Self-Assembly of Mesoscale Objects in ordered 2D Arrays - The ...
Self-Assembly of Mesoscale Objects in ordered 2D Arrays - The ...
Self-Assembly of Mesoscale Objects in ordered 2D Arrays - The ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
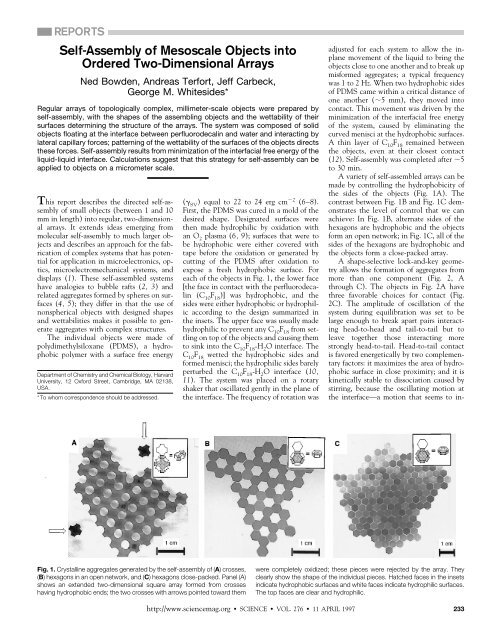
REPORTS<strong>Self</strong>-<strong>Assembly</strong> <strong>of</strong> <strong>Mesoscale</strong> <strong>Objects</strong> <strong>in</strong>toOrdered Two-Dimensional <strong>Arrays</strong>Ned Bowden, Andreas Terfort, Jeff Carbeck,George M. Whitesides*Regular arrays <strong>of</strong> topologically complex, millimeter-scale objects were prepared byself-assembly, with the shapes <strong>of</strong> the assembl<strong>in</strong>g objects and the wettability <strong>of</strong> theirsurfaces determ<strong>in</strong><strong>in</strong>g the structure <strong>of</strong> the arrays. <strong>The</strong> system was composed <strong>of</strong> solidobjects float<strong>in</strong>g at the <strong>in</strong>terface between perfluorodecal<strong>in</strong> and water and <strong>in</strong>teract<strong>in</strong>g bylateral capillary forces; pattern<strong>in</strong>g <strong>of</strong> the wettability <strong>of</strong> the surfaces <strong>of</strong> the objects directsthese forces. <strong>Self</strong>-assembly results from m<strong>in</strong>imization <strong>of</strong> the <strong>in</strong>terfacial free energy <strong>of</strong> theliquid-liquid <strong>in</strong>terface. Calculations suggest that this strategy for self-assembly can beapplied to objects on a micrometer scale.This report describes the directed self-assembly<strong>of</strong> small objects (between 1 and 10mm <strong>in</strong> length) <strong>in</strong>to regular, two-dimensionalarrays. It extends ideas emerg<strong>in</strong>g frommolecular self-assembly to much larger objectsand describes an approach for the fabrication<strong>of</strong> complex systems that has potentialfor application <strong>in</strong> microelectronics, optics,microelectromechanical systems, anddisplays (1). <strong>The</strong>se self-assembled systemshave analogies to bubble rafts (2, 3) andrelated aggregates formed by spheres on surfaces(4, 5); they differ <strong>in</strong> that the use <strong>of</strong>nonspherical objects with designed shapesand wettabilities makes it possible to generateaggregates with complex structures.<strong>The</strong> <strong>in</strong>dividual objects were made <strong>of</strong>polydimethylsiloxane (PDMS), a hydrophobicpolymer with a surface free energyDepartment <strong>of</strong> Chemistry and Chemical Biology, HarvardUniversity, 12 Oxford Street, Cambridge, MA 02138,USA.*To whom correspondence should be addressed.( SV) equal to 22 to 24 erg cm 2 (6–8).First, the PDMS was cured <strong>in</strong> a mold <strong>of</strong> thedesired shape. Designated surfaces werethen made hydrophilic by oxidation withan O 2plasma (6, 9); surfaces that were tobe hydrophobic were either covered withtape before the oxidation or generated bycutt<strong>in</strong>g <strong>of</strong> the PDMS after oxidation toexpose a fresh hydrophobic surface. Foreach <strong>of</strong> the objects <strong>in</strong> Fig. 1, the lower face[the face <strong>in</strong> contact with the perfluorodecal<strong>in</strong>(C 10F 18)] was hydrophobic, and thesides were either hydrophobic or hydrophilicaccord<strong>in</strong>g to the design summarized <strong>in</strong>the <strong>in</strong>sets. <strong>The</strong> upper face was usually madehydrophilic to prevent any C 10F 18from settl<strong>in</strong>gon top <strong>of</strong> the objects and caus<strong>in</strong>g themto s<strong>in</strong>k <strong>in</strong>to the C 10F 18-H 2O <strong>in</strong>terface. <strong>The</strong>C 10F 18wetted the hydrophobic sides andformed menisci; the hydrophilic sides barelyperturbed the C 10F 18-H 2O <strong>in</strong>terface (10,11). <strong>The</strong> system was placed on a rotaryshaker that oscillated gently <strong>in</strong> the plane <strong>of</strong>the <strong>in</strong>terface. <strong>The</strong> frequency <strong>of</strong> rotation wasadjusted for each system to allow the <strong>in</strong>planemovement <strong>of</strong> the liquid to br<strong>in</strong>g theobjects close to one another and to break upmisformed aggregates; a typical frequencywas 1 to 2 Hz. When two hydrophobic sides<strong>of</strong> PDMS came with<strong>in</strong> a critical distance <strong>of</strong>one another (5 mm), they moved <strong>in</strong>tocontact. This movement was driven by them<strong>in</strong>imization <strong>of</strong> the <strong>in</strong>terfacial free energy<strong>of</strong> the system, caused by elim<strong>in</strong>at<strong>in</strong>g thecurved menisci at the hydrophobic surfaces.A th<strong>in</strong> layer <strong>of</strong> C 10F 18rema<strong>in</strong>ed betweenthe objects, even at their closest contact(12). <strong>Self</strong>-assembly was completed after 5to 30 m<strong>in</strong>.A variety <strong>of</strong> self-assembled arrays can bemade by controll<strong>in</strong>g the hydrophobicity <strong>of</strong>the sides <strong>of</strong> the objects (Fig. 1A). <strong>The</strong>contrast between Fig. 1B and Fig. 1C demonstratesthe level <strong>of</strong> control that we canachieve: In Fig. 1B, alternate sides <strong>of</strong> thehexagons are hydrophobic and the objectsform an open network; <strong>in</strong> Fig. 1C, all <strong>of</strong> thesides <strong>of</strong> the hexagons are hydrophobic andthe objects form a close-packed array.A shape-selective lock-and-key geometryallows the formation <strong>of</strong> aggregates frommore than one component (Fig. 2, Athrough C). <strong>The</strong> objects <strong>in</strong> Fig. 2A havethree favorable choices for contact (Fig.2C). <strong>The</strong> amplitude <strong>of</strong> oscillation <strong>of</strong> thesystem dur<strong>in</strong>g equilibration was set to belarge enough to break apart pairs <strong>in</strong>teract<strong>in</strong>ghead-to-head and tail-to-tail but toleave together those <strong>in</strong>teract<strong>in</strong>g morestrongly head-to-tail. Head-to-tail contactis favored energetically by two complementaryfactors: it maximizes the area <strong>of</strong> hydrophobicsurface <strong>in</strong> close proximity; and it isk<strong>in</strong>etically stable to dissociation caused bystirr<strong>in</strong>g, because the oscillat<strong>in</strong>g motion atthe <strong>in</strong>terface—a motion that seems to <strong>in</strong>-Fig. 1. Crystall<strong>in</strong>e aggregates generated by the self-assembly <strong>of</strong> (A) crosses,(B) hexagons <strong>in</strong> an open network, and (C) hexagons close-packed. Panel (A)shows an extended two-dimensional square array formed from crosseshav<strong>in</strong>g hydrophobic ends; the two crosses with arrows po<strong>in</strong>ted toward themwere completely oxidized; these pieces were rejected by the array. <strong>The</strong>yclearly show the shape <strong>of</strong> the <strong>in</strong>dividual pieces. Hatched faces <strong>in</strong> the <strong>in</strong>sets<strong>in</strong>dicate hydrophobic surfaces and white faces <strong>in</strong>dicate hydrophilic surfaces.<strong>The</strong> top faces are clear and hydrophilic.http://www.sciencemag.org SCIENCE VOL. 276 11 APRIL 1997 233
fluence the objects primarily by shear—hasm<strong>in</strong>imal <strong>in</strong>fluence on pairs <strong>of</strong> objects onceassembled <strong>in</strong>to a head-to-tail configuration.A third method for self-assembly usesthe area <strong>of</strong> hydrophobic side surfaces, andthus the strength <strong>of</strong> the attractive capillaryforce, to direct the self-assembly <strong>of</strong> differentobjects (Fig. 3, A through D). A mixture <strong>of</strong>two types <strong>of</strong> PDMS objects with the samesquare bases, but heights that differed by afactor <strong>of</strong> 5, were agitated at the <strong>in</strong>terfacebetween C 10 F 18 and water. <strong>The</strong> order <strong>of</strong> theattractive forces <strong>in</strong> the system was talltall tall-short short-short. <strong>The</strong> degree<strong>of</strong> agitation was set to allow the tall objectsto form an array; when agitation wasFig. 2. Lock-and-key self-assembly. L<strong>in</strong>es areformed from one type <strong>of</strong> component (A) and fromtwo different types <strong>of</strong> components (B). (C) <strong>The</strong>three possibilities for favorable assembly <strong>of</strong> twoobjects <strong>in</strong> (A). Although <strong>in</strong> the tail-to-tail arrangement<strong>in</strong> (C) no hydrophobic surfaces are <strong>in</strong> contact,this <strong>in</strong>teraction was observed to be importantbecause the menisci are brought close enoughtogether to <strong>in</strong>teract. Dark faces <strong>in</strong> the <strong>in</strong>sets <strong>in</strong> (A) and (B) <strong>in</strong>dicate hydrophobic sides and white faces<strong>in</strong>dicate hydrophilic sides. <strong>The</strong> top faces are clear and hydrophilic.stopped, the short objects coagulatedaround this array <strong>in</strong> a dis<strong>ordered</strong> state.<strong>The</strong> above experiments were carried outwith objects hav<strong>in</strong>g dimensions <strong>of</strong> 1 to 10mm. We <strong>in</strong>vestigated the lower limits to thesize <strong>of</strong> the objects that could be assembledby lateral capillary forces at the C 10 F 18 -H 2 O<strong>in</strong>terface by calculat<strong>in</strong>g the change <strong>in</strong> <strong>in</strong>terfacialfree energy as two perpendicularsurfaces moved from <strong>in</strong>f<strong>in</strong>ite separation tosome f<strong>in</strong>ite separation, d. We calculated theheight h (<strong>in</strong> meters) <strong>of</strong> the C 10 F 18 -H 2 O<strong>in</strong>terface between the two objects us<strong>in</strong>g thel<strong>in</strong>earized Laplace equation (13) (Fig. 4A) 2 hx 2 1 gh P 0 (1)where (<strong>in</strong> joules per square meter) is the<strong>in</strong>terfacial free energy, (<strong>in</strong> kilograms percubic meter) is the difference <strong>in</strong> densitybetween the two fluids, the zero for h is setat the C 10 F 18 -H 2 O <strong>in</strong>terface far from theobjects, g (<strong>in</strong> meters per second per second)is the acceleration due to gravity, and P 0(<strong>in</strong> pascals) is the change <strong>in</strong> pressure acrossthe <strong>in</strong>terface at x 0. If we assign a value<strong>of</strong> h(0) 0, then the value <strong>of</strong> P 0 does notenter <strong>in</strong>to the solution <strong>of</strong> Eq. 1. Us<strong>in</strong>g theboundary conditions h(r) t, where t (<strong>in</strong>meters) is the thickness <strong>of</strong> the object and(h/x) x0 0, we f<strong>in</strong>d that the solution <strong>of</strong>Eq.1is2hxt ex/xc d/2xc e x/xc)1e d/xc e d/2xc (2) eFig. 3. <strong>Self</strong>-assembly based on different heights. <strong>The</strong>two components are identical except for their heights,and their sides are hydrophobic. <strong>The</strong> light gray objects<strong>in</strong> (A) and (B) have a height <strong>of</strong> 2.5 mm and thedark gray objects have a height <strong>of</strong> 0.5 mm. In (A) theobjects have just begun to assemble. After 20 m<strong>in</strong><strong>of</strong> agitation, the objects <strong>in</strong> (B) have segregated <strong>in</strong>totwo regions: a crystall<strong>in</strong>e central array <strong>of</strong> the tallerobjects, surrounded by a dis<strong>ordered</strong> collection <strong>of</strong> thesmaller ones. (C) A schematic view <strong>of</strong> the <strong>in</strong>teraction <strong>of</strong> the objects <strong>of</strong> different heights. (D) Side view <strong>of</strong>the three different <strong>in</strong>teractions when two pieces <strong>of</strong> PDMS with different heights are assembled. <strong>The</strong>strength <strong>of</strong> <strong>in</strong>teraction <strong>in</strong>creases from right to left.Fig. 4. (A) <strong>The</strong> coord<strong>in</strong>ate system for Eqs. 1 and 2.<strong>The</strong> objects have a height <strong>of</strong> t and a width <strong>of</strong> w 5t , and their proximate surfaces are separated byd. (B) <strong>The</strong> logarithm <strong>of</strong> the change <strong>in</strong> <strong>in</strong>terfacialfree energy—divided by thermal energy, kT—<strong>in</strong>br<strong>in</strong>g<strong>in</strong>g two surfaces from <strong>in</strong>f<strong>in</strong>ite separation to af<strong>in</strong>ite separation, d, is plotted for heights from t 1 mm to 100 nm.234SCIENCE VOL. 276 11 APRIL 1997 http://www.sciencemag.org
REPORTSwhere we have made the replacement x c (/g) 1/2 . For <strong>in</strong>f<strong>in</strong>ite d the capillary surfaceis given by a simple exponential decaywith h(x) e (x/x c) .To estimate the change <strong>in</strong> <strong>in</strong>terfacialenergy as a function <strong>of</strong> distance, we calculatedthe difference <strong>in</strong> the arc length (<strong>in</strong>meters), def<strong>in</strong>ed by h(x) for two surfacesseparated by d and two surfaces separated byan <strong>in</strong>f<strong>in</strong>ite distance; the change <strong>in</strong> arclength was then multiplied by the width <strong>of</strong>the object w (<strong>in</strong> meters), and the <strong>in</strong>terfacialfree energy to yield the change <strong>in</strong> <strong>in</strong>terfacialfree energy (14). As a model system, weassigned a length to the perpendicular surfaceequal to five times the height. Thismodel gave a change <strong>in</strong> <strong>in</strong>terfacial free energy,W, <strong>of</strong> W 5t. From thechange <strong>in</strong> <strong>in</strong>terfacial free energy for heightsfrom t 1 mm to 100 nm (Fig. 4B), weconclude that the energetics for self-assemblyare favorable for objects with t as smallas 100 nm. For the two-dimensional selfassembly<strong>of</strong> spheres, the radius at whichW/kT 1 (where kT is the thermal energy)has been calculated to be on the order<strong>of</strong> 1 to 10 m (15, 16). <strong>Self</strong>-assemblydriven by capillary forces between conformalsurfaces should therefore make possiblethe assembly <strong>of</strong> much smaller objectsthan would be possible with spheres; theability to control the shapes and <strong>in</strong>terfacialproperties <strong>of</strong> these objects makes itpossible to design the geometries <strong>of</strong> theresult<strong>in</strong>g arrays.Four factors contribute to the success <strong>of</strong>this strategy for the directed self-assembly <strong>of</strong>small objects. First, the aggregates are energeticallymore stable than the <strong>in</strong>dividualdissociated objects or dis<strong>ordered</strong> aggregates.Second, formation <strong>of</strong> the aggregates is reversiblewhen the system is agitated: formationand dissociation <strong>of</strong> the aggregates compete.<strong>The</strong> aggregates are therefore able toreach the energetically most stable form.Third, the hydrophobic sides are attractedto one another over large distances (abouttwo to three times the dimension <strong>of</strong> theheight), lead<strong>in</strong>g to relatively rapid assembly.Fourth, even when two hydrophobicsides are <strong>in</strong> close proximity, they can movelaterally from side to side, lubricated by the<strong>in</strong>terven<strong>in</strong>g film <strong>of</strong> C 10F 18, and can thusmaximize the amount <strong>of</strong> hydrophobic area<strong>in</strong> contact.REFERENCES AND NOTES___________________________1. <strong>The</strong>re are few methods for assembl<strong>in</strong>g arrays <strong>of</strong>small objects. See (2, 4, 5); J. K. Tu, J. J. Talghader,M. A. Hadley, J. S. Smith, Electron. Lett. 31, 1448(1995); A. Ashk<strong>in</strong> and J. M. Dziedzic, Science 235,1517 (1987); K. Svoboda and S. M. Block, Annu.Rev. Biophys. Biomol. Struct. 23, 247 (1994).2. A. W. Simpson and P. H. Hodk<strong>in</strong>son, Nature 237,320 (1972).3. S. T. Schober, J. Friedrich, A. Altmann, J. Appl.Phys. 71, 2206 (1992).4. M. Yamaki, J. Higo, K. Nagayama, Langmuir 11,2975 (1995).5. P. A. Kralchevsky and K. Nagayama, ibid. 10, 23(1994).6. PDMS is easy to fabricate and its solid-liquid <strong>in</strong>terfacialfree energy can be readily controlled by oxidation tomake the surfaces hydrophilic or hydrophobic. Weplaced the PDMS structure <strong>in</strong>to a plasma cleaner for 5m<strong>in</strong> under O 2at a pressure <strong>of</strong> 0.20 torr to oxidize thePDMS. <strong>The</strong> oxidation <strong>of</strong> PDMS is believed to result <strong>in</strong>a surface that comprises SiOH groups. <strong>The</strong> contactangle <strong>of</strong> water on oxidized PDMS is less than 15°.7. M. J. Owen, J. Coat<strong>in</strong>gs Technol. 53, 49 (1981).8. W. A. Zisman, <strong>in</strong> Symposium on Adhesion and Cohesion,P. Weiss, Ed. (Elsevier, New York, 1962), p.176.9. D. W. Fakes, M. C. Davies, A. Browns, J. M. Newton,Surf. Interface Anal. 13, 233 (1988).10. <strong>The</strong> perfluorodecal<strong>in</strong> wet the unoxidized PDMS andformed a meniscus; the water wet the higher energysurface <strong>of</strong> oxidized PDMS. <strong>The</strong> capillary forces act<strong>in</strong>gat the oxidized surfaces were very weakly attractivecompared to those <strong>of</strong> the hydrophobic surfaces,because the PDMS (density 1.05 g ml 1 ) does nots<strong>in</strong>k far enough <strong>in</strong>to the perfluorodecal<strong>in</strong> (density 1.91 g ml 1 ) to generate a meniscus with significantcurvature at the hydrophilic <strong>in</strong>terfaces. Other fluor<strong>in</strong>atedalkanes with properties similar to those <strong>of</strong> perfluorodecal<strong>in</strong>were used with equal success.11. C. D. Dashk<strong>in</strong>, P. A. Kralchevsky, W. N. Paunov, H.Yoshimura, K. Nagayama, Langmuir 12, 641 (1996).12. <strong>The</strong> evidence that a th<strong>in</strong> layer <strong>of</strong> C 10F 18rema<strong>in</strong>edbetween the PDMS solids at their closest contact is<strong>in</strong>direct: When two flat pieces <strong>of</strong> PDMS come <strong>in</strong>contact <strong>in</strong> water with no C 10F 18present, they stick toeach other strongly, and this process is effectivelyirreversible. If we add C 10F 18to the water after thePDMS solids have come <strong>in</strong>to contact, they rema<strong>in</strong>stuck to one another.13. H. T. Davis, Statistical Mechanics <strong>of</strong> Phases, Interfacesand Th<strong>in</strong> Films: Advances <strong>in</strong> Interfacial Eng<strong>in</strong>eer<strong>in</strong>g( VCH, New York, 1996).14. In perform<strong>in</strong>g these calculations, we used values <strong>of</strong> 0.05 J m 2 and 900 kg m 3 for the C 10F 18-H 2O <strong>in</strong>terface.15. P. A. Kralchevsky, V. N. Paunov, N. D. Denkov, I. B.Ivanov, K. Nagayama, J. Colloid Interface Sci. 155,420 (1993).16. V. N. Paunov, P. A. Kralchevsky, N. D. Denkov, K.Nagayama, ibid. 157, 100 (1993).17. We thank M. Mammen for helpful comments andsuggestions. Funded by NSF grant CHE-9122331,the Office <strong>of</strong> Naval Research, and the Defense AdvancedResearch Projects Agency. N.B. was supportedby a predoctoral fellowship from the Department<strong>of</strong> Defense. A.T. thanks the Deutsche Forschungsgeme<strong>in</strong>schaftfor a research grant.4 November 1996; accepted 24 February 1997Permo-Triassic Boundary Superanoxia andStratified Superocean: Records fromLost Deep SeaYukio IsozakiPelagic cherts <strong>of</strong> Japan and British Columbia, Canada, recorded a long-term and worldwidedeep-sea anoxic (oxygen-depleted) event across the Permo-Triassic (or Paleozoicand Mesozoic) boundary (251 2 million years ago). <strong>The</strong> symmetry <strong>in</strong> lithostratigraphyand redox condition <strong>of</strong> the boundary sections suggest that the superocean Panthalassabecame totally stratified for nearly 20 million years across the boundary. <strong>The</strong> tim<strong>in</strong>g <strong>of</strong>onset, climax, and term<strong>in</strong>ation <strong>of</strong> the oceanic stratification correspond to global bioticevents <strong>in</strong>clud<strong>in</strong>g the end-Guadalupian decl<strong>in</strong>e, the end-Permian ext<strong>in</strong>ction, and mid-Triassic recovery.<strong>The</strong> greatest mass ext<strong>in</strong>ction <strong>in</strong> the Phanerozoicoccurred at the tim<strong>in</strong>g <strong>of</strong> thePermo-Triassic (P-T) boundary; many hypothesesfor the ext<strong>in</strong>ction have focused onchanges <strong>in</strong> the ocean, <strong>in</strong>clud<strong>in</strong>g development<strong>of</strong> overturn <strong>of</strong> an anoxic ocean (1, 2).One problem has been that most records <strong>of</strong>the boundary are <strong>in</strong> shallow-water sedimentaryrocks that formed around the supercont<strong>in</strong>entPangea. Recently, however, P-Tboundary sections were discovered <strong>in</strong> deepseacherts that crop out extensively <strong>in</strong> theJurassic accretionary complex <strong>in</strong> southwestJapan (3, 4). <strong>The</strong> cherts represent ancientpelagic sediments primarily deposited <strong>in</strong> amid-oceanic deep sea <strong>of</strong> the superoceanPanthalassa and accreted onto the SouthCh<strong>in</strong>a (Yangtze) cont<strong>in</strong>ental marg<strong>in</strong> <strong>in</strong> theDepartment <strong>of</strong> Earth and Planetary Sciences, Tokyo Institute<strong>of</strong> Technology, O-okayama, Meguro, Tokyo 152,Japan. E-mail: yisozaki@geo.titech.ac.jpMiddle Jurassic (5). <strong>The</strong> Panthalassa superoceanoccupied nearly 70% <strong>of</strong> Earth’s surface<strong>in</strong> the Late Permian (6). Rocks nearthe P-T boundary are reduced, and arethought to have been deposited <strong>in</strong> an anoxicenvironment (3, 4, 7, 8). I refer tothese rocks as the P-T boundary unit(PTBU; Fig. 1). Across the PTBU, Paleozoicradiolarians (planktonic protozoans)are completely replaced by dist<strong>in</strong>ct Mesozoictypes. Similar rocks have also recentlybeen found <strong>in</strong> British Columbia, Canada. Iused the sections from Japan and BritishColumbia to evaluate Panthalassa oceandynamics across the P-T boundary.In the Japanese sections, Early to earlyLate Permian and Middle to Late Triassiccherts are composed ma<strong>in</strong>ly <strong>of</strong> siliceous radiolariantests (95% by weight) and aremostly brick red <strong>in</strong> color. X-ray diffractionand 57 Fe Mössbauer spectroscopy demon-http://www.sciencemag.org SCIENCE VOL. 276 11 APRIL 1997 235