Trilon® D Liquid - the Performance Chemicals division - BASF.com
Trilon® D Liquid - the Performance Chemicals division - BASF.com
Trilon® D Liquid - the Performance Chemicals division - BASF.com
- No tags were found...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
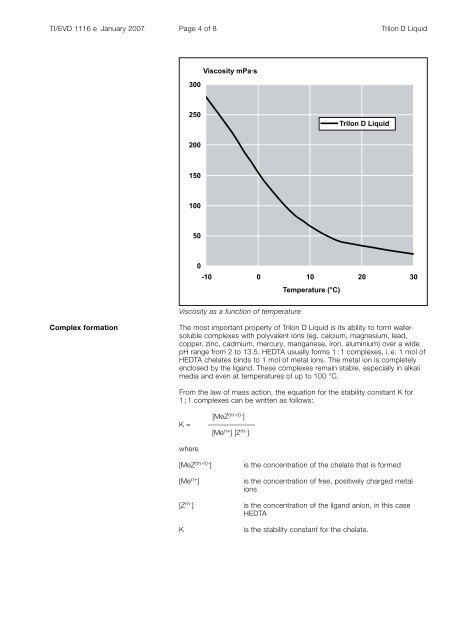
TI/EVD 1116 e January 2007 Page of 8 Trilon D <strong>Liquid</strong>300Viscosity mPa·s250Trilon D <strong>Liquid</strong>200150100500-10 0 10 20 30Temperature (°C)Viscosity as a function of temperatureComplex formationThe most important property of Trilon D <strong>Liquid</strong> is its ability to form watersoluble<strong>com</strong>plexes with polyvalent ions (eg. calcium, magnesium, lead, copper, zinc, cadmium, mercury, manganese, iron, aluminium) over a widepH range from 2 to 13.5. HEDTA usually forms 1 : 1 <strong>com</strong>plexes, i. e. 1 mol ofHEDTA chelates binds to 1 mol of metal ions. The metal ion is <strong>com</strong>pletelyenclosed by <strong>the</strong> ligand. These <strong>com</strong>plexes remain stable, especially in alkalimedia and even at temperatures of up to 100 °C. [MeZ (m-n)- ]K = ––––––––––––– [Me n+ ] [Z m- ]From <strong>the</strong> law of mass action, <strong>the</strong> equation for <strong>the</strong> stability constant K for1 : 1 <strong>com</strong>plexes can be written as follows:where[MeZ (m-n)- ][Me n+ ]is <strong>the</strong> concentration of <strong>the</strong> chelate that is formedis <strong>the</strong> concentration of free, positively charged metalions[Z m- ] is <strong>the</strong> concentration of <strong>the</strong> ligand anion, in this caseHEDTAKis <strong>the</strong> stability constant for <strong>the</strong> chelate.