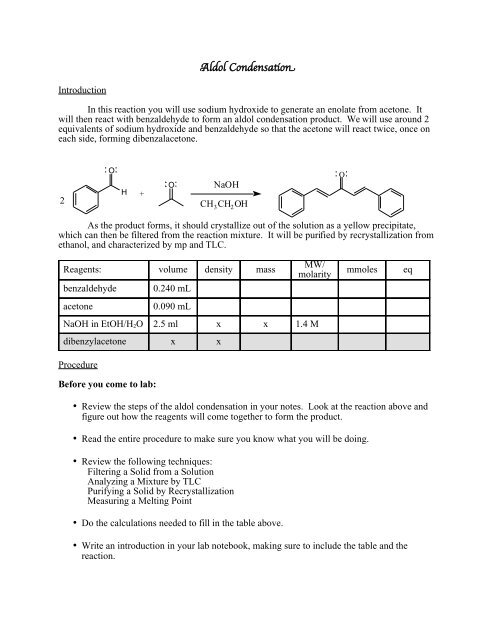
Aldol Condensation
Aldol Condensation
Aldol Condensation
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
<strong>Aldol</strong> CondensatonIntroductionIn this reaction you will use sodium hydroxide to generate an enolate from acetone. Itwill then react with benzaldehyde to form an aldol condensation product. We will use around 2equivalents of sodium hydroxide and benzaldehyde so that the acetone will react twice, once oneach side, forming dibenzalacetone.2OH+ONaOHCH 3 CH 2 OHOAs the product forms, it should crystallize out of the solution as a yellow precipitate,which can then be filtered from the reaction mixture. It will be purified by recrystallization fromethanol, and characterized by mp and TLC.Reagents: volume density massbenzaldehydeacetone0.240 mL0.090 mLMW/molarityNaOH in EtOH/H 2 O 2.5 ml x x 1.4 Mdibenzylacetone x xProcedureBefore you come to lab:mmoleseq• Review the steps of the aldol condensation in your notes. Look at the reaction above andfigure out how the reagents will come together to form the product.• Read the entire procedure to make sure you know what you will be doing.• Review the following techniques:Filtering a Solid from a SolutionAnalyzing a Mixture by TLCPurifying a Solid by RecrystallizationMeasuring a Melting Point• Do the calculations needed to fill in the table above.• Write an introduction in your lab notebook, making sure to include the table and thereaction.
Questions for <strong>Aldol</strong> ReactionName: _______________________1) Draw the mechanism for this reaction. You can abbreviate the addition of the secondbenzaldehyde if you should the first one completely.2) This reaction is an example of a mixed aldol condensation. Why is only one product formed?3) Draw the product that would have resulted if:a) cyclohexanone had been used instead of acetoneb) 2,2-dimethylpropanal had been used instead of benzaldehyde (heat may have beenrequired in this case)4) Explain why the NaOH is a catalyst in this reaction, rather than a reagent. (Hint - look at thereaction mechanism.)5) Usually an aldol condensation must be heated in order to dehydrate the beta-hydroxy ketoneto form the C=C. What is different about this product that causes it to dehydrate at roomtemperature?
6) How does the precipitation of the product from the reaction mixture help create more product?(hint – how does it affect the equilibrium)7) What type of isomers could result from this reaction?b) How would the melting point give you an idea of whether isomers are present?8) Look at the equivalents of the reagents used in this reaction.a) Why is benzaldehyde the limiting reagent in this case even though there are moreequivalents used than acetone?b) How does this affect the equivalents of product?9) Why does this product have a yellow color?