chronic-pain-opioid-treatment-report-140929
chronic-pain-opioid-treatment-report-140929
chronic-pain-opioid-treatment-report-140929
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
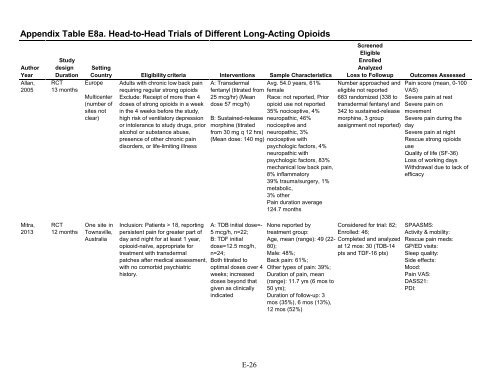
Appendix Table E8a. Head-to-Head Trials of Different Long-Acting OpioidsAuthorYearAllan,2005StudydesignDurationRCT13 monthsSettingCountry Eligibility criteria Interventions Sample CharacteristicsEurope Adults with <strong>chronic</strong> low back <strong>pain</strong> A: Transdermal Avg. 54.0 years, 61%requiring regular strong <strong>opioid</strong>s fentanyl (titrated from femaleMulticenter Exclude: Receipt of more than 4 25 mcg/hr) (Mean Race: not <strong>report</strong>ed, Prior(number of doses of strong <strong>opioid</strong>s in a week dose 57 mcg/h) <strong>opioid</strong> use not <strong>report</strong>edsites not in the 4 weeks before the study,35% nociceptive, 4%clear) high risk of ventilatory depression B: Sustained-release neuropathic, 46%or intolerance to study drugs, prior morphine (titrated nociceptive andalcohol or substance abuse, from 30 mg q 12 hrs) neuropathic, 3%presence of other <strong>chronic</strong> <strong>pain</strong> (Mean dose: 140 mg) nociceptive withdisorders, or life-limiting illnesspsychologic factors, 4%neuropathic withpsychologic factors, 83%mechanical low back <strong>pain</strong>,8% inflammatory39% trauma/surgery, 1%metabolic,3% otherPain duration average124.7 monthsScreenedEligibleEnrolledAnalyzedLoss to FollowupNumber approached andeligible not <strong>report</strong>ed683 randomized (338 totransdermal fentanyl and342 to sustained-releasemorphine, 3 groupassignment not <strong>report</strong>ed)Outcomes AssessedPain score (mean, 0-100VAS)Severe <strong>pain</strong> at restSevere <strong>pain</strong> onmovementSevere <strong>pain</strong> during thedaySevere <strong>pain</strong> at nightRescue strong <strong>opioid</strong>suseQuality of life (SF-36)Loss of working daysWithdrawal due to lack ofefficacyMitra,2013RCT12 monthsOne site inTownsville,AustraliaInclusion: Patients > 18, <strong>report</strong>ingpersistent <strong>pain</strong> for greater part ofday and night for at least 1 year,opiooid-naïve, appropriate for<strong>treatment</strong> with transdermalpatches after medical assessment,with no comorbid psychiatrichistory.A: TDB initial dose=-5 mcg/h, n=22;B: TDF initialdose=12.5 mcg/h,n=24;Both titrated tooptimal doses over 4weeks; increaseddoses beyond thatgiven as clinicallyindicatedNone <strong>report</strong>ed by<strong>treatment</strong> group:Age, mean (range): 49 (22-80);Male: 48%;Back <strong>pain</strong>: 61%;Other types of <strong>pain</strong>: 39%;Duration of <strong>pain</strong>, mean(range): 11.7 yrs (6 mos to50 yrs);Duration of follow-up: 3mos (35%), 6 mos (13%),12 mos (52%)Considered for trial: 82;Enrolled: 46;Completed and analyzedat 12 mos: 30 (TDB-14pts and TDF-16 pts)SPAASMS:Activity & mobility:Rescue <strong>pain</strong> meds:GP/ED visits:Sleep quality:Side effects:Mood:Pain VAS:DASS21:PDI:E-26