460-13923-01-B_COBRA Bipolar Surgical System.indd
460-13923-01-B_COBRA Bipolar Surgical System.indd
460-13923-01-B_COBRA Bipolar Surgical System.indd
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
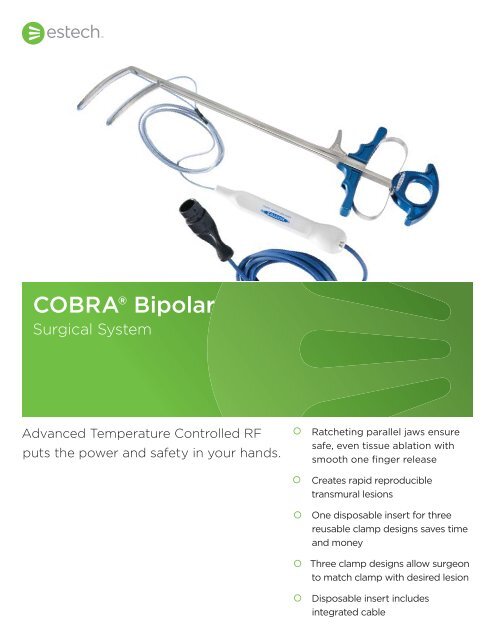
<strong>COBRA</strong>® <strong>Bipolar</strong><br />
<strong>Surgical</strong> <strong>System</strong><br />
Advanced Temperature Controlled RF<br />
puts the power and safety in your hands.<br />
Ratcheting parallel jaws ensure<br />
safe, even tissue ablation with<br />
smooth one fi nger release<br />
Creates rapid reproducible<br />
transmural lesions<br />
One disposable insert for three<br />
reusable clamp designs saves time<br />
and money<br />
Three clamp designs allow surgeon<br />
to match clamp with desired lesion<br />
Disposable insert includes<br />
integrated cable
<strong>COBRA</strong>® is the Clear Choice<br />
Any <strong>Bipolar</strong> approach with consistently reproducible results<br />
Reusable Clamps with Disposable <strong>Bipolar</strong> inserts provide signifi cant cost savings<br />
REUSABLE CLAMPS<br />
<strong>COBRA</strong>® Electrosurgical Unit<br />
Refer to Instructions for Use for Patent Information <strong>460</strong>-<strong>13923</strong>-<strong>01</strong>_Rev-B<br />
DISPOSABLE BIPOLAR INSERTS<br />
In the U.S., the Estech <strong>COBRA</strong> RF ablation products have been cleared for ablation of either cardiac<br />
or soft tissues. The AFfi rm Pacing Probe has been cleared to be used upon completion of the cardiac<br />
ablation procedure to assess the adequacy of cardiac lesions created in surgically treating the patient’s<br />
arrhythmia. Estech does not promote off-label use of its products and their use is at the discretion of<br />
the cardiac surgeon. Estech intends to undertake an IDE clinical trial and subsequent PMA submission<br />
to obtain a specifi c atrial fi brillation indication. In Europe, the Estech <strong>COBRA</strong> RF ablation products are<br />
CE marked with an indication for the treatment of atrial fi brillation by ablating cardiac tissue during<br />
surgery.<br />
Product Description Catalog Number<br />
<strong>COBRA</strong> <strong>Bipolar</strong> Clamp, Straight 604-N 1<strong>01</strong>61F<br />
<strong>COBRA</strong> <strong>Bipolar</strong> Clamp, Parallel, Right Curved 604-5<strong>01</strong><br />
<strong>COBRA</strong> <strong>Bipolar</strong> Clamp, Parallel, Left Curved 604-502<br />
<strong>COBRA</strong> Electrosurgical Unit (ESU) 115V 604-4810B<br />
<strong>COBRA</strong> Electrosurgical Unit (ESU) 230V 604-4811B<br />
<strong>COBRA</strong> Disposable <strong>Bipolar</strong> Inserts 600-006i<br />
2603 Camino Ramon, Suite 100, San Ramon, CA 94583 USA<br />
Phone: 1.925.866.7111 Fax: 1.925.866.7117 www.estech.com