Alergol Inmunol Clin 2001; 16: 294-296
Alergol Inmunol Clin 2001; 16: 294-296
Alergol Inmunol Clin 2001; 16: 294-296
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
The administration of unfractionated heparins<br />
(UFH) or of low-molecular weigh heparins<br />
(LMWH) may induce a non-necrotic eczematous<br />
reaction at the site of injection, in which a delayed hypersensitivity<br />
mechanism is involved 1 . Cross-reactivity<br />
has often been observed in these cases between the various<br />
heparins 2-5 or even with heparinoids 3-5 . This may<br />
constitute a problem, particularly when faced with an urgent<br />
need for anticoagulant therapy. There have recently<br />
been reports of cases in which the new recombinant hirudins<br />
represent a safe alternative for patients with this type<br />
of reaction 3,6,7,9 .<br />
CASE REPORT<br />
A 38-year old woman, obese but with no other salient<br />
features in her past history, was referred to the<br />
Allergy Outpatient <strong>Clin</strong>ic because of two episodes of abdominal<br />
skin rash which she described as vesicular-blistering<br />
within the past two years. The first episode had occurred<br />
ten days after the subcutaneous administration into<br />
the abdominal wall of enoxaparin (Clexane ® ) 40 mg, one<br />
dose every 24 hours, because of a tibioperoneal fracture<br />
suffered during a motoring accident. The administration of<br />
this low-molecular weight heparin was continued for five<br />
further days; the skin rash progressed to involve the whole<br />
abdomen, and remitted gradually in a number of days after<br />
withdrawal of the drug. The second episode occurred after<br />
the administration of the same low-molecular weight heparin<br />
because of a new tibial fracture after a further traumatism.<br />
In this case, the skin rash in the abdomen began<br />
several hours after the administration of the first dose and,<br />
as the drug was not immediately withdrawn, progressed to<br />
involve the whole abdomen with greater severity than in<br />
the previous occasion. The rash again remitted gradually<br />
after withdrawal of the drug.<br />
After recovering from this second episode the patient<br />
was seen for the first time at the Allergy Outpatient <strong>Clin</strong>ic,<br />
and the following allergologic study was performed (Table<br />
I): (1) skin prick tests at 1:1 dilution with unfractionated<br />
heparin (heparin sodium) and with two LMWH [enoxaparin<br />
and nadroparin (Fraxiparin ® )], which were read after 20<br />
minutes, 48 hours and 96 hours, and (2) epicutaneous tests,<br />
again at 1:1 dilution, with the same heparin preparations,<br />
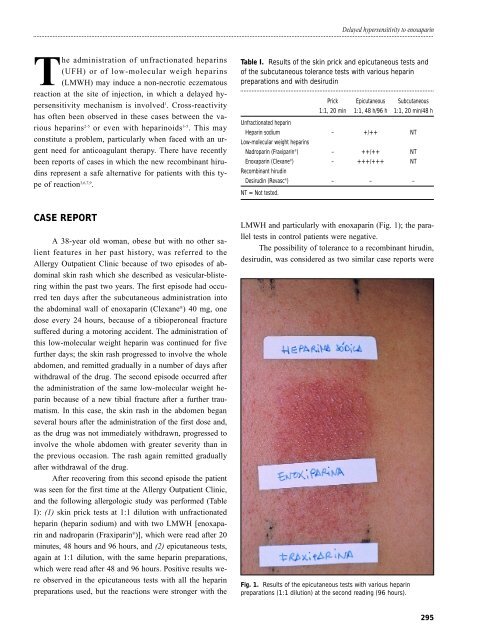
which were read after 48 and 96 hours. Positive results were<br />
observed in the epicutaneous tests with all the heparin<br />
preparations used, but the reactions were stronger with the<br />
Delayed hypersensitivity to enoxaparin<br />
Table I. Results of the skin prick and epicutaneous tests and<br />
of the subcutaneous tolerance tests with various heparin<br />
preparations and with desirudin<br />
Prick Epicutaneous Subcutaneous<br />
1:1, 20 min 1:1, 48 h/96 h 1:1, 20 min/48 h<br />
Unfractionated heparin<br />
Heparin sodium<br />
Low-molecular weight heparins<br />
– +/++ NT<br />
Nadroparin (Fraxiparin ® ) – ++/++ NT<br />
Enoxaparin (Clexane ® )<br />
Recombinant hirudin<br />
– +++/+++ NT<br />
Desirudin (Revasc ® )<br />
NT = Not tested.<br />
– – –<br />
LMWH and particularly with enoxaparin (Fig. 1); the parallel<br />
tests in control patients were negative.<br />
The possibility of tolerance to a recombinant hirudin,<br />
desirudin, was considered as two similar case reports were<br />
Fig. 1. Results of the epicutaneous tests with various heparin<br />
preparations (1:1 dilution) at the second reading (96 hours).<br />
295