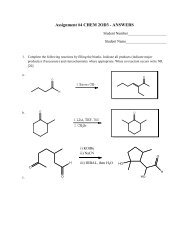
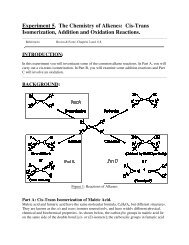
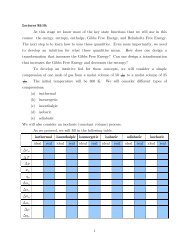
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
Worksheet 20: Multiple Choice; Short Answer. (80 pts.) We learned a ...
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
6. A 2.24 Liter flask contains 1 mole of a monatomic ideal gas at 10 atm pressure. The flask isplaced inside a piston; the height of the piston is held fixed so that the volume contained by thepiston is 22.4 Liters. The piston is evacuated and the piston is insulated from the environmentto prevent heat loss. So we have:(i) a flask of volume 2.24 Liters, with 1 mole of a monatomic ideal gas at 10 atminside(ii) a piston containing a vacuum, with the volume of the piston being 22.4 Liters.(1) The flask is broken and the gas undergoes a free expansion to fill the volume of the piston.(2) The piston is used to compress the gas back to the initial volume (22.4 Liters). Becausethe piston is insulated from the environment, this is an adiabatic compression.(a) Calculate the heat, work,Δ T , Δ U , and Δ S for step (i) and step (ii).