Homework - Chapter 12 Chem 2320 I. Addition reactions of alkenes
Homework - Chapter 12 Chem 2320 I. Addition reactions of alkenes
Homework - Chapter 12 Chem 2320 I. Addition reactions of alkenes
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
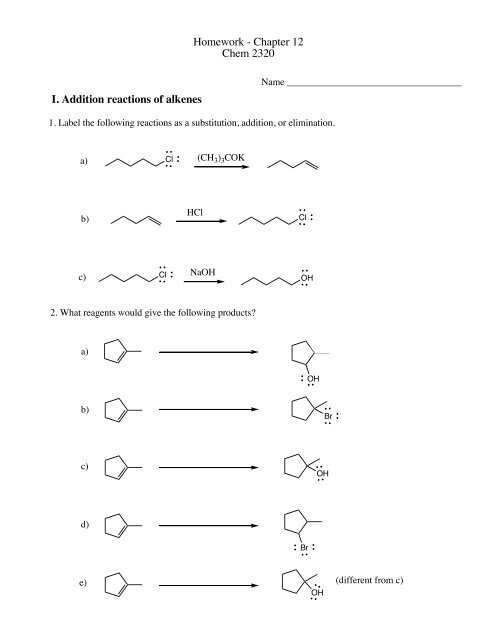
<strong>Homework</strong> - <strong>Chapter</strong> <strong>12</strong><strong>Chem</strong> <strong>2320</strong>I. <strong>Addition</strong> <strong>reactions</strong> <strong>of</strong> <strong>alkenes</strong>Name ____________________________________1. Label the following <strong>reactions</strong> as a substitution, addition, or elimination.a)Cl(CH 3 ) 3 COKb)HClClc)ClNaOHOH2. What reagents would give the following products?a)OHb)Brc)OHd)Bre)OH(different from c)
HW Ch <strong>12</strong> p 22. Give the products <strong>of</strong> the following <strong>reactions</strong>.a) H 2 , Pd/Cb)H 2 , Pd/Cc)H 2 , Pd/Cd)H 2 , Pd/Ce)H 2 , Pd/Cf)H 2 , Pd/C3. Give five isomers with the molecular formula C 6 H <strong>12</strong> that will react with H 2 and Pd/C to give hexane.4. Give the product <strong>of</strong> each <strong>of</strong> the following <strong>reactions</strong>.a)KMnO 4H 2 O, KOHb)OsO 4 , H 2 O 2
HW Ch <strong>12</strong> p 3c)KMnO 4H 2 O, KOHd)OsO 42 eq H 2 O 2e)KMnO 4H 2 O, NaOHf)OsO 4 , H 2 O 26. Give the products <strong>of</strong> the following <strong>reactions</strong>.a)PhCO 3 HOb)PhOOHc)PhCO 3 Hd)PhCO 3 HOe)PhOOH
HW Ch <strong>12</strong> p 47. What alkene could be reacted with peroxybenzoic acid to give the following epoxides?a)d)OOb)Oe)OOOc)f)8. Give the products <strong>of</strong> the following <strong>reactions</strong>.a)CHBr 3(CH 3 ) 3 COKb)CH 2 I 2Cu-Znc)CHCl 3(CH 3 ) 3 COKd)CH 2 N 2h!e)CH 2 I 2Cu-Zn
9. Give the product for each <strong>of</strong> the following <strong>reactions</strong>.HW Ch <strong>12</strong> p 5a)Cl 2b)Br 2 , H 2 Oc)Br 2CH 2 Cl 2d)Cl 2 , H 2 Oe)I 2 , H 2 Of)Br 2CH 2 Cl 2g)2 eq Cl 2 , H 2 O10. What starting material and reagents could be used to prepare each <strong>of</strong> the following products?a) b)IBrBrc)OHd)ClBrBrOHBrBr
HW Ch <strong>12</strong> p 611. Show the polymer that would be formed in the following <strong>reactions</strong>.a)H 2 SO 4b)ClCH 3 OOCH 3heat or lightc)OH 2 SO 4d)CH 3 OOCH 3heat or light<strong>12</strong>. Show the mechanism by which polybutylene could be made using a carbocation mechanism. Builda polymer three units long, showing all steps. (Note that this reaction will require heat, since thecarbocation is secondary.)H 2 SO 4heat13. What alkene could be used to synthesize each <strong>of</strong> the following polymers?a) polyacrylonitrile(used in making carpet)N N N
HW Ch <strong>12</strong> p 7b) teflonF FF FF FF F F F F F(used in making non-stick pans)c) polyethylene(used in making plastic bags)d) polyvinyl acetate(used in makingsafety glass)O O OO O O14. Write out the reaction which will occur when 1-ethyl-1-cyclopentene is treated with each <strong>of</strong> thefollowing.a) osmium tetroxide and hydrogen peroxideb) diiodomethane and copper-zinc couplec) mercuric acetate and water, followed by sodium borohydrided) peroxybenzoic acide) hydrogen gas and palladium on carbon
HW Ch <strong>12</strong> p 8f) borane-THF followed by hydrogen peroxide and hydroxideg) chlorine gash) hydrochloric acidi) potassium permanganate, water, and sodium hydroxidej) chlor<strong>of</strong>orm and potassium tert-butoxidek) hydrobromic acid with a trace <strong>of</strong> organic peroxidel) diazomethane and heatm) phosphoric acid and watern) iodine and water
II. Stereochemistry <strong>of</strong> alkene addition <strong>reactions</strong>HW Ch <strong>12</strong> p 915. Give all stereoisomers that would result from the following <strong>reactions</strong>.a)CH 2 N 2heat or lightb)H 2 Pt/Cc)Cl 2d)PhCO 3 He)I 2H 2 Of)HClg)OsO 4H 2 O 2h)H 2 SO 4H 2 Oi)1. BH 3 -THF2. H 2 O 2 , NaOH
HW Ch <strong>12</strong> p 10IV. Oxidative cleavage <strong>of</strong> <strong>alkenes</strong>16. Give the product <strong>of</strong> each <strong>of</strong> the following <strong>reactions</strong>.a)1. O 32. (CH 3 ) 2 Sb)1. O 32. (CH 3 ) 2 Sc)1. O 32. (CH 3 ) 2 S1. O 3d)2. (CH 3 ) 2 Se)1. O 32. (CH 3 ) 2 Sf)1. O 32. (CH 3 ) 2 S17. Which <strong>of</strong> the previous <strong>reactions</strong> above would give a different product if reacted with ozone followedby hydrogen peroxide? For those that will, draw the new products.a)d)b)e)c)f)
HW Ch <strong>12</strong> p 1118. What alkene would give the following products after ozonolysis?a)HOOc) andOOb)OHOd) andH OHOOHV. Synthesis using alkene <strong>reactions</strong>19. Fill in the starting material and reagent to form each <strong>of</strong> the following products.a)OHb)BrBrc)BrOHd)OH
HW Ch <strong>12</strong> p <strong>12</strong>OHe)Clf)OHg)HOOBrBrOh)i)OOj)+OH
HW Ch <strong>12</strong> p 1320. Three different types <strong>of</strong> peroxide are used in alkene <strong>reactions</strong>. Explain what each is useful for.Oa)O O Hb)H O O Hc)H 3 C O O CH 321. Explain where each <strong>of</strong> the atoms that is added to the C=C comes from in the following reagents.a)1. Hg(OAc) 2 , H 2 O2. NaBH 4OHHb)CHCl 3KOC(CH 3 ) 3ClClc)PhCO 3 HOd)1. BH 3 -THF2. H 2 O 2 , NaOHOHHe)H 2 SO 4