whichone gram of a substance is spread over the area of onesquare meter. It is equal to approximately 0.029 4935ounce per square yard. Also used as a dose calculationunit.(NCI)A dose calculation unit expressed in gram(s) persquare meter per period of time equal to twenty-fourhours.g/g/dayGram per Kilogramg/kg/dayGram per Square Meterg/m2/dayC73721 g/mol Gram per Mole A unit of mass commonly used to express the molarmass of a substance in gram(s) per mole. (NCI)C48497 grain Grain A unit of mass derived from the weight of a grain andequal to one seven-thousandth of a pound, or 1/480troy ounce, or 64.79891 milligrams. The originalEnglish grain unit based on the mass of a ripe grainbarleycorn was larger the corresponding grain units ofFrance and other European nations which were basedon the weight of the smaller wheat grain.(NCI)C69442 gtt Drop A unit of volume equal to 0.05 milliliter (20drops/ml).(NCI)C68871 in2 Square Inch A unit of area equal to the area of a square with sidesof one inch. It is equal to 6.4516 squarecentimeters.(NCI)C70511 kBq Kilobecquerel A unit of radioactivity equal to one thousand nucleardisintegrations or other nuclear transformations persecond, or to 10E3 Becquerels.(NCI)g/molGrainMedical DropSquare InchKilobecquerelC71168 kBq/uL Kilobecquerelper Microliter;GBq/L;Gigabecquerelper Liter;MBq/mL;Megabecquerelper MilliliterC70492 kIU KiloInternationalUnitA unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one thousand Becquerels per unitvolume equal to one millionth of a liter.(NCI)A unit equal to one thousand international units.(NCI)Kilobecquerel per MicroliterKilointernational UnitSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 404 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC92615 kN/cm2 Kilonewton perCentimeterSquared;kdyn/cm2The kilonewton per centimeter squared is an SI derivedunit of pressure; one newton is computed as the forcenecessary to accelerate a mass of one gram at the rateof one centimeter per second squared. One kilonewtonper centimeter squared is descriptive of the amount offorce exerted in a particular area. This measurement isfrequently used when describing conditions of cellularmovement. (NCI)Kilonewton per Centimeter SquaredC67284 kPa Kilopascal A SI derived unit of pressure equivalent to 1000newtons per square meter or 10000 bars or to 0.145pound per square inch. (NCI)kPaC7<strong>12</strong>02 kUSP Kilo UnitedStatesPharmacopoeiaUnitA unit of potency equal to one thousand USPharmacopoeia Units.(NCI)Kilo United States PharmacopeiaUnitC42566 kat Katal A unit for measuring catalytic (e.g. enzymatic) activity,the ability of the compound to accelerate the chemicalreaction by providing a lower energy pathway betweenthe reactants and the products. One katal is thatcatalytic activity which will raise the rate of reactionby one mole per second in a specified assay system.When the katal is used, the measurand should bespecified by reference to the measurement procedure;the measurement procedure must identify the indicatorreaction. The katal is not used to express a rate ofreaction itself, which should be expressed in moles persecond.(NCI)KatalC67194 kcal Kilogram-CalorieA unit of energy defined as the amount of heat requiredto raise the temperature of one kilogram of pure waterby one degree Centigrade under standard conditions(the specific heat of the water at 15 degrees Celsiusand the constant pressure of 101.325 kilopascals orone atm being defined as unity), equal toapproximately 4.1855 kJ. It is also is used bynutritionists in measuring the energy-producingpotential of food as a unit of potential energycontained by a substance, which can be liberated whenthe material is oxidized, usually by combustion in thepresence of oxygen.(NCI)CalorieC28252 kg Kilogram The basic SI unit of mass. It is defined as the mass ofan international prototype in the form of aplatinum-iridium cylinder kept at Sevres in France. It isthe only basic unit still defined in terms of a materialobject, and also the only one with a prefix [kilo]already in place. A kilogram is equal to 1,000 gramsand 2.204 622 6 pounds. (NCI)KilogramC64566 kg/L Kilogram perLiter; g/mL;Gram perMilliliter;gram/mL; kg/L;mg/uLA unit of mass concentration defined as theconcentration of one kilogram of a substance in unitvolume of the mixture equal to one liter. The conceptalso refers to the unit of mass density (volumic mass)defined as the density of substance which mass equalto one kilogram occupies the volume one liter.(NCI)Kilogram per LiterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 405 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC69094 kg/cm2 Kilogram perSquareCentimeterC49671 kg/m2 Kilogram perSquare MeterA unit of spread rate of a substance by mass expressedin kilograms per area unit equal to one squarecentimeter, used also as a measure of area density andas a dose calculation unit.(NCI)A unit expressed as kilogram of mass per square meterof area.(NCI)Kilogram Per Square CentimeterKilogram Per Square MeterC71177 km Kilometer A unit of distance equal to 1000 meters, 0.621 miles,1094 yards, or 3281 feet.(NCI)KilometerC7<strong>12</strong>03 km/h Kilometer PerHourA unit of both speed (scalar) and velocity (vector),defined as the distance of one thousand meterstravelled per unit time equal to one hour.(NCI)Kilometer per HourC42560 lm Lumen A unit of luminous flux. It is the amount of light thatfalls on a unit area at unit distance from a source ofone candela.(NCI)LumenC70480 log EID 50/dose Log10 50Percent EmbryoInfective Doseper DoseC70484 log10 CCID 50 Log10 50Percent CellCulture InfectiveDoseC70485 log10 CCID 50/dose Log10 50Percent CellCulture InfectiveDose per DoseA logarithmic-scale (base 10) potency unit formeasuring infectious activity of a biologic product orinfectious agent preparation equal to the potency atwhich one dose of infectious material contains one 50percent embryo infective dose.(NCI)A potency unit for measuring infectious activity of abiologic product or infectious agent preparation equalto a base-10 logarithm of amount of product or agentpreparation that causes infection in the 50% of the cellculture-containing vessels inoculated with that dilutionof infectious material in the product potency assay orpathogen activity assay.(NCI)A logarithmic-scale (base 10) potency unit formeasuring infectious activity of a biologic product orinfectious agent preparation equal to the potency atwhich one dose of infectious material contains one 50percent cell culture infective dose.(NCI)Log10 50 Percent Embryo InfectiveDose per DoseLog10 50 Percent Cell CultureInfective DoseLog10 50 Percent Cell CultureInfective Dose per DoseC102658 log10 CFU/g A logarithmic-scale (base 10) unit for measuringcolony forming units per unit of mass equal to onegram.C102659 log10 CFU/mL A logarithmic-scale (base 10) unit for measuringcolony forming units per unit of volume equal to onemilliliter.Log10 Colony Forming Units perGramLog10 Colony Forming Units perMilliliterC70479 log10 EID 50 Log10 50Percent EmbryoInfective DoseC70488 log10 TCID 50 Log10 50Percent TissueCulture InfectiveDoseA potency unit for measuring infectious activity of abiologic product or infectious agent equal to a base-10logarithm of amount of product or agent preparationthat causes infection in the 50% of embryos (such aschicken embryos) used in the product potency assay orpathogen activity assay.(NCI)A potency unit for measuring infectious activity of abiologic product or infectious agent preparation equalto a base-10 logarithm of amount of product or agentpreparation that causes infection in the 50% of thetissue culture-containing flasks inoculated with thatdilution of infectious material in the product potencyassay or pathogen activity assay.(NCI)Log10 50 Percent Embryo InfectiveDoseLog10 50 Percent Tissue CultureInfective DoseSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 406 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC70489 log10 TCID 50/dose Log10 50Percent TissueCulture InfectiveDose per DoseA logarithmic-scale (base 10) potency unit formeasuring infectious activity of a biologic product orinfectious agent preparation equal to the potency atwhich one dose of infectious material contains one 50percent tissue culture infective dose.(NCI)Log10 50 Percent Tissue CultureInfective Dose per DoseC42561 lx Lux A unit of illuminance equal to the direct illuminationon a surface that is everywhere one meter from auniform point source of one candela; a unit ofilluminance that is equal to one lumen per squaremeter.(NCI)C41139 m Meter A meter is defined as the length of the path traveled bylight in a vacuum during a time interval of 1/299 792458 of a second and is equal to 1.093 61 yards.(NCI)LuxMeterC42571 m/sec Meter PerSecondA unit of both speed (scalar) and velocity (vector),defined as the distance of one meter travelled per unittime equal to one second.(NCI)Meter Per SecondC42569 m2 Square Meter The SI unit of area measurement equal to a squarewhose sides are one meter long. Square meter is equalto 10,000 square centimeters; 0.01 are; 1.196 squareyards; 10.76 square feet; 1550 square inches. (NCI)C42570 m3 Cubic Meter A unit of volume or capacity equal to the volume of acube with edges one meter in length. It is equal to1,000 liters; 1,000 cubic decimeters; 10(E6) cubiccentimeters; 25.3 cubic feet; 6.29 barrels.(NCI)C48511 mCi Millicurie A unit of radioactivity equal to one thousandth of aCurie or 37 megabecquerels, and corresponding to aradioactivity of 37 millions of atomic disintegrationsper second.(NCI)Square MeterCubic MeterMillicurieC71174 mCi/L Millicurie perLiter; uCi/mL;Microcurie perMilliliterC70570 mCi/kg Millicurie perKilogram; uCi/g;Microcurie perGramA unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one thousandth of a Curie per unitvolume equal to one liter.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one millicurie of the sample with totalmass of one kilogram.(NCI)Millicurie per LiterMillicurie per KilogramC485<strong>12</strong> mEq Milliequivalent A unit of relative amount of a substance equal to onethousandth of an equivalent weight.(NCI)MilliequivalentC67474 mEq/L MilliequivalentPer Liter;mval/L;Millivalent perLiterC67473 mEq/dL Milliequivalentper DeciliterC67471 mEq/day Milliequivalentsper DayA concentration unit measured as a number ofmilliequivalents of solute per liter of solution.(NCI)A concentration unit measured as a number ofmilliequivalents of solute per deciliter of solution.(NCI)A unit of relative amount of substance flow rateequivalent to the rate at which one thousandth of anequivalent of substance travels to a given object orspace over a period of time equal to twenty fourhours.(NCI)Milliequivalent per LitermEq/dLMilliequivalent per 24 HoursSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 407 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC70580 mEq/g MilliequivalentPer GramC67475 mEq/kg MilliequivalentPer KilogramC73737 mEq/mL Milliequivalentper MilliliterC92616 mEq/mmol Milliequivalentper MillimoleC70578 mEq/uL MilliequivalentPer MicroliterC70581 mEq/ug MilliequivalentPer Microgram;mEq/mcgA unit of relative amount of substance contentequivalent to the content at which one gram of mixturecontains one thousandth of an equivalent of acomponent. The unit is also used as a dose calculationunit.(NCI)A unit of relative amount of substance contentequivalent to the content at which one kilogram ofmixture contains one thousandth of an equivalent of acomponent. The unit is also used as a dose calculationunit.(NCI)A concentration unit expressed in milliequivalent(s) ofsolute per milliliter of solution. (NCI)A concentration unit measured as a number of onethousandth of an equivalent weight per millimole ofsubstance. (NCI)A concentration unit measured as a number ofmilliequivalents of solute per microliter ofsolution.(NCI)A unit of relative amount of substance contentequivalent to the content at which one millionth of agram of mixture contains one thousandth of anequivalent of a component. The unit is also used as adose calculation unit.(NCI)Milliequivalent per GramMilliequivalent per KilogrammEq/mLMilliequivalent per MilliimoleMilliequivalent per MicroliterMilliequivalent per MicrogramC28254 mL Milliliter; cm3 The unit of volume equal to one thousandth of a liter,one cubic centimeter, 10E-6 cubic meter, orapproximately to 0.061 023 7 cubic inch.(NCI)MilliliterC73746 mL/animal Milliliter perAnimalC73747 mL/animal/day Milliliter perAnimal per DayC73748 mL/animal/wk Milliliter perAnimal per WeekC73749 mL/breath Milliliter perBreathC73750 mL/cage Milliliter perCageC73751 mL/cage/day Milliliter perCage per DayC73752 mL/cage/wk Milliliter perCage per WeekA unit of measure expressed in milliliter(s) per animal.A unit of measure expressed in milliliter(s) per animalper period of time equal to twenty-four hours.A unit of measure expressed in milliliter(s) per animalper period of time equal to seven days.A unit of measure expressed in milliliter(s) perinspiration or expiration of breath.A unit of measure expressed in milliliter(s) per cage.A unit of measure expressed in milliliter(s) per cageper period of time equal to twenty-four hours.A unit of measure expressed in milliliter(s) per cageexpressed per period of time equal to seven days.mL/animalmL/animal/dayMilliliter per Animal per WeekmL/breathmL/cagemL/cage/dayMilliliter per Cage per WeekC98755 mL/cm H2O A unit of pressure expressed in milliliter(s) percentimeter of water. (NCI)Milliliter per Centimeter of WaterC67410 mL/day Milliliter perDayC73754 mL/g Milliliter perGramMilliliters per day.A unit of measure expressed in milliliter(s) per gram.mL/daymL/gSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 408 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC73755 mL/g/day Milliliter perGram per Day;mL/g/day;uL/mg/day;L/day/kg;mL/day/g;uL/day/mg;L/kg/dC73756 mL/g/h Milliliter perGram per HourC73757 mL/g/min Milliliter perGram per MinuteC66962 mL/h Milliliters perHourC67411 mL/kg Milliliter perKilogramC73758 mL/kg/day Milliliter perKilogram perDayC73759 mL/kg/h Milliliter perKilogram perHourC73760 mL/kg/min Milliliter perKilogram perMinute;uL/g/min;mL/min/kg;uL/min/g;mL/kg/minC73761 mL/m2 Milliliter perSquare MeterC66977 mL/m2/day Milliliter perSquare Meter perDayC73762 mL/m2/h Milliliter perSquare Meter perHourC73763 mL/m2/min Milliliter perSquare Meter perMinuteC64777 mL/min Milliliters perMinuteLiters per kilogram per day.Milliliters per gram per hour.Milliliters per gram per minute.A unit of volumetric flow rate defined as the rate atwhich one milliliter of matter travels during the periodof time equal to one hour.(NCI)A dose calculation unit equal to one milliliter ofpreparation per one kilogram of body mass. (NCI)Milliliters per kilogram per day.Milliliters per kilogram per hour.Milliliters per kilogram per minute.A unit of measure expressed in milliliter(s) per squaremeter.A dose calculation unit expressed in milliliter(s) persquare meter per period of time equal to twenty-fourhours. (NCI)A dose calculation unit expressed in milliliter(s) persquare meter per period of time equal to sixty minutes.(NCI)A dose calculation unit expressed in milliliter(s) persquare meter per period of time equal to sixty seconds.(NCI)A metric unit of volumetric flow rate defined as therate at which one milliliter of matter travels during theperiod of time equal to one minute.(NCI)Milliliter per Gram per DaymL/g/hmL/g/minMilliliter per HourmL/kgmL/kg/daymL/kg/hmL/kg/minmL/m2mL/m2/daymL/m2/hmL/m2/minMilliliter per MinuteC674<strong>12</strong> mL/min/1.73m2 A metric unit of volumetric flow rate defined as therate at which one milliliter of matter travels during theperiod of time equal to one minute per 1.73 meterssquared of body surface area.Milliliter per Minute per 1.73 m2 ofBody Surface AreaC69073 mL/s mL/sec Milliliters per second. Milliliter per SecondSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 409 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC67318 mOsm Milliosmole A unit of osmotic pressure equal to one thousandth ofan osmole or osmotic pressure of 0.001 molarsolution of a substance that does not dissociate. (NCI)mOsmC67427 mOsm/kg Milliosmole perKilogramA unit of osmotic pressure equal to one thousandth ofan osmole per kilogram substance.mOsm/kgC73765 mPa Millipascal A SI derived unit of pressure equivalent to onethousandth of one pascal. (NCI)C67408 mU/L An arbitrary unit of substance concentration equal tothe concentration at which one liter of mixturecontains one thousandth of a unit of a substance.C67324 mV Millivolt A unit of electric potential and electromotive forceequal to one thousandth of a volt.(NCI)C28253 mg Milligram The unit of mass equal to one thousandth of a gram or1000 micrograms. One milligram equalsapproximately 0.015432 grain or 35.274 x 10E-6ounce.(NCI)mPaMicrounit per MilliliterMillivoltMilligramC64572 mg/L g/m3; Gram perCubic Meter;mg/L;Microgram perMilliliter;Milligram perLiter; mcg/mL;ng/uL; ug/mLC73738 mg/animal Milligram perAnimalC67015 mg/dL Milligram perDeciliter; mg%C67399 mg/day Milligram perDayC73740 mg/g/h Milligram perGram per HourC73741 mg/g/min Milligram perGram per MinuteC66969 mg/h Milligram perHourC67401 mg/kg Milligram perKilogramC66976 mg/kg/day Milligram perKilogram perDayA unit of mass concentration equal to theconcentration of one gram of a substance per unitvolume of the mixture equal to one cubic meter. Theconcept also refers to the metric unit of mass density(volumic mass) defined as the density of substancewhich mass equal to one gram occupies the volumeone cubic meter.(NCI)A unit of measure expressed in milligram(s) peranimal.A unit of mass concentration defined as theconcentration of one milligram of a substance in unitvolume of the mixture equal to one cubic deciliter or100 cubic centimeters. It is also a unit of mass density(volumic mass) defined as the density of substancewhich mass equal to one milligram occupies thevolume one cubic deciliter or 100 cubiccentimeters.(NCI)A unit of measure referring to the ratio between massexpressed in milligrams per day.(NCI)A dose calculation unit expressed in milligram(s) pergram per period of time equal to sixty minutes. (NCI)A dose calculation unit expressed in milligram(s) pergram per period of time equal to sixty seconds. (NCI)A unit of mass flow rate equivalent to the rate at whichone thousandth of a gram of matter travels to a givenobject or space over a period of time equal to onehour.(NCI)A unit of a mass fraction expressed as a number ofmilligrams of substance per kilogram of mixture. Theunit is also used as a dose calculation unit.(NCI)A dose calculation unit expressed in milligram(s) perkilogram per period of time equal to twenty-fourhours. (NCI)Microgram per Millilitermg/animalMilligram per DeciliterMilligram per 24 Hoursmg/g/hmg/g/minMilligram per HourMilligram per Kilogrammg/kg/daySource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 410 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC71362 mg/kg/h Milligram perKilogram perHourC7<strong>12</strong>07 mg/kg/min Milligram perKilogram perMinuteC67402 mg/m2 Milligram perSquare MeterC66974 mg/m2/day Milligram perSquare Meter perDayC73743 mg/m2/h Milligram perSquare Meter perHourC73744 mg/m2/min Milligram perSquare Meter perMinuteC73742 mg/min Milligram perMinuteC48154 min Minute Unit ofTimeA dose calculation unit equal to one thousandth of agram of a preparation per one kilogram of body massadministered per unit of time equal to one hour.(NCI)A dose calculation unit equal to one thousandth of agram of a preparation per one kilogram of body massadministered per unit of time equal to oneminute.(NCI)A unit of area density equal to approximately2.94935E-5 ounce per square yard. Also used as adose calculation unit.(NCI)A dose calculation unit expressed in milligram(s) persquare meter per period of time equal to twenty-fourhours. (NCI)A dose calculation unit expressed in milligram(s) persquare meter per period of time equal to sixty minutes.(NCI)A dose calculation unit expressed in milligram(s) persquare meter per period of time equal to sixty seconds.(NCI)Milligrams per minute.A unit measure of time equal to 60 seconds.(NCI)Milligram per Kilogram per HourMilligram per Kilogram per MinuteMilligram per Square Metermg/m2/daymg/m2/hmg/m2/minmg/minMinuteC70507 mkat Millikatal A unit of catalytic activity measurement equal to onethousandth of one katal (10E-3 katal).(NCI)C28251 mm Millimeter A unit of measure equal to one thousandth of a meter.(NCI)MillikatalMillimeterC67419 mm/h Millimeter perHourC65104 mm2 SquareMillimeterC49670 mmHg Millimeter ofMercuryC73764 mmHg/sec Millimeter ofMercury perSecondA unit of both speed (scalar) and velocity (vector),defined as the distance of one millimeter travels perunit time equal to one hour.(NCI)A unit of area measurement equal to a squaremeasuring one millimeter on each side. One squaremillimeter is equal to 10(E-2) square centimeter and10(E-6) square meter.(NCI)A unit of pressure equal to 0.001316 atmosphere andequal to the pressure indicated by one millimeter riseof mercury in a barometer at the Earth's surface. (NCI)A rate of inflation or deflation of a manometric devicebased on the unit of pressure equal to 133,332 Pa or1.316E10-3 standard atmosphere during period oftime equal to one sixtieth of a minute. (NCI)Millimeter per HourSquare MillimeterMillimeter of MercurymmHg/secC48513 mmol Millimole A unit of amount of substance equal to 0.001mole.(NCI)MillimoleSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 411 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC64387 mmol/L Millimole perLiter; umol/mL;Micromole perMilliliter;mol/m3; Moleper Cubic Meter;mcmol/mL;mmol/L;nmol/uLC67420 mmol/day Millimole per 24HoursC68740 mmol/g Millimole perGramC68892 mmol/kg Millimole perKilogramA unit of concentration (molarity unit) equal to onethousandth of a mole (10E-3 mole) of solute per oneliter of solution.(NCI)A unit of amount of substance flow rate equivalent tothe rate at which one thousandth of a mole ofsubstance travels or is delivered to a given object orspace over a period of time equal to 24 hours.(NCI)A unit amount of substance content (molality unit)defined as one mole of solute per one kilogram ofsolvent.(NCI)A unit of amount of substance content (molality unit)defined as one thousandth of mole (10E-3 mole) ofsolute per one kilogram of solvent.(NCI)Millimole per LiterMillimole per 24 HoursMole per KilogramMillimole per KilogramC42539 mol Mole A unit defined as the amount of substance that containsas many elementary units as there are atoms in 0.0<strong>12</strong>kg of carbon-<strong>12</strong>. When the mole is used, theelementary entities must be specified and may beatoms, molecules, ions, electrons, other particles, orspecified groups of such particles.(NCI)MoleC48555 mol/L Mole per Liter;mol/L; mmol/mLA unit of concentration (molarity unit) equal to onemole of solute in one liter of solution.(NCI)Mole per LiterC68893 mol/g Mole per Gram A unit of amount of substance content (molality unit)defined as one mole of solute per one gram ofsolvent.(NCI)Mole per GramC68891 mol/mL Mole perMilliliterC68894 mol/mg Mole perMilligramA unit of concentration (molarity unit) equal to onemole of solute in one milliliter of solution.(NCI)A unit of amount of substance content (molality unit)defined as one mole of solute per one milligram ofsolvent.(NCI)Mole per MilliliterMole per MilligramC70455 mol/mol Mole per Mole A unit of fraction expressed as the ratio of the amountof substance of solute in moles to the amount ofsubstance of the mixture in moles.(NCI)C41140 msec Millisecond A unit of time, which is equal to one thousandth of asecond.(NCI)C67352 nCi Nanocurie A unit of radioactivity equal to one billionth of Curieor 37 Becquerels, and corresponding to a radioactivityof 37 atomic disintegrations per second.(NCI)C69188 nL Nanoliter A unit of volume equal to one billionth of a liter(10E-9 liter).(NCI)C48516 ng Nanogram A unit of mass equal to one billionth (10E-9) of agram, or one millionth (10E-6) of a milligram.(NCI)Mole per MoleMillisecondNanocurieNanoliterNanogramSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 4<strong>12</strong> of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC67327 ng/L ug/m3;Microgram perCubic Meter;ng/L; pg/mLC67326 ng/dL Nanogram perDeciliterA unit of mass concentration defined as theconcentration of one nanogram of a substance per oneliter of the mixture, or one picogram of a substance inunit volume of the mixture equal to one milliliter, orone microgram of a substance per one cubic meter ofthe mixture. The concept also refers to the metric unitof mass density (volumic mass) defined as the densityof substance which mass equal to one nanogramoccupies the volume one liter.(NCI)A unit of mass concentration defined as theconcentration of one nanogram of a substance in unitvolume of the mixture equal to one deciliter. Theconcept also refers to the unit of mass density(volumic mass) defined as the density of substancewhich mass equal to one nanogram occupies thevolume one deciliter.(NCI)Nanogram per LiterNanogram per DeciliterC70508 nkat Nanokatal A unit of catalytic activity measurement equal to onebillionth of one katal (10E-9 katal).(NCI)NanokatalC70510 nkat/L Nanokatal perLiterA unit of catalytic activity concentration defined as thecatalytic activity of the component equal to onebillionth of one katal (10E-9 katal) in the unit volumeof the system equal to one liter.(NCI)Nanokatal per LiterC67328 nm Nanometer A unit of length equal to one billionth of a meter(10E-9 meter). Nanometer is used as a unit for lightwavelength measurement.(NCI)C48517 nmol Nanomole A unit of amount of substance equal to one billionth(10E-9) of a mole.(NCI)NanometerNanomoleC67432 nmol/L Nanomole perLiterC92613 nmol/mL/min Nanomole perMilliliter perMinuteA unit of concentration (molarity unit) equal to onebillionth of a mole (10E-9 mole) of solute in one literof solution.(NCI)A unit of concentration (molarity unit) equal to onebillionth of a mole (10E-9 mole) of solute in onemilliliter of solution to be administered per minute oftime.Nanomole per LiterNanomole per Minute per MilliliterC73767 nsec Nanosecond A unit of time equal to one billionth of a second. (NCI) nsecC42554 ohm Ohm A unit of electrical resistance equal to the resistancebetween two points on a conductor when a potentialdifference of one volt between them produces acurrent of one Ampere. Ohm is also used to measureimpedance and reactance for complex resistance. Ameasurement in ohms is the reciprocal of ameasurement in Siemens. (NCI)C67330 osm Osmole A unit of osmotic pressure equal to that of an idealsolution of a nondissociating substance that has aconcentration of one mole of solute per liter ofsolution.(NCI)C48519 oz Ounce A unit of mass, the avoirdupois ounce is equal to 1/16pound, or 28.3495 grams, or 0.911 457 troyounce.(NCI)C45997 pH pH A measure of the acidity or alkalinity of a fluid on ascale of 0 to 14.(NCI)OhmOsmoleOuncepHSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 413 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC69189 pL Picoliter A unit of volume equal to one trillionth of a liter(10E-<strong>12</strong> liter). (NCI)C66967 per min Per Minute The number of occurrences of something within aminute of time.(NCI)C66965 per sec Per Second The number of occurrences of something within asecond of time.(NCI)C64551 pg Picogram A unit of mass equal to one trillionth of a gram(10E-<strong>12</strong> gram).(NCI)pLPer MinutePer SecondPicogramC67331 pg/dL Picogram perDeciliterPicograms per deciliter.pg/dLC70509 pkat Picokatal A unit of catalytic activity measurement equal totrillionth of one katal (10E-<strong>12</strong> katal).(NCI)C69148 pm Picometer A unit of length equal to one trillionth of a meter(10E-<strong>12</strong> meter).(NCI)C65045 pmol Picomole A unit of amount of substance equal to a trillionth(10E-<strong>12</strong>) of a mole.(NCI)PicokatalPicometerPicomoleC67434 pmol/L Picomole perLiter; fmol/mL;Femtomole perMilliliterA unit of concentration (molarity unit) equal to onetrillionth of a mole (10E-<strong>12</strong> mole) in one liter ofsolution.(NCI)Picomole per LiterC70565 ppb Part per Billion A unit of measure referring to one entity counted perone billion entities.(NCI)C48523 ppm Part per Million A unit of measurement referring to one entity countedper one million entities.(NCI)Per BillionPart Per MillionC691<strong>12</strong> ppth Part perThousandA unit of proportion equal to 10E-3.(NCI)Part per ThousandC70566 pptr Parts per Trillion A unit of measure referring to one entity counted perone trillion entities.(NCI)C73768 psec Picosecond A unit of time equal to one trillionth of a second.(NCI)Per TrillionpsecC67334 psi Pounds perSquare InchC70469 rpm Revolution perMinuteC68858 scm Standard CubicMeterA unit of pressure equivalent to 6.894757 kilopascals,or 703.0696 kilograms per square meter, or 51.71507millimeters of mercury.(NCI)A unit of frequency equal to one revolution per unit oftime equal to one minute.(NCI)A unit used in physical chemistry to express theamount of substance of an ideal gas in one cubic meterat standard conditions: temperature 273.15 K andpressure of one atmosphere (101.325kilopascals).(NCI)Pound per Square InchRevolution per MinuteStandard Cubic MeterC42535 sec Second The second is a unit of time with a duration of 919 263177 0 periods of the specified light radiationcorresponding to the transition between the twohyperfine levels of the cesium 133 atom in its groundstate at 0 K. According to the convention, 60 secondsconstitute one minute; 3,600 seconds constitute onehour. Abbreviation Sec. is acceptable in non-scientificusage only.(NCI)SecondSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 414 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC48544 tsp Teaspoon DosingUnitA unit of volume used in pharmacy equal to 5milliliters.(NCI)Teaspoon Dosing UnitC65132 tuberculin unit Tuberculin Unit An arbitrary unit of tuberculin dosage defined bycomparison of clinical response with a preparation ofthe purified protein derivative standardized for use inhumans for tuberculin skin test reaction.(NCI)Tuberculin UnitC70506 tuberculin unit/mL Tuberculin Unitper MilliliterC48507 uCi Microcurie;mcCiC71173 uCi/L Microcurie perLiter; mcCi/LC70571 uCi/kg Microcurie perKilogram;mcCi/kgA unit of biologic activity of tuberculin expressed as anumber of arbitrary units of tuberculin in one milliliterof preparation.(NCI)A unit of radioactivity equal to one millionth of aCurie or 37 kilobecquerels, and corresponding to aradioactivity of 37 000 atomic disintegrations persecond.(NCI)A unit of volumetric radioactivity concentrationdefined as a concentration of a radionuclide with anactivity equal to one millionth of a Curie per unitvolume equal to one liter.(NCI)A unit of specific radioactivity (massic activity) equalto activity of one microcurie of the sample with totalmass of one kilogram.(NCI)Tuberculin Unit per MilliliterMicrocurieMicrocurie per LiterMicrocurie per KilogramC73726 uEq Microequivalent A unit of relative amount of a substance equal to onemillionth of an equivalent weight.(NCI)uEqC67405 uIU/mL Micro-International Unit permilliliter; mIU/L;mcIU/mL;uIU/mLC48153 uL mcL; Microliter;mm3C69175 uL/mL Microliter perMilliliter;mcL/mLMicrointernational units per milliliter.A unit of volume accepted for use with the SI and equalto one millionth of a liter (10E-6 liter).(NCI)A unit of volume fraction expressed as a number ofmicroliters of the constituent per the volume of thesystem represented in milliliters.(NCI)uIU/mLuLMicroliter per MilliliterC73736 uOsM Microosmole A unit of osmotic pressure equal to one millionth of anosmole or the osmotic pressure of a 10E-6 molarsolution of a substance that does not dissociate. (NCI)C71175 uV Microvolt; mcV A unit of an electric potential and electromotive forceequal to one millionth of a volt.(NCI)C48152 ug Microgram; mcg The unit of mass equal to one millionth of a gram orone thousandth of a milligram.(NCI)uOsMMicrovoltMicrogramC67306 ug/L mg/m3;Microgram perLiter; Milligramper Cubic Meter;Nanogram perMilliliter; ug/L;ng/mL; mcg/LC73728 ug/animal Microgram perAnimalA unit of mass concentration defined as theconcentration of one microgram of a substance perunit volume of the mixture equal to one liter. Theconcept also refers to the unit of mass density(volumic mass) defined as the density of substancewhich mass equal to one microgram occupies thevolume one liter.(NCI)A unit of measure expressed in microgram(s) peranimal.Microgram per Literug/animalSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 415 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC67305 ug/dL Microgram perDeciliterC7<strong>12</strong>05 ug/day Microgram perDay; mcg/dayC74921 ug/g/day Microgram perGram per DayC74922 ug/g/h Microgram perGram per HourC74923 ug/g/min Microgram perGram per MinuteC67394 ug/h Micrograms perHour; mcg/hC67396 ug/kg mcg/kg;Microgram perKilogram; ng/g;ug/kgC73729 ug/kg/day Microgram perKilogram perDayC73730 ug/kg/h Microgram perKilogram perHourC7<strong>12</strong>10 ug/kg/min Microgram perKilogram perMinute; Gammaper Kilogram perMinute;gamma/kg/min;mcg/kg/minC673<strong>12</strong> ug/m2 Microgram perSquare MeterC73787 ug/m2/day Microgram perSquare Meter perDayC73727 ug/m2/h Microgram perSquare Meter perHourA unit of mass concentration defined as theconcentration of one microgram of a substance perunit volume of the mixture equal to one deciliter. Theconcept also refers to the unit of mass density(volumic mass) defined as the density of substancewhich mass equal to one microgram occupies thevolume one deciliter. (NCI)A unit of mass flow rate equivalent to the rate at whichone millionth of a gram of matter travels to a givenobject or space over a period of time equal to twentyfour hours. Microgram per day is also a doseadministration rate unit equal to the rate at which onemillionth of a gram of a product is administered perunit of time equal to twenty four hours.(NCI)A dose calculation unit expressed in microgram(s) pergram per period of time equal to twenty-four hours.(NCI)A dose calculation unit expressed in microgram(s) pergram per period of time equal to sixty minutes. (NCI)A dose calculation unit expressed in microgram(s) pergram per period of time equal to sixty seconds. (NCI)A unit of mass flow rate equivalent to the rate at whichone millionth of a gram of matter travels to a givenobject or space over a period of time equal to onehour.(NCI)A unit of a mass fraction expressed as a number ofmicrograms of substance per kilogram of mixture. Theunit is also used as a dose calculation unit.(NCI)A dose calculation unit expressed in microgram(s) perkilogram per period of time equal to twenty-fourhours. (NCI)A dose calculation unit expressed in microgram(s) perkilogram per period of time equal to sixty minutes.(NCI)A dose calculation unit equal to one millionth of agram of a preparation per one kilogram of body massadministered per unit of time equal to oneminute.(NCI)A dose calculation unit expressed in microgram(s) persquare meter.A dose calculation unit expressed in microgram(s) persquare meter per period of time equal to twenty-fourhours. (NCI)A dose calculation unit expressed in microgram(s) persquare meter per period of time equal to sixty minutes.(NCI)ug/dLMicrogram per Dayug/g/dayug/g/hug/g/minMicrogram per HourMicrogram per Kilogramug/kg/dayug/kg/hMicrogram per Kilogram per Minuteug/m2ug/m2/dayug/m2/hSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 416 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C71620 - UNIT - UnitCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC73733 ug/m2/min Microgram perSquare Meter perMinuteC75905 ug/mL/h Microgram perMilliliter perHourC7<strong>12</strong>11 ug/min Micrograms perMinute; mcg/minC70562 ukat Microkatal;mckatC67397 ukat/L Microkatal perLiter; mckat/LA dose calculation unit expressed in microgram(s) persquare meter per period of time equal to sixty seconds.(NCI)A dose calculation unit expressed in microgram(s) permilliliter of solution per period of time equal to sixtyminutes. (NCI)A unit of mass flow rate equivalent to the rate at whichone millionth of a gram of matter travels to a givenobject or space over a period of time equal to oneminute. Microgram per minute is also a doseadministration rate unit equal to the rate at which onemillionth of a gram of a product is administered perunit of time equal to one minute.(NCI)A unit of catalytic activity measurement equal to onemillionth of katal (10E-6 katal).(NCI)Unit of catalytic activity concentration defined asactivity equal to one millionth of katal per one liter ofthe system volume.(NCI)ug/m2/minug/mL/hMicrogram per MinuteMicrokatalMicrokatal per LiterC48510 um Micron; mcm A unit of length in metric system equal to 10E-6meter, or micrometer.(NCI)MicronC73770 um2 MicroSquareMeterC67565 um3 CubicMicrometerC48509 umol Micromole;mcmolC48508 umol/L Micromole perLiterC67406 umol/day Micromoles perDay; mcmol/dayC73735 umol/mg/min Micromole perMilligram perMinuteA SI unit of area measurement equal to a square whosesides are one micrometer long. (NCI)A unit of volume equal to 10E-18 liter or one cubicmicrometer.A unit of amount of substance equal to one millionth(10E-6) of a mole.(NCI)A unit of concentration (molarity unit) equal to oneone-millionth of a mole (10E-6 mole) of solute perone liter of solution. (NCI)A unit of amount of substance flow rate equivalent tothe rate at which one millionth of a mole of substancetravels to a given object or space over a period of timeequal to 24 hours.(NCI)A unit of concentration (molarity unit) equal to onemillionth of a mole (10E-6 mole) per milligram of asubstance per period of time equal to sixty seconds.(NCI)Square MicrometerAttoliterMicromoleumol/LMicromole per 24 Hoursumol/mg/minC69149 usec Microsecond A unit of time equal to one millionth of a second.(NCI)C48553 yd Yard A unit of length equal to 3 feet, or 36 inches, or0.9144 meter.(NCI)usecYardSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 417 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66770 - VSRESU - Units for Vital Signs ResultsCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC25613 % Percentage A fraction or ratio with 100 understood as thedenominator. (NCI)C49673 BEATS/MIN Beats per Minute The number of heartbeats measured per minute time.(NCI)PercentageBeats per MinuteC49674 BREATHS/MIN Breaths perMinuteThe number of breaths (inhalation and exhalation)taken per minute time. (NCI)Breaths per MinuteC42559 C Degree Celsius A unit of temperature of the temperature scaledesigned so that the freezing point of water is 0degrees and the boiling point is 100 degrees atstandard atmospheric pressure. The current officialdefinition of the Celsius sets 0.01 C to be at the triplepoint of water and a degree to be 1/273.16 of thedifference in temperature between the triple point ofwater and absolute zero. One degree Celsiusrepresents the same temperature difference as oneKelvin. (NCI)Degree CelsiusC44277 F DegreeFahrenheitThe Fahrenheit temperature scale is named after theGerman physicist Gabriel Fahrenheit (1686-1736),who proposed it in 1724. In this scale, the freezingpoint of water is 32 degrees Fahrenheit and the boilingpoint is 2<strong>12</strong> degrees, placing the boiling and meltingpoints of water 180 degrees apart. In this scale adegree Fahrenheit is 5/9ths of a Kelvin (or of a degreeCelsius), and minus 40 degrees Fahrenheit is equal tominus 40 degrees Celsius. (NCI)Degree FahrenheitC48500 IN Inch A traditional unit of length equal to 2.54 centimeters.(NCI)C48531 LB Pound A traditional unit of mass. By international agreement,one avoirdupois pound is equal to exactly 0.453 59237 kilogram, 16 ounces, or 1.215 28 troy pounds.(NCI)C49668 cm Centimeter A basic unit of length in the former CGS version ofmetric system, equal to one hundredth of a meter orapproximately 0.393 700 787 inch. (NCI)C48155 g Gram A metric unit of mass equal to one one thousandth of akilogram. (NCI)C28252 kg Kilogram The basic SI unit of mass. It is defined as the mass ofan international prototype in the form of aplatinum-iridium cylinder kept at Sevres in France. It isthe only basic unit still defined in terms of a materialobject, and also the only one with a prefix [kilo]already in place. A kilogram is equal to 1,000 gramsand 2.204 622 6 pounds. (NCI)InchPoundCentimeterGramKilogramC49671 kg/m2 Kilogram PerSquare MeterThe SI derived unit of spread rate of a substance bymass, used also as a measure of area density. (NCI)Kilogram Per Square MeterC42569 m2 Square Meter The SI unit of area measurement equal to a squarewhose sides are one meter long. Square meter is equalto 10,000 square centimeters; 0.01 ares; 1.196 squareyards; 10.76 square feet; 1550 square inches. (NCI)Square MeterSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 418 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66770 - VSRESU - Units for Vital Signs ResultsCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC49670 mmHg Millimeter ofMercuryA unit of pressure equal to 0.001316 atmosphere andequal to the pressure indicated by one millimeter riseof mercury in a barometer at the Earth's surface. (NCI)Millimeter of MercuryC42554 ohm Ohm A unit of electrical resistance equal to the resistancebetween two points on a conductor when a potentialdifference of one volt between them produces acurrent of one Ampere. Ohm is also used to measureimpedance and reactance for complex resistance. Ameasurement in ohms is the reciprocal of ameasurement in Siemens. (NCI)OhmSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 419 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67153 - VSTEST - Vital Signs Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103346 Abdominal Skinfold Thickness AbdominalSkinfoldThicknessC<strong>12</strong>472 Adipose Tissue Adipose Tissue;Body Fat; FatTissueA measurement for determining the subcutaneous fatlayer thickness whereby a pinch of skin approximatelyfive centimeters to the right of the umbilicus ismeasured using calipers. (NCI)A specialized form of connective tissue consistingprimarily of adipocytes (fat cells), surrounded by ameshwork of collagen fibers.Abdominal Skinfold ThicknessAdipose TissueC49680 Body Frame Size Body Frame Size The categorization of a person's body frame into small,medium and large based on the measurement of wristcircumference or the breadth of the elbow. (NCI)C16358 Body Mass Index Body Mass Index A general indicator of the body fat an individual iscarrying based upon the ratio of weight to height.(NCI)Body Frame SizeBody Mass IndexC25157 Body Surface Area Body SurfaceAreaC25299 Diastolic Blood Pressure Diastolic BloodPressureC100946 Forearm Circumference ForearmCircumferenceC8<strong>12</strong>55 Head Circumference HeadCircumferenceA measure of the 2-dimensional extent of the bodysurface (i.e., the skin). Body surface area (BSA) can becalculated by mathematical formula or from a chartthat relates height to weight. BSA is often an importantfactor in dosing. (NCI)The blood pressure after the contraction of the heartwhile the chambers of the heart refill with blood.(NCI)The distance around an individual's forearm.A circumferential measurement of the head at thewidest point, which is traditionally above theeyebrows.Body Surface AreaDiastolic Blood PressureForearm CircumferenceHead CircumferenceC49677 Heart Rate Heart Rate The number of heartbeats per unit of time, usuallyexpressed as beats per minute. (NCI)C25347 Height Height The vertical measurement or distance from the base tothe top of an object; the vertical dimension ofextension. (NCI)Heart RateHeightC100947 Hip Circumference HipCircumferenceC84372 Knee to Heel Length Knee to HeelLength; LowerLeg LengthThe distance around an individual's pelvic area or hips.A measurement of the length of the lower leg from thetop of the knee to the bottom of the heel. Thismeasurement may be taken with a knemometer orcalipers. (NCI)Hip CircumferenceKnee to Heel Length MeasurementC7<strong>12</strong>58 Lean Body Mass Lean Body Mass The weight of all organs and tissue in an individual lessthe weight of the individual's body fat.Lean Body MassC49679 Mean Arterial Pressure Mean ArterialPressureThe mean pressure of the blood within the arterialcirculation. The arterial pressure may be directlymeasured by insertion of an intra-arterial catheterconnected to a transducer. The mean arterial pressure(MAP) can be calculated by subsequent analysis of thewaveform. MAP can be approximated without aninvasive procedure using the following formula:diastolic pressure plus 1/3 of the pulse pressure,where pulse pressure is systolic pressure - diastolicpressure. (NCI)Mean Arterial PressureSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 420 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C67153 - VSTEST - Vital Signs Test NameCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC60832 Oxygen Saturation OxygenSaturationA measurement of the oxygen-hemoglobin saturationof a volume of blood.Oxygen Saturation MeasurementC100945 Pulse Pressure Pulse Pressure The change in systolic to diastolic pressure whichproduces a pulse.C49676 Pulse Rate Pulse Rate The rate of the pulse as observed in an artery,expressed as beats per minute. It can be measured atseveral anatomical sites, including the wrist, neck,temple, groin, behind the knees, or on top of the foot.(NCI)C49678 Respiratory Rate Respiratory Rate The rate of breathing (inhalation and exhalation)measured within in a unit time, usually expressed asbreaths per minute. (NCI)Pulse PressurePulse RateRespiratory RateC87054 Sagittal Abdominal Diameter SagittalAbdominalDiameterA standard measure of visceral obesity, or abdominalfat, which is measured from the patient's back to upperabdomen between the bottom of the rib cage and thetop of the pelvic area. This measurement may be takenwith the patient standing or in the supine position.(NCI)Sagittal Abdominal DiameterC98785Subscapular SkinfoldThicknessSubscapularSkinfoldThicknessA measurement of the thickness of a pinch of skinsituated below or on the underside of the scapula.(NCI)Subscapular Skinfold ThicknessC25298 Systolic Blood Pressure Systolic BloodPressureThe blood pressure during the contraction of the leftventricle of the heart.Systolic Blood PressureC25206 Temperature Temperature The property of a body or region of space thatdetermines whether or not there will be a net flow ofheat into it or out of it from a neighboring body orregion and in which direction (if any) the heat willflow, perceptible by living organism as a somaticsensation of cold or heat. It is a measure of the averagetranslational kinetic energy associated with thedisordered microscopic motion of atoms andmolecules. Temperature is measured in one of thethree standard temperature scales: Celsius, Kelvin, andFahrenheit. (NCI)TemperatureC98793 Triceps Skinfold Thickness Triceps SkinfoldThicknessC100948 Waist Circumference WaistCircumferenceA measurement of the thickness of a pinch of skin onthe triceps. (NCI)The distance around an individual's midsection orwaist.Triceps Skinfold ThicknessWaist CircumferenceC25208 Weight Weight The vertical force exerted by a mass as a result ofgravity. (NCI)WeightSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 421 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66741 - VSTESTCD - Vital Signs Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC103346 ABSKNF AbdominalSkinfoldThicknessA measurement for determining the subcutaneous fatlayer thickness whereby a pinch of skin approximatelyfive centimeters to the right of the umbilicus ismeasured using calipers. (NCI)Abdominal Skinfold ThicknessC16358 BMI Body Mass Index A general indicator of the body fat an individual iscarrying based upon the ratio of weight to height.(NCI)Body Mass IndexC<strong>12</strong>472 BODYFAT Adipose Tissue;Body Fat; FatTissueC25157 BSA Body SurfaceAreaC25299 DIABP Diastolic BloodPressureC100946 FARMCIR ForearmCircumferenceA specialized form of connective tissue consistingprimarily of adipocytes (fat cells), surrounded by ameshwork of collagen fibers.A measure of the 2-dimensional extent of the bodysurface (i.e., the skin). Body surface area (BSA) can becalculated by mathematical formula or from a chartthat relates height to weight. BSA is often an importantfactor in dosing. (NCI)The blood pressure after the contraction of the heartwhile the chambers of the heart refill with blood.(NCI)The distance around an individual's forearm.Adipose TissueBody Surface AreaDiastolic Blood PressureForearm CircumferenceC49680 FRMSIZE Body Frame Size The categorization of a person's body frame into small,medium and large based on the measurement of wristcircumference or the breadth of the elbow. (NCI)Body Frame SizeC8<strong>12</strong>55 HDCIRC HeadCircumferenceA circumferential measurement of the head at thewidest point, which is traditionally above theeyebrows.Head CircumferenceC25347 HEIGHT Height The vertical measurement or distance from the base tothe top of an object; the vertical dimension ofextension. (NCI)HeightC100947 HIPCIR HipCircumferenceThe distance around an individual's pelvic area or hips.Hip CircumferenceC49677 HR Heart Rate The number of heartbeats per unit of time, usuallyexpressed as beats per minute. (NCI)Heart RateC84372 KNEEHEEL Knee to HeelLength; LowerLeg LengthA measurement of the length of the lower leg from thetop of the knee to the bottom of the heel. Thismeasurement may be taken with a knemometer orcalipers. (NCI)Knee to Heel Length MeasurementC7<strong>12</strong>58 LBM Lean Body Mass The weight of all organs and tissue in an individual lessthe weight of the individual's body fat.Lean Body MassC49679 MAP Mean ArterialPressureThe mean pressure of the blood within the arterialcirculation. The arterial pressure may be directlymeasured by insertion of an intra-arterial catheterconnected to a transducer. The mean arterial pressure(MAP) can be calculated by subsequent analysis of thewaveform. MAP can be approximated without aninvasive procedure using the following formula:diastolic pressure plus 1/3 of the pulse pressure,where pulse pressure is systolic pressure - diastolicpressure. (NCI)Mean Arterial PressureSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 422 of 423 -
CDISC <strong>SEND</strong> Controlled <strong>Terminology</strong>, <strong>20<strong>12</strong></strong>-<strong>12</strong>-21C66741 - VSTESTCD - Vital Signs Test CodeCodelist extensible: YesNCI CodeCDISC Submission ValueCDISCSynonym CDISC Definition NCI Preferred TermC60832 OXYSAT OxygenSaturationA measurement of the oxygen-hemoglobin saturationof a volume of blood.Oxygen Saturation MeasurementC49676 PULSE Pulse Rate The rate of the pulse as observed in an artery,expressed as beats per minute. It can be measured atseveral anatomical sites, including the wrist, neck,temple, groin, behind the knees, or on top of the foot.(NCI)C100945 PULSEPR Pulse Pressure The change in systolic to diastolic pressure whichproduces a pulse.C49678 RESP Respiratory Rate The rate of breathing (inhalation and exhalation)measured within in a unit time, usually expressed asbreaths per minute. (NCI)Pulse RatePulse PressureRespiratory RateC87054 SAD SagittalAbdominalDiameterC98785 SSSKNF SubscapularSkinfoldThicknessC25298 SYSBP Systolic BloodPressureA standard measure of visceral obesity, or abdominalfat, which is measured from the patient's back to upperabdomen between the bottom of the rib cage and thetop of the pelvic area. This measurement may be takenwith the patient standing or in the supine position.(NCI)A measurement of the thickness of a pinch of skinsituated below or on the underside of the scapula.(NCI)The blood pressure during the contraction of the leftventricle of the heart.Sagittal Abdominal DiameterSubscapular Skinfold ThicknessSystolic Blood PressureC25206 TEMP Temperature The property of a body or region of space thatdetermines whether or not there will be a net flow ofheat into it or out of it from a neighboring body orregion and in which direction (if any) the heat willflow, perceptible by living organism as a somaticsensation of cold or heat. It is a measure of the averagetranslational kinetic energy associated with thedisordered microscopic motion of atoms andmolecules. Temperature is measured in one of thethree standard temperature scales: Celsius, Kelvin, andFahrenheit. (NCI)TemperatureC98793 TRSKNF Triceps SkinfoldThicknessA measurement of the thickness of a pinch of skin onthe triceps. (NCI)Triceps Skinfold ThicknessC25208 WEIGHT Weight The vertical force exerted by a mass as a result ofgravity. (NCI)WeightC100948 WSTCIR WaistCircumferenceThe distance around an individual's midsection orwaist.Waist CircumferenceSource: NCI <strong>EVS</strong> <strong>Terminology</strong> Resources website: http://www.cancer.gov/cancertopics/terminologyresources/page6Source Date: <strong>20<strong>12</strong></strong>-<strong>12</strong>-21- page 423 of 423 -