Dr. Frances Shepherd CV - (NCRI) is
Dr. Frances Shepherd CV - (NCRI) is
Dr. Frances Shepherd CV - (NCRI) is
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
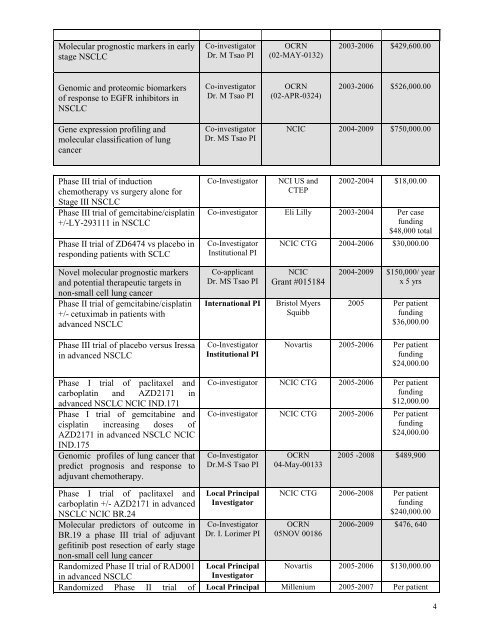
Molecular prognostic markers in early<br />
stage NSCLC<br />
Genomic and proteomic biomarkers<br />
of response to EGFR inhibitors in<br />
NSCLC<br />
Gene expression profiling and<br />
molecular classification of lung<br />
cancer<br />
Phase III trial of induction<br />
chemotherapy vs surgery alone for<br />
Stage III NSCLC<br />
Phase III trial of gemcitabine/c<strong>is</strong>platin<br />
+/-LY-293111 in NSCLC<br />
Phase II trial of ZD6474 vs placebo in<br />
responding patients with SCLC<br />
Novel molecular prognostic markers<br />
and potential therapeutic targets in<br />
non-small cell lung cancer<br />
Phase II trial of gemcitabine/c<strong>is</strong>platin<br />
+/- cetuximab in patients with<br />
advanced NSCLC<br />
Phase III trial of placebo versus Iressa<br />
in advanced NSCLC<br />
Phase I trial of paclitaxel and<br />
carboplatin and AZD2171 in<br />
advanced NSCLC NCIC IND.171<br />
Phase I trial of gemcitabine and<br />
c<strong>is</strong>platin increasing doses of<br />
AZD2171 in advanced NSCLC NCIC<br />
IND.175<br />
Genomic profiles of lung cancer that<br />
predict prognos<strong>is</strong> and response to<br />
adjuvant chemotherapy.<br />
Phase I trial of paclitaxel and<br />
carboplatin +/- AZD2171 in advanced<br />
NSCLC NCIC BR.24<br />
Molecular predictors of outcome in<br />
BR.19 a phase III trial of adjuvant<br />
gefitinib post resection of early stage<br />
non-small cell lung cancer<br />
Randomized Phase II trial of RAD001<br />
in advanced NSCLC<br />
Co-investigator<br />
<strong>Dr</strong>. M Tsao PI<br />
Co-investigator<br />
<strong>Dr</strong>. M Tsao PI<br />
Co-investigator<br />
<strong>Dr</strong>. MS Tsao PI<br />
OCRN<br />
(02-MAY-0132)<br />
OCRN<br />
(02-APR-0324)<br />
Co-Investigator NCI US and<br />
CTEP<br />
2003-2006 $429,600.00<br />
2003-2006<br />
$526,000.00<br />
NCIC 2004-2009 $750,000.00<br />
2002-2004 $18,00.00<br />
Co-investigator Eli Lilly 2003-2004 Per case<br />
funding<br />
$48,000 total<br />
Co-Investigator<br />
Institutional PI<br />
Co-applicant<br />
<strong>Dr</strong>. MS Tsao PI<br />
NCIC CTG 2004-2006 $30,000.00<br />
NCIC<br />
Grant #015184<br />
International PI Br<strong>is</strong>tol Myers<br />
Squibb<br />
Co-Investigator<br />
Institutional PI<br />
2004-2009 $150,000/ year<br />
x 5 yrs<br />
2005 Per patient<br />
funding<br />
$36,000.00<br />
Novart<strong>is</strong> 2005-2006 Per patient<br />
funding<br />
$24,000.00<br />
Co-investigator NCIC CTG 2005-2006 Per patient<br />
funding<br />
$12,000.00<br />
Co-investigator NCIC CTG 2005-2006 Per patient<br />
funding<br />
$24,000.00<br />
Co-Investigator<br />
<strong>Dr</strong>.M-S Tsao PI<br />
Local Principal<br />
Investigator<br />
Co-Investigator<br />
<strong>Dr</strong>. I. Lorimer PI<br />
Local Principal<br />
Investigator<br />
OCRN<br />
04-May-00133<br />
2005 -2008 $489,900<br />
NCIC CTG 2006-2008 Per patient<br />
funding<br />
$240,000.00<br />
OCRN<br />
05NOV 00186<br />
2006-2009 $476, 640<br />
Novart<strong>is</strong> 2005-2006 $130,000.00<br />
Randomized Phase II trial of Local Principal Millenium 2005-2007 Per patient<br />
4