7 - World Journal of Gastroenterology
7 - World Journal of Gastroenterology
7 - World Journal of Gastroenterology
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
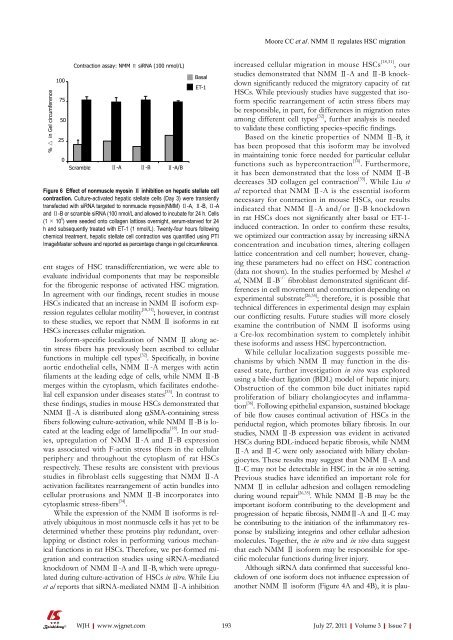
Moore CC et al. NMM Ⅱ regulates HSC migration% △ in Gel circumference1007550250Contraction assay: NMM Ⅱ siRNA (100 nmol/L)Scramble Ⅱ-A Ⅱ-B Ⅱ-A/BBasalET-1Figure 6 Effect <strong>of</strong> nonmuscle myosin Ⅱ inhibition on hepatic stellate cellcontraction. Culture-activated hepatic stellate cells (Day 3) were transientlytransfected with siRNA targeted to nonmuscle myosin(NMM) Ⅱ-A, Ⅱ-B, Ⅱ-Aand Ⅱ-B or scramble siRNA (100 nmol/L and allowed to incubate for 24 h. Cells(1 × 10 5 ) were seeded onto collagen lattices overnight, serum-starved for 24h and subsequently treated with ET-1 (1 nmol/L). Twenty-four hours followingchemical treatment, hepatic stellate cell contraction was quantified using PTIImageMaster s<strong>of</strong>tware and reported as percentage change in gel circumference.ent stages <strong>of</strong> HSC transdifferentiation, we were able toevaluate individual components that may be responsiblefor the fibrogenic response <strong>of</strong> activated HSC migration.In agreement with our findings, recent studies in mouseHSCs indicated that an increase in NMM Ⅱ is<strong>of</strong>orm expressionregulates cellular motility [18,31] ; however, in contrastto these studies, we report that NMM Ⅱ is<strong>of</strong>orms in ratHSCs increases cellular migration.Is<strong>of</strong>orm-specific localization <strong>of</strong> NMM Ⅱ along actinstress fibers has previously been ascribed to cellularfunctions in multiple cell types [32] . Specifically, in bovineaortic endothelial cells, NMM Ⅱ-A merges with actinfilaments at the leading edge <strong>of</strong> cells, while NMM Ⅱ-Bmerges within the cytoplasm, which facilitates endothelialcell expansion under diseases states [33] . In contrast tothese findings, studies in mouse HSCs demonstrated thatNMM Ⅱ-A is distributed along αSMA-containing stressfibers following culture-activation, while NMM Ⅱ-B is locatedat the leading edge <strong>of</strong> lamellipodia [18] . In our studies,upregulation <strong>of</strong> NMM Ⅱ-A and Ⅱ-B expressionwas associated with F-actin stress fibers in the cellularperiphery and throughout the cytoplasm <strong>of</strong> rat HSCsrespectively. These results are consistent with previousstudies in fibroblast cells suggesting that NMM Ⅱ-Aactivation facilitates rearrangement <strong>of</strong> actin bundles intocellular protrusions and NMM Ⅱ-B incorporates intocytoplasmic stress-fibers [34] .While the expression <strong>of</strong> the NMM Ⅱ is<strong>of</strong>orms is relativelyubiquitous in most nonmuscle cells it has yet to bedetermined whether these proteins play redundant, overlappingor distinct roles in performing various mechanicalfunctions in rat HSCs. Therefore, we per-formed migrationand contraction studies using siRNA-mediatedknockdown <strong>of</strong> NMM Ⅱ-A and Ⅱ-B, which were upregulatedduring culture-activation <strong>of</strong> HSCs in vitro. While Liuet al reports that siRNA-mediated NMM Ⅱ-A inhibitionincreased cellular migration in mouse HSCs [18,31] , ourstudies demonstrated that NMM Ⅱ-A and Ⅱ-B knockdownsignificantly reduced the migratory capacity <strong>of</strong> ratHSCs. While previously studies have suggested that is<strong>of</strong>ormspecific rearrangement <strong>of</strong> actin stress fibers maybe responsible, in part, for differences in migration ratesamong different cell types [32] , further analysis is neededto validate these conflicting species-specific findings.Based on the kinetic properties <strong>of</strong> NMM Ⅱ-B, ithas been proposed that this is<strong>of</strong>orm may be involvedin maintaining tonic force needed for particular cellularfunctions such as hypercontraction [14] . Furthermore,it has been demonstrated that the loss <strong>of</strong> NMM Ⅱ-Bdecreases 3D collagen gel contraction [35] . While Liu etal reported that NMM Ⅱ-A is the essential is<strong>of</strong>ormnecessary for contraction in mouse HSCs, our resultsindicated that NMM Ⅱ-A and/or Ⅱ-B knockdownin rat HSCs does not significantly alter basal or ET-1-induced contraction. In order to confirm these results,we optimized our contraction assay by increasing siRNAconcentration and incubation times, altering collagenlattice concentration and cell number; however, changingthese parameters had no effect on HSC contraction(data not shown). In the studies performed by Meshel etal, NMM Ⅱ-B -/- fibroblast demonstrated significant differencesin cell movement and contraction depending onexperimental substrate [26,35] ; therefore, it is possible thattechnical differences in experimental design may explainour conflicting results. Future studies will more closelyexamine the contribution <strong>of</strong> NMM Ⅱ is<strong>of</strong>orms usinga Cre-lox recombination system to completely inhibitthese is<strong>of</strong>orms and assess HSC hypercontraction.While cellular localization suggests possible mechanismsby which NMM Ⅱ may function in the diseasedstate, further investigation in vivo was exploredusing a bile-duct ligation (BDL) model <strong>of</strong> hepatic injury.Obstruction <strong>of</strong> the common bile duct initiates rapidproliferation <strong>of</strong> biliary cholangiocytes and inflammation[36] . Following epithelial expansion, sustained blockage<strong>of</strong> bile flow causes continual activation <strong>of</strong> HSCs in theperiductal region, which promotes biliary fibrosis. In ourstudies, NMM Ⅱ-B expression was evident in activatedHSCs during BDL-induced hepatic fibrosis, while NMMⅡ-A and Ⅱ-C were only associated with biliary cholangiocytes.These results may suggest that NMM Ⅱ-A andⅡ-C may not be detectable in HSC in the in vivo setting.Previous studies have identified an important role forNMM Ⅱ in cellular adhesion and collagen remodelingduring wound repair [26,35] . While NMM Ⅱ-B may be theimportant is<strong>of</strong>orm contributing to the development andprogression <strong>of</strong> hepatic fibrosis, NMMⅡ-A and Ⅱ-C maybe contributing to the initiation <strong>of</strong> the inflammatory responseby stabilizing integrins and other cellular adhesionmolecules. Together, the in vitro and in vivo data suggestthat each NMM Ⅱ is<strong>of</strong>orm may be responsible for specificmolecular functions during liver injury.Although siRNA data confirmed that successful knockdown<strong>of</strong> one is<strong>of</strong>orm does not influence expression <strong>of</strong>another NMM Ⅱ is<strong>of</strong>orm (Figure 4A and 4B), it is plauWJH|www.wjgnet.com 193July 27, 2011|Volume 3|Issue 7|