Mycoplasma Synoviae Fact Sheet - Veterinary Diagnostic Laboratory
Mycoplasma Synoviae Fact Sheet - Veterinary Diagnostic Laboratory
Mycoplasma Synoviae Fact Sheet - Veterinary Diagnostic Laboratory
- No tags were found...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
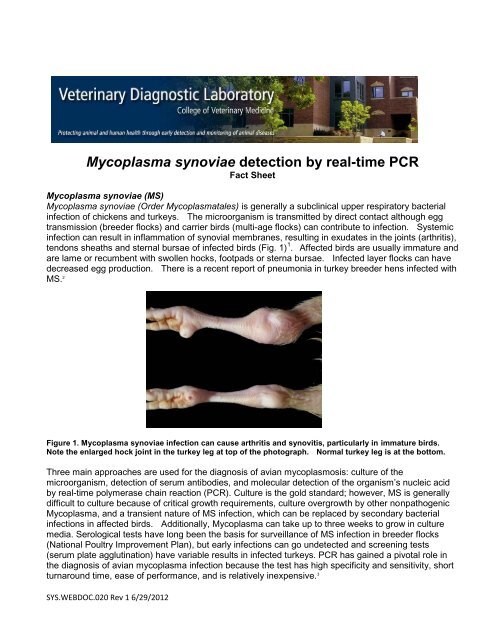
<strong>Mycoplasma</strong> synoviae detection by real-time PCR<strong>Fact</strong> <strong>Sheet</strong><strong>Mycoplasma</strong> synoviae (MS)<strong>Mycoplasma</strong> synoviae (Order <strong>Mycoplasma</strong>tales) is generally a subclinical upper respiratory bacterialinfection of chickens and turkeys. The microorganism is transmitted by direct contact although eggtransmission (breeder flocks) and carrier birds (multi-age flocks) can contribute to infection. Systemicinfection can result in inflammation of synovial membranes, resulting in exudates in the joints (arthritis),tendons sheaths and sternal bursae of infected birds (Fig. 1) 1 . Affected birds are usually immature andare lame or recumbent with swollen hocks, footpads or sterna bursae. Infected layer flocks can havedecreased egg production. There is a recent report of pneumonia in turkey breeder hens infected withMS. 2Figure 1. <strong>Mycoplasma</strong> synoviae infection can cause arthritis and synovitis, particularly in immature birds.Note the enlarged hock joint in the turkey leg at top of the photograph. Normal turkey leg is at the bottom.Three main approaches are used for the diagnosis of avian mycoplasmosis: culture of themicroorganism, detection of serum antibodies, and molecular detection of the organism’s nucleic acidby real-time polymerase chain reaction (PCR). Culture is the gold standard; however, MS is generallydifficult to culture because of critical growth requirements, culture overgrowth by other nonpathogenic<strong>Mycoplasma</strong>, and a transient nature of MS infection, which can be replaced by secondary bacterialinfections in affected birds. Additionally, <strong>Mycoplasma</strong> can take up to three weeks to grow in culturemedia. Serological tests have long been the basis for surveillance of MS infection in breeder flocks(National Poultry Improvement Plan), but early infections can go undetected and screening tests(serum plate agglutination) have variable results in infected turkeys. PCR has gained a pivotal role inthe diagnosis of avian mycoplasma infection because the test has high specificity and sensitivity, shortturnaround time, ease of performance, and is relatively inexpensive. 3SYS.WEBDOC.020 Rev 1 6/29/2012
The Minnesota <strong>Veterinary</strong> <strong>Diagnostic</strong> <strong>Laboratory</strong> has standardized and validated the use of a recentlypublished real-time TaqMan® PCR assay 3 for detection of <strong>Mycoplasma</strong> synoviae in clinical samples.This test is currently available for routine testing of respiratory and joint samples of avian origin.Test validationSpecificity: The analytical specificity of the real-time TaqMan® PCR assay was evaluated by testingDNA extracted from 10 field strains of M. synoviae and 17 additional avian <strong>Mycoplasma</strong> species, mostof which are nonpathogenic. Tracheal swabs from specific mycoplasma-free chickens and turkeys alsotested negative. 3Sensitivity: The real-time TaqMan® PCR detected 2 x 10 3 copies of M. synoviae per reaction. Testingwas performed by doing serial 10-fold dilutions of a pure culture (ATTC strain 25204) in PBS and inSP4 media4 (specific <strong>Mycoplasma</strong> culture broth). SP4 media is present in swabs frequently used byfield veterinarians to collect and submit samples for testing. Table 1 shows the effect of pooling, media,and storage time under refrigeration in the analytical sensitivity of the new M. synoviae real-timeTaqMan® test. Three concentrations of M. synoviae were tested (10 3 , 10 5 , 10 7 ), along with threedifferent diluents (phosphate-buffered saline [PBS] and SP4 broth) and two time points (2, 6, and 7days). All samples were maintained at 4°C until testing.Results from this experiment demonstrated that there was no significant effect of the diluents, storagetime, or pooling of up to 5 swabs in the sensitivity of the test. As expected, samples containing a higherinitial concentration (2 x 10 7 copies/ reaction) were detected earlier in the real-time reaction (lower CTvalue) compared with samples with a lower initial concentration (2 x 10 5 copies/ reaction). Samplescontaining the lowest initial concentration (2 x 10 3 copies/ reaction) were all negative in this experiment.ConclusionsThe new M. synoviae real-time TaqMan® PCR test has a detection limit of 2 x 10 3 copies/ reactionunder laboratory conditions. Pooling of up to 5 swabs in PBS or SP4 broth, or storage time of up to 7days under refrigeration does not affect the sensitivity of the test when samples contain an initialconcentration between 10 5 to 10 7 copies/ reaction.RecommendationsSamples: Appropriate swabs to use are synthetic (Dacron or Rayon) on plastic shafts (bacterialculturettes). The type of media can vary (most commonly used are liquid Stuarts and Hank's media),but be careful to avoid any agar gel-type media. One inexpensive, multi-purpose swab is BBLCultureSwab Liquid Stuart, Single Swab (pkg of 50), Product Number 220099. 5 should be applied totrachea, sinus/nasal cavity and swollen joints or tendon sheaths (hock tendon or foot pads). Theculturettes should be labeled according to anatomic site to facilitate pooling.Sample submission: Ideally, samples should be submitted immediately after collection, usingovernight delivery. Samples can be maintained under refrigeration until shipping, but should be testedwithin 7 days of collection.Pooling: Pooling of up to 5 swabs is acceptable and does not affect the sensitivity of the test. Swabscollected from the same anatomic site can be pooled at the VDL prior to testing. Swabs that arepooled in the field should be collected into 2ml of PBS or SP4 broth.SYS.WEBDOC.020 Rev 1 6/29/2012
Frequently Asked Questions:1. Is PCR “better” than culture?Answer: PCR can detect a lower quantity of <strong>Mycoplasma</strong> than current culture methods and PCRshould be able to detect MS that is no longer alive or that is present, but overgrown by othernonpathogenic <strong>Mycoplasma</strong>s. Culture of MS can be difficult because of critical growth requirementsand overgrowth by nonpathogenic <strong>Mycoplasma</strong>s. Nevertheless, culture of MS remains the goldstandard for diagnosis.2. Can I pool swab specimens in the field?Answer: Yes, but for best results it is recommended that swabs be maintained in individualculturettes, labeled as to site of collection and shipped to the VDL overnight. The swabs will be pooledat VDL prior to testing. Swabs pooled in the field should be collected into 2 ml phosphate-bufferedsaline or SP4 broth in unbreakable tubes. Swabs collected from similar anatomic sites should bepooled together; do not mix sample sites. Tubes should be labeled as to anatomic site of collection,placed on ice packs or refrigerated prior to shipping.3. It is the end of the week. Can I still collect swabs for testing even if samples will not be sent untilthe next week?Answer: It is recommended that swabs be packed and shipped to the VDL immediately aftercollection; however, validation studies at VDL indicate that the nucleic acid of MS will be stable anddetectable for up to seven days after collection.4. What samples should I collect?Answer: This depends on the flock situation. If you are performing surveillance and the birds are notshowing clinical signs, the trachea, nasal cavity or infraorbital sinus (dead bird) should be swabbed. Ifthe birds are showing signs of lameness (e.g., reluctance to move, swollen hock joints or footpads,breast blisters) the hock joint, gastrocnemius tendon (hock tendon) sheath, incised footpad andenlarged sterna bursa should be swabbed.5. I want to identify MS by both PCR and culture. Can I do this with one sample?Answer: Yes, if you submit the correct sample. Commercial bacterial culturettes (good) or SP4 broth(best) can stabilize <strong>Mycoplasma</strong> and reduce secondary contamination of the sample. The bestmethod for isolation of <strong>Mycoplasma</strong> is to immediately collect the sample into SP4 broth. Do not collectswabs in PBS if you want samples to be cultured for <strong>Mycoplasma</strong>. All of these samples, culturette,SP4 and PBS, will be sufficient for PCR.6. Does the PCR detect other pathogenic <strong>Mycoplasma</strong>s besides MS?No, the PCR test is specific for MS; however, the VDL offers separate real-time PCR tests for<strong>Mycoplasma</strong> gallisepticum (MG), <strong>Mycoplasma</strong> meleagridis (MM) and <strong>Mycoplasma</strong> iowae (MI). Swabsto be tested for MG, MM and MI will not be pooled because this method has not been validated at theVDLReferences:1. Kang MS, Gadzinski P and Kleven SH. Virulence of recent isolates of <strong>Mycoplasma</strong> synoviae inturkeys. Avian Dis. 46:102-110, 2002.2. Osorio C, Fletcher K, Abdul-Aziz T, et al. Penumonia of turkey breeder hens associated with<strong>Mycoplasma</strong> synoviae. Avian Dis. 51:791-796, 2007.SYS.WEBDOC.020 Rev 1 6/29/2012
3. Raviv Z, Kleven SH. The development of diagnostic real-time TaqMan PCRs for the four pathogenicavian mycoplasmas. Avian Dis. 53(1):103-7, 2009.4. Hardy <strong>Diagnostic</strong>s, 1430 West McCoy Lane, Santa Maria, CA 93455; Hardydiagnostics.com5. BD <strong>Diagnostic</strong> Systems, 7 Loveton Cir, Sparks, MD 21152. Customer Service: (800) 675-0908.Tech: (800) 638-8663SYS.WEBDOC.020 Rev 1 6/29/2012