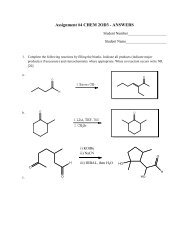
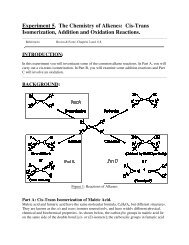
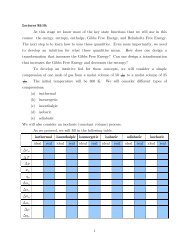
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
“Thinking” Problem. (40 <strong>pts</strong>.)Consider the reaction:( ) + ( g) ( g):C=C=C: g 4H CH CH CH2 3 2 3Suppose that you start with 5 moles of C31 mole of H2and find that, in equilibrium, the molefraction of propane is .01. La Chatelier’s principle indicates that if you increase the amount of C3,then equilibrium will shift in such a way to reduce the chemical potential of C3.Compute the mole fractions of each substance in equilibrium when you start with 4.99, 5.00,or 5.01 moles of C3. Comment on any anomalous behavior. You will earn extra marks ifyou can derive a general mathematical description of the phenomenon you observe.