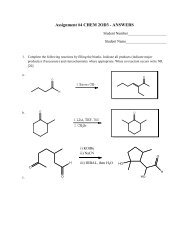
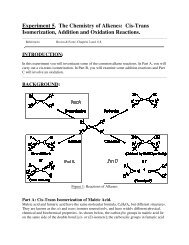
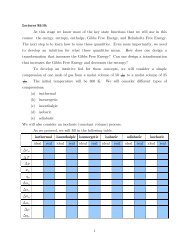
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Worksheet</strong> <strong>20</strong>:<strong>Multiple</strong> <strong>Choice</strong>; <strong>Short</strong> <strong>Answer</strong>. (<strong>80</strong> <strong>pts</strong>.)<strong>We</strong> <strong>learned</strong> a lot about pressure-volume work in this course. There are also other types of work.For example, if one applies a force F to a rubber band (or any linear elastic substance) to stretch it,the work is Fdl , where dl is the change in length.1. Write an expression for the change in Helmholtz free energy of a rubber band. Yourexpression should depend on (i) the length of the rubber band, (ii) the temperature ofthe rubber band, (iii) the volume of the rubber-like substance.2. Write an expression for the change in internal energy of the rubber band.3. Write the Maxwell relations corresponding to your equations in question 1.4. What is the expression for the chemical potential of a component in an ideal mixture.5. Starting from the second law of thermodynamics, derive an inequality for the enthalpychange in an irreversible transformation.
6. This is a Carnot cycle. Suppose one builds a machine that performs this particularCarnot cycle. That machine would be(a) a refrigerator. [I.e., the environment does work on the system.](b) an engine. [The system does work on the environment.]7. What is the temperature at the beginning of the fourth step in the process? (What is thetemperature at the beginning of the isothermal compression step?)8. How much heat is put into/taken out of the system during the Carnot cycle?9. How much work is put into/taken out of the system during the Carnot cycle?10. What is the entropy change in step two of the cycle. (What is the entropy change in theisothermal expansion.)11. Which of the following is true. [Mark all that apply.](a) Δ Hstep 1+Δ Hstep 2+Δ Sstep 3+Δ Sstep 4= 0 .(b) Δ Hstep 1+Δ Sstep 2+Δ Hstep 3+Δ Sstep 4= 0 .(c) Δ Hstep 1+Δ Sstep 2+Δ Sstep 3+Δ Hstep 4= 0 .(d) Δ Sstep 1+Δ Hstep 2+Δ Hstep 3+Δ Sstep 4= 0 .(e) Δ Hstep 1+Δ Sstep 2+Δ Hstep 3+Δ Sstep 4= 0 .(f) Δ Sstep 1+Δ Sstep 2+Δ Hstep 3+Δ Hstep 4= 0 .
12. You begin with 1 kilogram of water; a large amount of AgCl is added, forming asaturated solution. If you add 10 grams of NaCl to the water,(a) the solubility of AgCl increases.(b) the solubility of AgCl decreases.13. You begin with 1 kilogram of water; a large amount of AgCl is added, forming asaturated solution. If you add 10 grams of NaBr to the water,(a) the solubility of AgCl increases.(b) the solubility of AgCl decreases.kJ14. At 25 Celsius, for the reaction given below, ΔrxnH =− 2.5 mol and the equilibriumconstant is 8.2. What is the entropy of reaction?15. A rigid (volume = constant) reaction vessel is immersed in a heat bath (temperature =373.15 K). The reaction is a dissociation reaction,AB2 2A+ B K eq( 373.15 K,1 atm)= 3.0and all of the molecules behave as ideal gasses. Initially, .5 moles of molecule AB isadded to the reaction vessel; after this is done, the pressure is 1 atm. After three days,the system is in thermodynamic equilibrium and the concentrations of the componentsare measured. The mole fraction of A will be:3(a) greater than xA= .2+33(b) equal to xA= .2+33(c) less than = .xA 2+316. A isomerization reaction is performed in an insulated (heat transfer = zero) and rigid(volume = constant) container. Enough reactant is added for the system to be at 1atmosphere in pressure; the initial temperature of the reactant is 298.15 K.After the system has come into equilibrium, there is less product than would havebeen expected based on the equilibrium constant at 298.15 K and 1 atm and(a) The temperature of the reaction vessel is greater than 298.15 Kelvin.(b) The temperature of the reaction vessel is less than 298.15 Kelvin.(c) The temperature of the reaction vessel is equal to 298.15 Kevlin.
Derivation and Problems. (<strong>80</strong> <strong>pts</strong>.)1. Derive the formula for the dependence of the temperature on the pressure in anadiabatic expansion of an ideal gas.TfTiPfc ,( P )i=To derive this formula, use a fundamental equation for the heat gained in a process inwhich the temperature and the pressure changes. Remember that the latent heat ofvpressure change is =−T ( ∂∂ ) .p T pRvm+R
2. You should remember that the entropy is always maximized in equilibrium. Use this to−1 ⎛∂v⎞mshow that the isothermal compressibility, κT= , is always positive.v ⎜⎝∂p⎟⎠ mT
3. What is the isothermal compressibility of water at 500 K. Assume the van der Waalsequation of state.
4. After graduation, you sell your services to the highest bidder and you end up working ona uranium enrichment project. To test your new gas centrifuge, you decide to start with82 84 86Krypton gas. After enrichment, you obtain 75% Kr, <strong>20</strong>% Kr, and 5% Kr . Whatis ΔenrichmentS and ΔenrichmentG . Assume your gas centrifuges work at 500 K and have avolume of 1 Liter; assume that you start with 1 mole of Krypton gas in the natural82 84 86isotopic abundances, 10% Kr, 70% Kr, and <strong>20</strong>% Kr .
5. Consider the following reaction,2COF2 CO2 + CF4All reagents are in the gas phase. The reaction is performed at 10 atm and 1000 K; thenthe system is quenched (i.e., the reaction is stopped by rapid cooling and removal of thecatalyst). Afterwards, it is observed that the combined volume of COF2 and CO2(together) is 300 mL; the reaction vessel held a total of 500 mL.(a) What is the equilibrium constant.(b) If the equilibrium constant increases by 1% per degree Kelvin at 10 atm and 1000 K,what are Δ , Δ S , and rxnΔ G rxnat 10 atm and 1000 K.rxn H
6. A 2.24 Liter flask contains 1 mole of a monatomic ideal gas at 10 atm pressure. The flask isplaced inside a piston; the height of the piston is held fixed so that the volume contained by thepiston is 22.4 Liters. The piston is evacuated and the piston is insulated from the environmentto prevent heat loss. So we have:(i) a flask of volume 2.24 Liters, with 1 mole of a monatomic ideal gas at 10 atminside(ii) a piston containing a vacuum, with the volume of the piston being 22.4 Liters.(1) The flask is broken and the gas undergoes a free expansion to fill the volume of the piston.(2) The piston is used to compress the gas back to the initial volume (22.4 Liters). Becausethe piston is insulated from the environment, this is an adiabatic compression.(a) Calculate the heat, work,Δ T , Δ U , and Δ S for step (i) and step (ii).
7. For a reaction occurring at constant volume and temperature, the Helmholtz free energyis the key indicator of spontaneity. Using the formula for the change in Helmholtz freeenergy in terms of changes in the numbers of moles of substance, derive an expressionfor (a) an expressionfor the the equilibrium constant for a reaction occurring at constanttemperature and volume in terms of ΔrxnA , (b) formulas for the way this equilibriumconstant changes with respect to changes in temperature and volume.
8. Derive the Gibbs-Duhem-like equation for the partial molar enthalpy,⎛c∂H⎞⎛∂H⎞dT ⎜+ dp = nidhi⎝ T⎟⎠⎜ p ⎟ ∑ .∂ ⎝∂⎠ i=1pn , iTn , i
“Thinking” Problem. (40 <strong>pts</strong>.)Consider the reaction:( ) + ( g) ( g):C=C=C: g 4H CH CH CH2 3 2 3Suppose that you start with 5 moles of C31 mole of H2and find that, in equilibrium, the molefraction of propane is .01. La Chatelier’s principle indicates that if you increase the amount of C3,then equilibrium will shift in such a way to reduce the chemical potential of C3.Compute the mole fractions of each substance in equilibrium when you start with 4.99, 5.00,or 5.01 moles of C3. Comment on any anomalous behavior. You will earn extra marks ifyou can derive a general mathematical description of the phenomenon you observe.
Problems 6-10 pertained to the Carnot Cycle. Depending on the direction of the process, itis either a “Carnot refrigerator” or a “Carnot engine.” The Carnot engine is often called aheat pump.(a) Some Canadians heat their homes using what is called a water-exchange heat pump. Inthis case, heat is taken out of well water (at an approximate temperature of 10 C) andtransferred to a cosy Canadian cottage (at an approximate temperature of <strong>20</strong> C). Theefficiency of a heat pump is defined as the amount of work that must be done on thesystem to transfer a given amount of heat to the inside of the cottage. What is theefficiency of the cottage’s heat pump?
(b) It is a deep result from Thermodynamics that the Carnot cycle is the most efficientpossible way to construct a heat pump. Try your best to explain why.