Homework - Chapter 12 Chem 2320 I. Addition reactions of alkenes
Homework - Chapter 12 Chem 2320 I. Addition reactions of alkenes
Homework - Chapter 12 Chem 2320 I. Addition reactions of alkenes
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
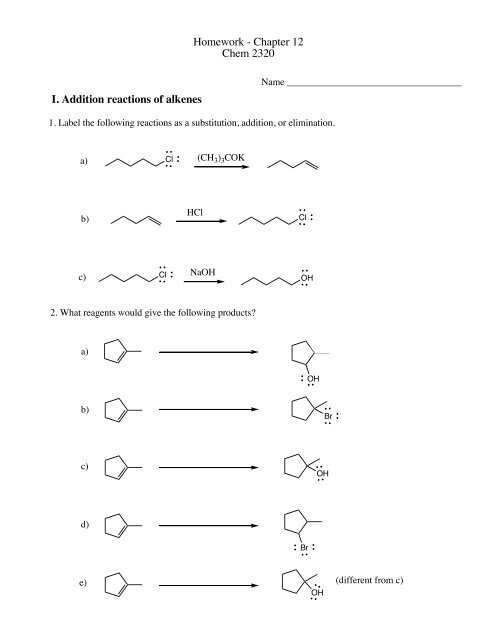
<strong>Homework</strong> - <strong>Chapter</strong> <strong>12</strong><strong>Chem</strong> <strong>2320</strong>I. <strong>Addition</strong> <strong>reactions</strong> <strong>of</strong> <strong>alkenes</strong>Name ____________________________________1. Label the following <strong>reactions</strong> as a substitution, addition, or elimination.a)Cl(CH 3 ) 3 COKb)HClClc)ClNaOHOH2. What reagents would give the following products?a)OHb)Brc)OHd)Bre)OH(different from c)
HW Ch <strong>12</strong> p 22. Give the products <strong>of</strong> the following <strong>reactions</strong>.a) H 2 , Pd/Cb)H 2 , Pd/Cc)H 2 , Pd/Cd)H 2 , Pd/Ce)H 2 , Pd/Cf)H 2 , Pd/C3. Give five isomers with the molecular formula C 6 H <strong>12</strong> that will react with H 2 and Pd/C to give hexane.4. Give the product <strong>of</strong> each <strong>of</strong> the following <strong>reactions</strong>.a)KMnO 4H 2 O, KOHb)OsO 4 , H 2 O 2
HW Ch <strong>12</strong> p 3c)KMnO 4H 2 O, KOHd)OsO 42 eq H 2 O 2e)KMnO 4H 2 O, NaOHf)OsO 4 , H 2 O 26. Give the products <strong>of</strong> the following <strong>reactions</strong>.a)PhCO 3 HOb)PhOOHc)PhCO 3 Hd)PhCO 3 HOe)PhOOH
HW Ch <strong>12</strong> p 47. What alkene could be reacted with peroxybenzoic acid to give the following epoxides?a)d)OOb)Oe)OOOc)f)8. Give the products <strong>of</strong> the following <strong>reactions</strong>.a)CHBr 3(CH 3 ) 3 COKb)CH 2 I 2Cu-Znc)CHCl 3(CH 3 ) 3 COKd)CH 2 N 2h!e)CH 2 I 2Cu-Zn
9. Give the product for each <strong>of</strong> the following <strong>reactions</strong>.HW Ch <strong>12</strong> p 5a)Cl 2b)Br 2 , H 2 Oc)Br 2CH 2 Cl 2d)Cl 2 , H 2 Oe)I 2 , H 2 Of)Br 2CH 2 Cl 2g)2 eq Cl 2 , H 2 O10. What starting material and reagents could be used to prepare each <strong>of</strong> the following products?a) b)IBrBrc)OHd)ClBrBrOHBrBr
HW Ch <strong>12</strong> p 611. Show the polymer that would be formed in the following <strong>reactions</strong>.a)H 2 SO 4b)ClCH 3 OOCH 3heat or lightc)OH 2 SO 4d)CH 3 OOCH 3heat or light<strong>12</strong>. Show the mechanism by which polybutylene could be made using a carbocation mechanism. Builda polymer three units long, showing all steps. (Note that this reaction will require heat, since thecarbocation is secondary.)H 2 SO 4heat13. What alkene could be used to synthesize each <strong>of</strong> the following polymers?a) polyacrylonitrile(used in making carpet)N N N
HW Ch <strong>12</strong> p 7b) teflonF FF FF FF F F F F F(used in making non-stick pans)c) polyethylene(used in making plastic bags)d) polyvinyl acetate(used in makingsafety glass)O O OO O O14. Write out the reaction which will occur when 1-ethyl-1-cyclopentene is treated with each <strong>of</strong> thefollowing.a) osmium tetroxide and hydrogen peroxideb) diiodomethane and copper-zinc couplec) mercuric acetate and water, followed by sodium borohydrided) peroxybenzoic acide) hydrogen gas and palladium on carbon
HW Ch <strong>12</strong> p 8f) borane-THF followed by hydrogen peroxide and hydroxideg) chlorine gash) hydrochloric acidi) potassium permanganate, water, and sodium hydroxidej) chlor<strong>of</strong>orm and potassium tert-butoxidek) hydrobromic acid with a trace <strong>of</strong> organic peroxidel) diazomethane and heatm) phosphoric acid and watern) iodine and water
II. Stereochemistry <strong>of</strong> alkene addition <strong>reactions</strong>HW Ch <strong>12</strong> p 915. Give all stereoisomers that would result from the following <strong>reactions</strong>.a)CH 2 N 2heat or lightb)H 2 Pt/Cc)Cl 2d)PhCO 3 He)I 2H 2 Of)HClg)OsO 4H 2 O 2h)H 2 SO 4H 2 Oi)1. BH 3 -THF2. H 2 O 2 , NaOH
HW Ch <strong>12</strong> p 10IV. Oxidative cleavage <strong>of</strong> <strong>alkenes</strong>16. Give the product <strong>of</strong> each <strong>of</strong> the following <strong>reactions</strong>.a)1. O 32. (CH 3 ) 2 Sb)1. O 32. (CH 3 ) 2 Sc)1. O 32. (CH 3 ) 2 S1. O 3d)2. (CH 3 ) 2 Se)1. O 32. (CH 3 ) 2 Sf)1. O 32. (CH 3 ) 2 S17. Which <strong>of</strong> the previous <strong>reactions</strong> above would give a different product if reacted with ozone followedby hydrogen peroxide? For those that will, draw the new products.a)d)b)e)c)f)
HW Ch <strong>12</strong> p 1118. What alkene would give the following products after ozonolysis?a)HOOc) andOOb)OHOd) andH OHOOHV. Synthesis using alkene <strong>reactions</strong>19. Fill in the starting material and reagent to form each <strong>of</strong> the following products.a)OHb)BrBrc)BrOHd)OH
HW Ch <strong>12</strong> p <strong>12</strong>OHe)Clf)OHg)HOOBrBrOh)i)OOj)+OH
HW Ch <strong>12</strong> p 1320. Three different types <strong>of</strong> peroxide are used in alkene <strong>reactions</strong>. Explain what each is useful for.Oa)O O Hb)H O O Hc)H 3 C O O CH 321. Explain where each <strong>of</strong> the atoms that is added to the C=C comes from in the following reagents.a)1. Hg(OAc) 2 , H 2 O2. NaBH 4OHHb)CHCl 3KOC(CH 3 ) 3ClClc)PhCO 3 HOd)1. BH 3 -THF2. H 2 O 2 , NaOHOHHe)H 2 SO 4