Notes for Exp. 34: UV-VIS/Fluorescence Spectroscopy S. K. Buratto ...
Notes for Exp. 34: UV-VIS/Fluorescence Spectroscopy S. K. Buratto ...
Notes for Exp. 34: UV-VIS/Fluorescence Spectroscopy S. K. Buratto ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
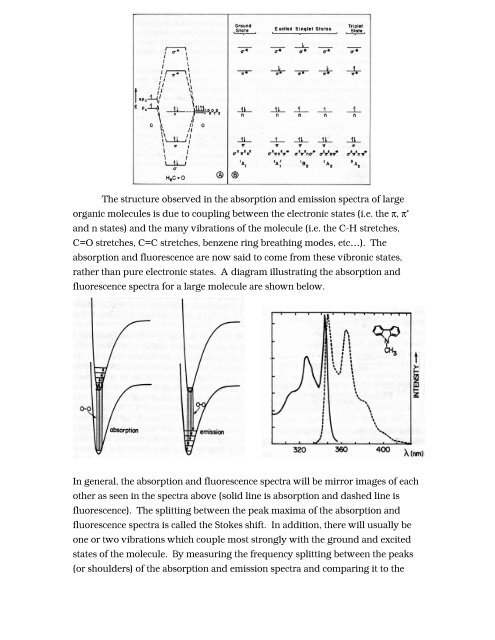
The structure observed in the absorption and emission spectra of largeorganic molecules is due to coupling between the electronic states (i.e. the π, π *and n states) and the many vibrations of the molecule (i.e. the C-H stretches,C=O stretches, C=C stretches, benzene ring breathing modes, etc…). Theabsorption and fluorescence are now said to come from these vibronic states,rather than pure electronic states. A diagram illustrating the absorption andfluorescence spectra <strong>for</strong> a large molecule are shown below.In general, the absorption and fluorescence spectra will be mirror images of eachother as seen in the spectra above (solid line is absorption and dashed line isfluorescence). The splitting between the peak maxima of the absorption andfluorescence spectra is called the Stokes shift. In addition, there will usually beone or two vibrations which couple most strongly with the ground and excitedstates of the molecule. By measuring the frequency splitting between the peaks(or shoulders) of the absorption and emission spectra and comparing it to the