Membrane Protein Crystallization From Cubic Lipid Matrices
Membrane Protein Crystallization From Cubic Lipid Matrices
Membrane Protein Crystallization From Cubic Lipid Matrices
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
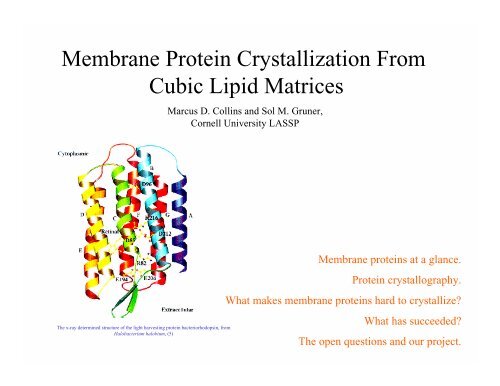
<strong>Membrane</strong> <strong>Protein</strong> <strong>Crystallization</strong> <strong>From</strong><strong>Cubic</strong> <strong>Lipid</strong> <strong>Matrices</strong>Marcus D. Collins and Sol M. Gruner,Cornell University LASSP<strong>Membrane</strong> proteins at a glance.<strong>Protein</strong> crystallography.What makes membrane proteins hard to crystallize?The x-ray determined structure of the light harvesting protein bacteriorhodopsin, fromHalobacterium halobium, (5)What has succeeded?The open questions and our project.
<strong>Protein</strong> crystallographyJust like minerals and salts, proteins can form crystals. And, justlike other crystals, proteins will diffract X-rays. <strong>From</strong> the X-raysdiffraction patterns, the protein structures can be determined.X-ray diffraction images of bacteriorhodopsin crystals, (3)However, proteins are much harder to grow than salts, andby 1975 only 37 structures had been placed in the <strong>Protein</strong>Data Bank. These crystals tend to be extremely fragileand sensitive to conditions such as pH, specific ionconcentrations, and other factors. They are also easilydamaged by the X-rays used to probe their structure.Now, as techniques of growing crystals and inverting the X-ray scattering data haveimproved, more than 12,000 structures have been posted to the PDB.bR crystals surrounded by lipids, (5)
And then... <strong>Membrane</strong> protein crystallization in cuboIn late 1996, a Swiss group led by E. M. Landaudropped a bombshell on the world of membraneprotein crystallography. They had succeeded incrystallizing a bacterial light harvesting proteinout of a lipid cubic liquid crystalline matrix.bR crystals, (3)The principle is quite simple: if you want to make a membrane proteinstable, why not put it in a membrane? But the task of crystallization ismore difficult than that. Purity of the protein in the crystal isparamount, and in any case, one must deliver the protein to theartificial membrane somehow. Once the protein is in the membrane, itthen must come out and crystallize. These are not trivial matters.What interests our group is that, though the Landau group hassucceeded in extending their technique to a handful of other proteins ina mere few years, no one yet knows how the technique actuallyworks. One idea, pictured at left, depends directly on the chemicalprecipitants Landau’s group used.A hypothetical sketch of protein crystals forming from a lipidic cubic phase, (1)
Our projectThere are two intriguing questions that are raised by theLandau groups experiments. The first is whether thecubic structure they used is important, or whethersimply being in a membrane allows the membraneprotein to crystallize. Second, and more fundamental,is how the protein actually forms crystals. There areseveral clues to how this might work, but there are nohard answers.Two important lipid phases, (1)It may be that the precipitant is not directlyresponsible for the change in solubility that leadsto crystallization. There is indirect evidence thatthe crystals form due to structural changes in thesurrounding lipid matrix. We are exploringwhether changes in lipid crystal phase and latticeparameters lead to protein crystal formation.One of the challenges we face is that the complicated detergent-salt system which permits us to solubilizethe protein initially can interfere with the structural behavior of the lipids. Indeed, the designer detergentsused in membrane protein experiments are designed to have properties quite similar to those of lipids. It isnow well known that these detergents do alter the phase behavior of common lipids significantly. However,it may be possible to avoid the use of these detergents entirely. This is a question which remainsunanswered.(1)
References1. Caffrey, M, Current Opinion in Structural Biology, 2000, 10:486-4972. Bowie, JU, Current Opinion in Structual Biology, 2000, 10:435-4373. Landau, EM and JP Rosenbusch, PNAS, 1996, 93:14532-145354. Pebay-Peyroula, E et al, Biochimica et Biophysica Acta, 2000, 119-1325. Rummel, G et al, Journal of Structural Biology, 1998, 121:82-916. Chiu, ML et al, Acta Crystallographica, 2000, D56:781-784