Activities - Benjamin-Mills
Activities - Benjamin-Mills
Activities - Benjamin-Mills
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
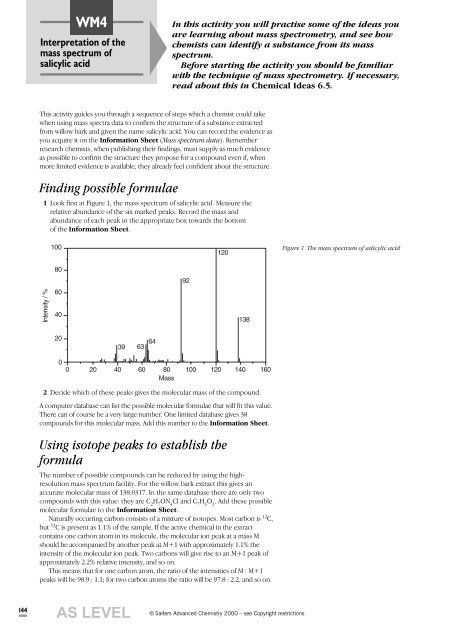
WM4Interpretation of themass spectrum ofsalicylic acidIn this activity you will practise some of the ideas youare learning about mass spectrometry, and see howchemists can identify a substance from its massspectrum.Before starting the activity you should be familiarwith the technique of mass spectrometry. If necessary,read about this in Chemical Ideas 6.5.This activity guides you through a sequence of steps which a chemist could takewhen using mass spectra data to confirm the structure of a substance extractedfrom willow bark and given the name salicylic acid. You can record the evidence asyou acquire it on the Information Sheet (Mass spectrum data). Rememberresearch chemists, when publishing their findings, must supply as much evidenceas possible to confirm the structure they propose for a compound even if, whenmore limited evidence is available, they already feel confident about the structure.Finding possible formulae1 Look first at Figure 1, the mass spectrum of salicylic acid. Measure therelative abundance of the six marked peaks. Record the mass andabundance of each peak in the appropriate box towards the bottomof the Information Sheet.100120Figure 1 The mass spectrum of salicylic acid80Intensity / %60402039 63 64 9213800 20 40 60 80 100 120 140 160Mass2 Decide which of these peaks gives the molecular mass of the compound.A computer database can list the possible molecular formulae that will fit this value.There can of course be a very large number. One limited database gives 38compounds for this molecular mass. Add this number to the Information Sheet.Using isotope peaks to establish theformulaThe number of possible compounds can be reduced by using the highresolutionmass spectrum facility. For the willow bark extract this gives anaccurate molecular mass of 138.0317. In the same database there are only twocompounds with this value: they are C 2H 7ON 4Cl and C 7H 6O 3. Add these possiblemolecular formulae to the Information Sheet.Naturally occurring carbon consists of a mixture of isotopes. Most carbon is 12 C,but 13 C is present as 1.1% of the sample. If the active chemical in the extractcontains one carbon atom in its molecule, the molecular ion peak at a mass Mshould be accompanied by another peak at M+1 with approximately 1.1% theintensity of the molecular ion peak. Two carbons will give rise to an M+1 peak ofapproximately 2.2% relative intensity, and so on.This means that for one carbon atom, the ratio of the intensities of M : M+1peaks will be 98.9 : 1.1; for two carbon atoms the ratio will be 97.8 : 2.2, and so on.144AS LEVEL„ Salters Advanced Chemistry 2000 – see Copyright restrictions















![ISI Web of Knowledge [v.4.10] - All Databases Results - Benjamin-Mills](https://img.yumpu.com/39253071/1/184x260/isi-web-of-knowledge-v410-all-databases-results-benjamin-mills.jpg?quality=85)