Stability Indicating RP-HPLC Assay Method Development and ...
Stability Indicating RP-HPLC Assay Method Development and ...
Stability Indicating RP-HPLC Assay Method Development and ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
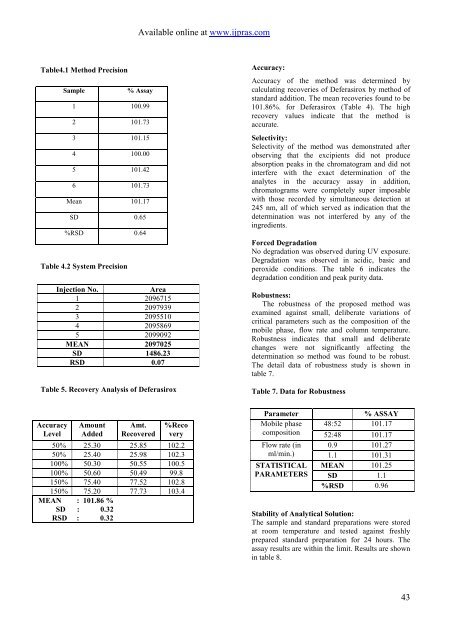
Available online at www.ijpras.comTable4.1 <strong>Method</strong> PrecisionSampleTable 4.2 System Precision% <strong>Assay</strong>1 100.992 101.733 101.154 100.005 101.426 101.73Mean 101.17SD 0.65%RSD 0.64Injection No.Area1 20967152 20979393 20955104 20958695 2099092MEAN 2097025SD 1486.23RSD 0.07Table 5. Recovery Analysis of DeferasiroxAccuracy:Accuracy of the method was determined bycalculating recoveries of Deferasirox by method ofst<strong>and</strong>ard addition. The mean recoveries found to be101.86%. for Deferasirox (Table 4). The highrecovery values indicate that the method isaccurate.Selectivity:Selectivity of the method was demonstrated afterobserving that the excipients did not produceabsorption peaks in the chromatogram <strong>and</strong> did notinterfere with the exact determination of theanalytes in the accuracy assay in addition,chromatograms were completely super imposablewith those recorded by simultaneous detection at245 nm, all of which served as indication that thedetermination was not interfered by any of theingredients.Forced DegradationNo degradation was observed during UV exposure.Degradation was observed in acidic, basic <strong>and</strong>peroxide conditions. The table 6 indicates thedegradation condition <strong>and</strong> peak purity data.Robustness:The robustness of the proposed method wasexamined against small, deliberate variations ofcritical parameters such as the composition of themobile phase, flow rate <strong>and</strong> column temperature.Robustness indicates that small <strong>and</strong> deliberatechanges were not significantly affecting thedetermination so method was found to be robust.The detail data of robustness study is shown intable 7.Table 7. Data for RobustnessAccuracyLevelAmountAddedAmt.Recovered%Recovery50% 25.30 25.85 102.250% 25.40 25.98 102.3100% 50.30 50.55 100.5100% 50.60 50.49 99.8150% 75.40 77.52 102.8150% 75.20 77.73 103.4MEAN : 101.86 %SD : 0.32RSD : 0.32ParameterMobile phasecompositionFlow rate (inml/min.)STATISTICALPARAMETERS% ASSAY48:52 101.1752:48 101.170.9 101.271.1 101.31MEAN 101.25SD 1.1%RSD 0.96<strong>Stability</strong> of Analytical Solution:The sample <strong>and</strong> st<strong>and</strong>ard preparations were storedat room temperature <strong>and</strong> tested against freshlyprepared st<strong>and</strong>ard preparation for 24 hours. Theassay results are within the limit. Results are shownin table 8.43