Plant Sterols and Stanols as Cholesterol-Lowering Ingredients in ...
Plant Sterols and Stanols as Cholesterol-Lowering Ingredients in ...
Plant Sterols and Stanols as Cholesterol-Lowering Ingredients in ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
10 Recent Patents on Food, Nutrition & Agriculture, 2009, Vol. 1, No. 1 Kamal-Eld<strong>in</strong> <strong>and</strong> Moazzami<br />
(Table 2) Contd….<br />
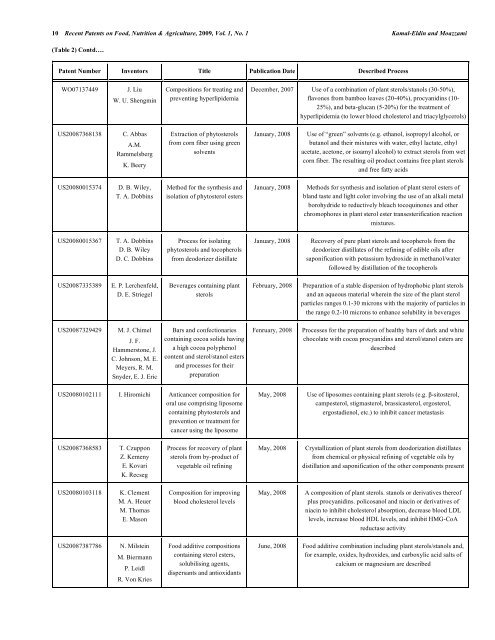
Patent Number Inventors Title Publication Date Described Process<br />
WO07137449 J. Liu<br />
W. U. Shengm<strong>in</strong><br />
US20087368138 C. Abb<strong>as</strong><br />
A.M.<br />
Rammelsberg<br />
K. Beery<br />
US20080015374 D. B. Wiley,<br />
T. A. Dobb<strong>in</strong>s<br />
US20080015367 T. A. Dobb<strong>in</strong>s<br />
D. B. Wiley<br />
D. C. Dobb<strong>in</strong>s<br />
US20087335389 E. P. Lerchenfeld,<br />
D. E. Striegel<br />
US20087329429 M. J. Chimel<br />
J. F.<br />
Hammerstone, J.<br />
C. Johnson, M. E.<br />
Meyers, R. M.<br />
Snyder, E. J. Eric<br />
Compositions for treat<strong>in</strong>g <strong>and</strong><br />
prevent<strong>in</strong>g hyperlipidemia<br />
Extraction of phytosterols<br />
from corn fiber us<strong>in</strong>g green<br />
solvents<br />
Method for the synthesis <strong>and</strong><br />
isolation of phytosterol esters<br />
Process for isolat<strong>in</strong>g<br />
phytosterols <strong>and</strong> tocopherols<br />
from deodorizer distillate<br />
Beverages conta<strong>in</strong><strong>in</strong>g plant<br />
sterols<br />
Bars <strong>and</strong> confectionaries<br />
conta<strong>in</strong><strong>in</strong>g cocoa solids hav<strong>in</strong>g<br />
a high cocoa polyphenol<br />
content <strong>and</strong> sterol/stanol esters<br />
<strong>and</strong> processes for their<br />
preparation<br />
US20080102111 I. Hiromichi Anticancer composition for<br />
oral use compris<strong>in</strong>g liposome<br />
conta<strong>in</strong><strong>in</strong>g phytosterols <strong>and</strong><br />
prevention or treatment for<br />
cancer us<strong>in</strong>g the liposome<br />
US20087368583<br />
T. Czuppon<br />
Z. Kemeny<br />
E. Kovari<br />
K. Recseg<br />
US20080103118 K. Clement<br />
M. A. Heuer<br />
M. Thom<strong>as</strong><br />
E. M<strong>as</strong>on<br />
US20087387786 N. Milste<strong>in</strong><br />
M. Biermann<br />
P. Leidl<br />
R. Von Kries<br />
Process for recovery of plant<br />
sterols from by-product of<br />
vegetable oil ref<strong>in</strong><strong>in</strong>g<br />
Composition for improv<strong>in</strong>g<br />
blood cholesterol levels<br />
Food additive compositions<br />
conta<strong>in</strong><strong>in</strong>g sterol esters,<br />
solubilis<strong>in</strong>g agents,<br />
dispersants <strong>and</strong> antioxidants<br />
December, 2007 Use of a comb<strong>in</strong>ation of plant sterols/stanols (30-50%),<br />
flavones from bamboo leaves (20-40%), procyanid<strong>in</strong>s (10-<br />
25%), <strong>and</strong> beta-glucan (5-20%) for the treatment of<br />
hyperlipidemia (to lower blood cholesterol <strong>and</strong> triacylglycerols)<br />
January, 2008 Use of “green” solvents (e.g. ethanol, isopropyl alcohol, or<br />
butanol <strong>and</strong> their mixtures with water, ethyl lactate, ethyl<br />
acetate, acetone, or isoamyl alcohol) to extract sterols from wet<br />
corn fiber. The result<strong>in</strong>g oil product conta<strong>in</strong>s free plant sterols<br />
<strong>and</strong> free fatty acids<br />
January, 2008 Methods for synthesis <strong>and</strong> isolation of plant sterol esters of<br />
bl<strong>and</strong> t<strong>as</strong>te <strong>and</strong> light color <strong>in</strong>volv<strong>in</strong>g the use of an alkali metal<br />
borohydride to reductively bleach tocoqu<strong>in</strong>ones <strong>and</strong> other<br />
chromophores <strong>in</strong> plant sterol ester transesterification reaction<br />
mixtures.<br />
January, 2008 Recovery of pure plant sterols <strong>and</strong> tocopherols from the<br />
deodorizer distillates of the ref<strong>in</strong><strong>in</strong>g of edible oils after<br />
saponification with pot<strong>as</strong>sium hydroxide <strong>in</strong> methanol/water<br />
followed by distillation of the tocopherols<br />
February, 2008 Preparation of a stable dispersion of hydrophobic plant sterols<br />
<strong>and</strong> an aqueous material where<strong>in</strong> the size of the plant sterol<br />
particles ranges 0.1-30 microns with the majority of particles <strong>in</strong><br />
the range 0.2-10 microns to enhance solubility <strong>in</strong> beverages<br />
Fenruary, 2008 Processes for the preparation of healthy bars of dark <strong>and</strong> white<br />
chocolate with cocoa procyanid<strong>in</strong>s <strong>and</strong> sterol/stanol esters are<br />
described<br />
May, 2008 Use of liposomes conta<strong>in</strong><strong>in</strong>g plant sterols (e.g. �-sitosterol,<br />
campesterol, stigm<strong>as</strong>terol, br<strong>as</strong>sic<strong>as</strong>terol, ergosterol,<br />
ergostadienol, etc.) to <strong>in</strong>hibit cancer met<strong>as</strong>t<strong>as</strong>is<br />
May, 2008<br />
Crystallization of plant sterols from deodorization distillates<br />
from chemical or physical ref<strong>in</strong><strong>in</strong>g of vegetable oils by<br />
distillation <strong>and</strong> saponification of the other components present<br />
May, 2008 A composition of plant sterols. stanols or derivatives thereof<br />
plus procyanid<strong>in</strong>s. policosanol <strong>and</strong> niac<strong>in</strong> or derivatives of<br />
niac<strong>in</strong> to <strong>in</strong>hibit cholesterol absorption, decre<strong>as</strong>e blood LDL<br />
levels, <strong>in</strong>cre<strong>as</strong>e blood HDL levels, <strong>and</strong> <strong>in</strong>hibit HMG-CoA<br />
reduct<strong>as</strong>e activity<br />
June, 2008 Food additive comb<strong>in</strong>ation <strong>in</strong>clud<strong>in</strong>g plant sterols/stanols <strong>and</strong>,<br />
for example, oxides, hydroxides, <strong>and</strong> carboxylic acid salts of<br />
calcium or magnesium are described