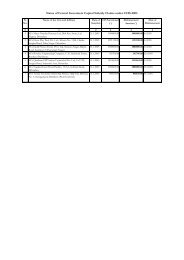
License Under Drug/Cosmetics Act - Doiuk.org
License Under Drug/Cosmetics Act - Doiuk.org
License Under Drug/Cosmetics Act - Doiuk.org
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
LokLF; lsok egkfuns'kky;] pUnj uxj] nsgjknwu¼vkS"kf/k d{k½Mªx ykblsUl gsrq psd&fyLV1- vkosnu&i= iw.kZ :i ls Hkjk tkuk gksxkA2- okafNr 'kqYd ds lca/k esa pkyku dh ewy izfr A3- xBu ds laca/k esa ?kks"k.kk fd vkosnd ,d dEiuh gS vFkok lgHkkfxrk ;qDrQeZ gS vFkok ,dy LokfeRo gS ds Øe esa vkfVZfdy vkWQ eseksjs.Me]lgHkkfxrk i= dh Nk;k izfr e; vf/kÑr O;fDr dh ?kks"k.kk ds ;fn vko';dgS@LokfeRo ds laca/k esa 'kiFk i= tks fd uksVjh }kjk izekf.kr gks] gksukpkfg;sA4- iznw"k.k foHkkx ls vukifÙk izek.k&i=A5- Hkou dk ekufp= ¼Cyw fizaV½ tks fd fdlh vf/kÑr okLrqfon }kjk izekf.kr gksrhu izfr;ksa esa okafNr gksxkA6- is;ty ds laca/k esa is;rk dh ijh{k.k fjiksVZ] dSfedy rRoksa ls lEcaf/kr ijh{k.kfjiksVZ rFkk gkfudkjd thok.kqvksa ds lEcU/k esa fjiksVZ fd os is; ;ksX; gS] fdlhvuqeksfnr iz;ksx'kkyk vFkok jkT; LokLF; laLFkku ls izekf.kr gksA7- rduhdh deZpkfj;ksa] fuekZ.k ,oa fo'ys'k.k nksuksa ds lEcU/k esa 'kSf{kd ;ksX;rklEca/kh izek.k&i=] ftl Hkh vuqKkiu izkfÌdkjh ls vuqeksfnr dh izekf.kr izfr]fu;qfDr i= ,oa izHkkj xzg.k djus dh fLFkfr tksfd 'kiFk iwoZd izekf.kr gksokafNr gksxhA ;gk¡ ;g Hkh mYys[kuh; gS fd fuekZ.k ,oa fo'ys"k.k osÙkkvksa dsrhu ikliksVZ layXu ds izekf.kr QksVks Hkh okafNr gksaxsA8- Hkou ds laca/k esa LokfeRo ds laca/k esa izek.k i= fd og fdjk;s dk gS vFkokfuth gSA9- fuekZ.k midj.kksa dh lwphA10- fo'ys"k.k midj.kksa dh lwph tks fd oxkZokj 'kM~;wy esa fn;s gq, fooj.k dsvuqlkj gksxhA ;fn fdlh okg; iz;ksx'kkyk ls fdlh fo'ks"k ijh{k.k ds fy;sijh{k.k djk;k tkuk gks rks mldk mYys[kA11- izR;sd vkS"kf/k ds laca/k esa layXu ifjf'k"V ij okafNr lwpukvksa dk iw.kZr;kmYys[k fd;k tkukA12- fuekZ.k'kkyk ds deZpkfj;ksa ds lEca/k esa LokLF; ijh{k.k dh fjiksVZ] mudsoSDlhus'ku rFkk bu vkdqys'ku ds lEca/k esa izek.ki= rFkk lkef;d :i lsmuds fpfdRldh; ijh{k.k] oSDlhus'ku] bu vkdqys'ku fd;s tkus dh'kiFkiwoZd ?kks"k.kkA
13- vfXu'keu ds mik; tksfd iznku fd;s x;sa gksa dk fooj.kA14- Å"ek ds fy, iz;ksx fd;s tkus okys lk/ku fd fo|qr 'kfDr ls Å"ek dk iz;ksxfd;k tk;sxk vFkok dks;ys ds }kjk fd;k tk;sxk vFkok LVhe ds }kjk fd;ktk;sxk ,oa bl lEca/k esa fdl izdkj ds midj.k iz;qDr gksxs tSls LVhe dslEcU/k esa OokW;yj vyx vU; midj.k McytSdsV oSlsVl vkfn ds }kjk fd;ktk;sxkA;gk¡ ;g mYys[kuh; gS fd fuEu laoxksZa ds lEca/k esa %1½2½oSDlhu ,oa lhjk(yktZ okWY;we isjUVyl ds lEca/k esa vkosnu i= dh ,d ewy izfrvkids dk;kZy; esa fy;k tkuk leqfpr gksxk ftlesa fd lHkh ewyvfHkys[k gksaxs rFkk nks Nk;k izfr;ksa lfgr vkosnd dks egkvkS"kf/kfu;a=d] Hkkjr ljdkj] fuekZ.k Hkou] ubZ fnYyh rFkk nwljh izfrlgk;d vkS"kf/k fu;a=d] Hkkjr ljdkj lc&tksu] y[kuÅ] 364pUnzyksd] vyhxat y[kuÅ dks vkosnu }kjk izsf"kr djk;k tkuk gksxkA15- Qhl layXu lwph ds vuqlkj foHkkx }kjk izekf.kr [kkrk 'kh"kZd esa Vsªtjh esapkyku ls foHkkxh; vf/kdkjh }kjk ;g muds }kjk izfrfu/kk;fur O;fDr }kjktek dh tk;sxhA
¼rhu izfr;ksa esa½FORM 24[See Rule 69]Application for the grant of or renewal of a licence to manufacture of sale[or for distribution of] drugs other than those specified in [Schedules C,C(1) and X ]1. I/We________________________________________________of___________________________________________hereby applyfor the grant/renewal of a licence to manufacture on the premisessituated at___________________________________________thefollowing drugs, being drugs other than those specified in[Schedules C, C(1), and X] to the <strong>Drug</strong>s and <strong>Cosmetics</strong> Rules, 1945.2. Name of drugs categorized according to Schedule M.________________________________________________________________________________________________________________________________________________________________________________________________________________________3. Names, qualifications and experience of techincal staff employed formanufacture and testing.4. A fee of rupees____________________________has been creditedto Government under the head of account__________________________Date ____________________ Signature___________________Note: The application should be accompanied by a plan of the premises.
¼rhu izfr;ksa esa½FORM 24-A[ See Rule 69-A ]Application for the grant of or renewal of a licence to manufacture of sale [orfor distribution of] drugs other than those specified in [Schedules C, C(1) and X]1. I/We*_______________________________________________of___________________________________________hereby apply for thegrant/renewal of a loan licence to manufacture on the premises situatedat_______________C/o** _______________the unermentioned drugs,other than those specified in [Schedules C, C(1), and X] to the <strong>Drug</strong>s and<strong>Cosmetics</strong> Rules, 1945.Name of drugs (each substance to be separately specified).2. The namees, qualifications and experience of the expert staff actuallyconnected with the manufacture and testing of the specified products inthe manufacturing premises.__________________________________________________________________________________________________________________________________________________________________3. I/We enclose(a) A true copy of a letter from me/us to the manufacturing concernwhose manufacturing capacity is intended to be utilised by me/us.(b) A true copy of a letter from the manufacturing concern that theyagree to lend the services of their expert staff, equipment andpremises for the manufacture of each item required by me/us andthat they will analyse every batch of finished product and maintainthe registers of raw materials, finished products and reports ofanalysis separately in this behalf.(c)Specimens of labels, cartoons of the products proposed to bemanufactured.4. A fee of rupees__________________________________has beencredited to Government under the head of account___________________Date ____________________Signature___________________* Enter here tha name of the proprietor, partners or Managing Director asthe case may be.• Enter here the name of the applicant firm and the address or the prinicpalplace of business.** Enter here the name and address of the manufactring concern where themanufacture will be actually carried out and also the Licence numberunder which the letter operates.
FORM 24-B[ See Rule 69 ]Application for grant or renewal of a licence to repack for sale ordistribution of drugs, being drugs otehr than those specified in SchedulesC and C (1) [excluding those specified in Sch. X]1. I/We_______________________________________________of___________________________________________hereby applyfor the grant/renewal of a licence to repack the following durgs atthe premises situtated at ___________________________________________________________________2. Names of the drugs to be repacked._____________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________3. Name, qualification and experience of competent staff______________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________________4. A fee rupees forty has been credited to Government under the headof account_____________Date _________________Signature of applicant______________Note: - The application should be accompained by a plan of the premises.
FORM 24-C[ See Rule 85-B ]Application for grant or renewal of a licence to manufacture for sale [orfor distribution] of Homeopathic medicines or a licence to manufacturepreparations from back potencies by licenses holding in Form 20-C.1. I/We________________________________________________of______________________________________________holder ofLicence No.___________________________________________ inForm 20-C hereby apply for the grant/renewal of licence tomanufacture the under mentioned Homeopathic MotherTincture/Potentised and other preparations on the premises situatedat _________________________________________.Name of Homeopathic preparations___________________________________________________________________________________________________________________________________________________________________________________________(each item to be separately specified)2. Names, qualifications and experience to technical staff employed formanufacture and testing of Homeopathic medicines.3. A fee of Rupees_________________________________has beencredited to Government under the head ofaccount__________________________________________________________________________________________________________________________________________________________Date _______________Signature of applicant_______________Note: - 1) Delete whichever portion is not applicable2) The application should be accompanied by a plan of thepremises.
FORM 24-D[ See Rule 153 ]Application for the grant/renewal of a licence to manufacture for Sale ofAyurvedic/Siddha or Unani drugs1. I/we________________________________________________of___________________________________________hereby applyfor the grant/renewal of a licence to manufacture Ayurvedic(including Siddha) or Unani drugs on the premises situated at______________________________2. Name of drugs to be manufactured (with details).________________________________________________________________________________________________________________________________________________________________________________________________________________________3. Name qualifications and experience of technical staff employed formanufacture and testing of Ayurvedic (including Siddha) or Unanidrugs___________________________________________________________________________________________________________________________________________________________________________________________________________________4. A fee of rupees______________________________________hasbeen credited to the Government under the head ofaccount_________________________and the relevant TreasuryChallan is enclosed herewithDate _______________Signature of applicant______________Note: -The application should be accompanied by a plan of thepremises.
FORM 24-F[ See Rule 69 ]Application for the grant or renewal of a licence to manufacture for sale[or for distribution of ] drugs specified in Schedule X and not specified inSchedules C and C(1)1. I/We________________________________________________of___________________________________________hereby applyfor the grant/renewal of a licence to manufacture on premisessituated at ___________________________________________theunder mentioned drugs, specified in Schedule X to the <strong>Drug</strong>s and<strong>Cosmetics</strong> Rules, 1945.2. Name of drugs.________________________________________________________________________________________________________________________________________________________________________________________________________________________3. Name qualifications and experience of technical staff employed formanufacture and testing____________________________________________________________________________________________________________________________________________________________________________________________________4. A fee of rupees__________________________________has beencredited to the Government under the head ofaccount_____________________________________________________________________________________________________Date ____________________Signature ____________________Designation__________________
FORM 27Application for grant or renewal of a licence to manufacture for sale[or for distribution] drugs specified in Schedules C and C(1)[excluding those specified in [Part XB and] Sch. X]1. I/We_______________________________________hereby applyfor the grant/renewal of a licence to manufacture on the premisessituated at___________________________________________theunder mentioned drugs, being drugs specified in Schedules C and C(1), [excluding those specified in [Part XB and] Sch. X] to the <strong>Drug</strong>sand <strong>Cosmetics</strong> Rules, 1945.Name of <strong>Drug</strong>s.(each item to be separately specified).2. The names, qualifications and experience of the expert staffresponsible for the manufacture and testing of the above-mentioneddrugs:(a) Name (s) of staff responsible for test___________________(b) Name (s) of staff responsible for manufacture____________3. The premises and plan____________________________are readyfor inspection____________________________________will beready for inspection on ____________________________4. A fee rupees______________________________________and aninspection fee of rupees __________________________has beencredited to Government under the head of account _____________Date ____________________Signature ____________________Designation__________________Note: -premises.The application should be accompanied by a plan of the
FORM 27-A[ See Rule 75-A ]Application for Grant or Renewal of a Loan Licence to Manufacture for Sale [or fordistribution of] drugs specified in Schedules C and C(1) [excluding those specified in[Part XB and] Sch. X].1. I/We*_______________________________________________of___________________________________________hereby apply for thegrant/renewal of a loan licence to manufacture on the premises situatedat_______________C/o** ______________the under mentioned drugs, beingdrugs specified in Schedules C, C(1) [excluding those specified in [Part Xb and]Sch. X] to the <strong>Drug</strong>s and <strong>Cosmetics</strong> Rules, 1945.2. Name of drugs (each substance to be separately specified)__________________________________________________________________________________________________________________________________________________________________3. The names, qualifications and experience of the expert staff actually connectedwith the manufacture and testing of the specified products in the manufacturingpremises.(a) Name (s) of expert staff responsible for manufacture_______(b) Name (s) of expert staff responsible for testing___________4. I/We enclose(a) A true copy of a letter from me/us to the manufacturing concern whosemanufacturing capacity is intended to be utilised by me/us.(b) A true copy of a letter from the manufacturing concern that they agree tolend the services of their expert staff, equipment and premises for themanufacture of each item required by me/us and that they will analyseevery batch of finished product and maintain the registers of rawmaterials, finished products and reports of analysis separately in thisbehalf.(c) Specimens of labels, cartoons of the products proposed to bemanufactured.5. A fee of rupees_________________________________has been credited toGovernment under the head of account______________Date _______________Signature of Applicant______________* Enter here tha name of the proprietor, partners or Managing Director as the casemay be.• Enter here the name of the applicant firm and the address or the prinicpal placeof business.** Enter here the name and address of the manufactring concern where themanufacture will be actually carried out and also the Licence number underwhich the letter operates.
FORM 27-BApplication for grant or renewal of a licence to manufacture of sale [or fordistribution of] drugs specified in Schedules C and C (1) and X1. I/We________________________________________________of___________________________________________hereby applyfor the grant/renewal of a loan licence to manufacture on thepremises situated at____________________________________ theunder mentioned drugs, being drugs specified in Schedules C, C(1)and X to the <strong>Drug</strong>s and <strong>Cosmetics</strong> Rules, 1945.2. Name of drugs._____________________________________________________________________________________________________________________________________________________3. The names, qualifications and experience of the expert staffresponsible for the manufacture and testing of the above-mentioneddrugs.(a) Name (s) of expert staff responsible for manufacture_______(b) Name (s) of expert staff responsible for testing___________4. The premises and plan* are ready for inspection/will be ready forinspection on_____________5. A fee of rupees___________________________and an inspectionfee of rupees________________ has been credited to Governmentunder the head of account__________________________Date _____________Signature ____________The application shall be accompanied by a plan of the premises.* Delete whichever is not applicable.
FORM 27-C[ See Rule 122-F ]Application for grant or renewal of licence for the operation of BloodBank, processing of whole human blood for components and/ormanufacture of blood products.1. I/We________________________________________________of__________________________________________hereby apply forthe grant/renewal of licence to operate a Blood Bank, processing ofwhole human blood for components and/or manufacture of bloodproducts.2. The names of the Human Blood Components intended to beprocessed shall be specified.__________________________________________________________________________________________________________________________________________________________________3. The name, qualifications and experience of the expert staff.(a) Name (s) of Medical Officer _________________________(b) Name (s) of Registered Nurse ________________________(c) Name (s) of Blood Bank Technician____________________4. The premises and plan* are ready for inspection/will be ready forinspection on_________________________________________________________________________________________________5. A fee of rupees_______________________and an inspection fee ofrupees_________ has been credited to Government under the headof account__________________________Date ______________Signature _______________Designation______________
FORM 27-D[ See Rule 75 ]Application for grant or renewal of a licence to manufacture for sale or fordistribution of Large Volume Parenterals/Sera and Vaccines excluding thosespecified in Schedule X.1. I/We________________________________________________of___________________________________________hereby apply for thegrant/renewal of licence to manufacture for sale or distribution onpremises situated at _____________________________________theundermentioned Large Volume Parenterals / Sera and Vaccines, specifiedin Schedules C and C (1) to the <strong>Drug</strong>s and <strong>Cosmetics</strong> Rules, 1945.2. Name (s) of drugs (s)____________________________________________________________ (each item to be separately specified)3. The Name (s), qualifications and experience of competent technical staffresponsible for the manufacture of the above mentioned drugs.(a) Name (s) of staff responsible for testing_________________(b) Name (s) of staff responsible for manufacturing__________4. The premises and plan* are ready for inspection/will be ready forinspection on_________________________________________________________________________________________________5. A fee of rupees___________________________and an inspection fee ofrupees________________ has been credited to Government under thehead of account________________________Date ______________Signature _______________Designation_____________Note:1. The application is to be accompanied by a plan of the premises; list ofequipments and machinery to be employed for manufacture and testing;memorandum of association/constitution of the firm; copies ofqualification and experience of competent technical staff and documentsrelating to ownership or tenancy of the premises.2. A copy of the application together with relevant enclosures shall also besent each to Central Licence Approving Authority and concernedZonal/Sub-Zonal Officers of Central <strong>Drug</strong>s Standard ControlOrganisation.
INFORMATION DATA SUBMITTED WITH THE APPLICATIONFOR GRANT OF DRUG MANUFACTURING LICENCEREGARDING ITEMS TO BE APPROVED1 Name & Address of the Firm :2 Licence No. and Date : New Licence Case3 Categories of items permitted : Not applicable (New Licenceunder the licenceCase)4 For Pharmacopoeial <strong>Drug</strong>s : Not applicable(a) Name of the Product :(b) Pharmacopoeial Reference :(Indicate the edition and page ofPharmacopoeia)5 Patent and Proprietory <strong>Drug</strong>s(a) ame of the drug :(b) Complete formula: Kindly see overleaf.(c) If the product is a combination, : Not applicable as similarthe data of the rationals, efficacy product exists in the marketand safety of each of the ingredientsingally or in combination.(d) Whether a similar product is : Yesbeing manufactured by any otherfirm in India.If so, details thereof.(e) Proposed Dosage :(f) The therapeutic claims : NILproposed to to made on thelabel/carton and insert literature.(g) Certificate that the proposedname does not infringe the TradeMark <strong>Act</strong> for the time being inforce.: Mfd. By M/s. _____________________________________________________________________________________________________________: It is certified that the proposedname does not infringe theTrade Mark <strong>Act</strong> for the timebeing in force. An affidavit inthis regard, is enclosed withthe application.
FORM 30[ See Rule 90 ]Application for licence to manufacture drugs for purposes of examination,test or analysis.1. I/We________________________________________________of______________________________________________________by occupation__________________________________________hereby apply for a licence to manufacture the drugs specified belowfor purposes of examination, test or analysis at_________________________________and I undertake to complywith the conditions applicable to the licence.Name of <strong>Drug</strong>sDate _______________Signature ______________
FORM 31[ See Rule 138 ]Application for the grant or renewal of a licence to manufacture <strong>Cosmetics</strong>for Sale[ or for distribution ]1. I/We____________________of__________________________ofhereby apply for the grant/renewal of a licence to manufacture onthe premises situated at _________________________________ thefollowing cosmetics:-2. Name of <strong>Cosmetics</strong> _____________________________________3. Names, qualifications and experience of technical staff employed formanufacture and testing _________________________________________________________________________________________________________________________________________________________________________________________________4. A fee of Rupees __________________________has been creditedto Government under the head of account ____________________Date ____________Signature of Applicant ______________Note :- The application should be accompanied by a plan of the premises.
FORM 31-A[ See Rule 138-A ]Application for Grant or Renewal of a Loan Licence to Manufacture cosmeticsfor sale [or for distribution]1. I/We________________________________________________of___________________________________________hereby apply for thegrant/renewal of a loan licence to manufacture cosmetics, for sale on thepremises situated at_______________________________________________C/o________________________________________________the following cosmetics:-2. Name of <strong>Cosmetics</strong> _________________________________________________________________________________________________________________________________________________3. The names, qualifications and experience of the expert staff actuallyconnected with the manufacture and testing of the specified products inthe manufacturing premises.__________________________________________________________________________________________________________________________________________________________________4. I/We enclose(a) A true copy of a letter from me/us to the manufacturing concernwhose manufacturing capacity is intended to be utilised by me/us.(b) A true copy of a letter from the manufacturing concern that theyagree to lend the services of their expert staff, equipment andpremises for the manufacture of each item required by me/us andthat they will analyse every batch of finished product and maintainthe registers of raw materials, finished products and reports ofanalysis separately in this behalf.(c) Specimens of labels, cartoons of the products proposed to bemanufactured.5. A fee of rupees__________________________________has beencredited to Government under the head of account______________Date ____________________Signature ___________________* Enter here the name and address of the manufacturing concern where themanufacture will be actually carried out and also their licence number.
FORM 36[ See Rule 150-B ]Application for grant or renewal of approval for carrying out tests ondrugs/cosmetics or raw materials used in the manufacture thereof onbehalf of licensees for manufacture for sale of drugs/cosmetics.1. I/We_________________________________________________of_________________________________________hereby applyfor the grant or renewal of approval for carrying out tests of identity,purity, quality and strengths on the following categories ofdrugs/items of cosmetics or raw materials used in the manufacturethereof on behalf of licensees for manufacture for sale ofdrugs/cosmetics.2. *Categories of drugs, items of cosmetics:a] <strong>Drug</strong>s other than those specified in Schedule C and C(1) andalso excluding Homeopathic <strong>Drug</strong>s:-(i)(ii)(iii)(iv)(v)(iv)Crude Vegetable <strong>Drug</strong>sMechanical ContraceptivesSurgical Dressings<strong>Drug</strong>s requiring the use of ultraviolet/Infra RedSpectro-Photometer or chromatography.DisinfectantsOther <strong>Drug</strong>s.b] <strong>Drug</strong>s Specified in Schedules C and C (1) :-(i) Sera, Vaccines, Antigens, Toxins, Antitoxins, Toxoids,Bacteriophages and similar Immunological Products.(ii) Antibiotics(iii) Vitamins(iv) Parenteral Preparations(v) Sterilised Surgical Ligature/Suture.(vi) Sterilised Surgical Ligature/Sulture.(vii) <strong>Drug</strong>s requiring microbiological tests.(viii) <strong>Drug</strong>s requiring the use of Ultraviolet/Infra RedSpectrophotometer or Chromatography.(ix) Other <strong>Drug</strong>sC] Homeopathic <strong>Drug</strong>sD] <strong>Cosmetics</strong>
3. Names, qualifications and experience of expert staff employed fortesting and the person-in-change testing _______________________________________________________________________________________________________________________________________________________________________________________4. List of testing of equipment provided _________________________________________________________________________________________________________________________________________________________________________________________5. I/We enclose a plan of the testing premises showing the location andarea of the different sections thereof.6. A inspection fee of Rs. ___________________________has beencredited to Government unde the head of Account______________Date ____________________ Signature ___________________* Delete whichever is not applicable.
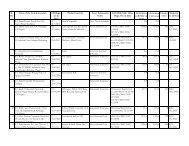
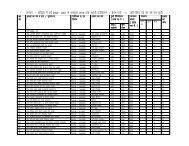
Schedule of Fees For Grant of <strong>Drug</strong>/Cosmetic LicencesSl. Form Rule SCH Fee Rs. Requirement For Manufacture/No. No.Testing/ Re packing Etc.1 24 Rule 69 'M' 7500/- Application for the grant of orrenewal of a licence tomanufacture for sale (or fordistribution of) drugs other thanthose specified in (Sch. C. C(1)and x).2 24 A Rule 69AM 7500/- Application for the grant of orrenewal of a loan licence tomanufacture for sale (fordistribution of) drugs other thanthose specified (Schedules C, C(1)and x).3 24 B Rule 69 M 700/- Application for the grant of orrenewal of a licence to repack forsale of distribution of dmgs, beingdmgs other than those specified inschedule C and C(1) (Excludingthose specified in Sch. X).4 24 C Rule 85-B5 24 F Rule 75 M +MIII6 27 Rule 75A7 27-A Rule 75AM-1 300/- Application for the grant of orrenewal of a licence tomanufacture for sale (or fordistribution) of Homoeopathicmedicines or a licence tomanufacture potentised prepartionsfrom back potencies by licenseesholding licence in Form 20G.7500/- Application for the grant of orrenewal of a licence to maufacturefor sale (or for distribution of)drugs specified in Schedule C andC(1) (excluding those specified in(Part XB and) Sch. X).M 7500/- Application for the grant of orrenewal of a loan licence tomanufacture for sale (or fordistribution of) dmg specified inSchedules C and C(1) (excludingthose specified in (Part XB) Sch.X).M 7500/- Application for the grant of orrenewal of a laon licence tomanufacture for sale (or for
distribution of) dmg specified inSchedules C and C(1) (excludingthose specified in (Part XB) Sch.X).8 27-B Rule 75 M 7500/- Application for the grant of orrenewal of a licence tomanufacture for sale (or fordistribution of) drugs specified inSchedules C, C(1) and X.9 27-C Rule122 FM &MFXII10 27-D Rule 75 M &F11 30 Rule 90 TESTLIC12 31 Rule13813 31 A Rule138 A14 36 Rule150-B7500/- Application for the grant of orrenewal of a licence for theoperation of Blood Bankprocessing of whole Human bloodfor components and/ormanufacture of Blood Products.7500/- Application for the grant of orrenewal of a licence tomanufacture for sale or fordistribution of large volumeparentrals/sera and vaccinesexcluding those specified inschedule X.250/- Application for licence tomanufacture drugs for purpuses ofexamination, test or analysis.MII 3500/- Application for the grant orrenewal of a licence tomanufacture cosmetics for sale (orfor distribution)MII 3500/- Application for the grant orrenewal of a licence tomanufacture cosmetics for sale (orfor distribution)7500/- Application for the grant of orrenewal of approval for carryingout tests on drugs/cosmetics or rawmaterials used in the manufacturethere of on behalf of licensees formanufacture for sale ofdrugs/cosmetics." ENCLOSED " INFORMATION DATA TO BE SUBMITTED WITHAPPLICATION FOR GRANT OF DRUG MFG.LIC REGARDING ITEM TO BE APROVED."