Personalised Cancer TherapyWhat is KRAS and What Does it Mean for PatientsWith Metastatic Colorectal Cancer?Liesbeth Lemmens, BSc, MSc, Coordinator Clinical Trials, Digestive <strong>Oncology</strong> Department of Gastroenterology,University Hospitals Leuven, BelgiumIntroductionEGFR as a Therapeutic Target in Colorectal CancerRecent advances in understanding <strong>the</strong> molecular basis of cancer The EGFR pathway plays a critical role in tumour growth andhave revolutionised medical oncology. Scientists have identified progression (7, 8). The EGFR-mediated signalling activates multiplefunctionally important proteins that are involved in regulating <strong>the</strong> pathways that result in cell proliferation and survival (Figure 1). Thegrowth, survival, and metastatic properties of tumour cells. These abnormal activation of EGFR is implicated in many types of cancers,proteins have served as targets for <strong>the</strong> rational design and discovery including 75% to 90% of CRC, and seems to reflect a more aggressiveof novel treatments, referred to as “targeted <strong>the</strong>rapies” (1, 2). pathology and clinical behaviour, such as more tumour angiogenesis,Although many of <strong>the</strong>se novel targeted agents have been shown to proliferation, metastasis, and survival (9-11). As a result, EGFR hasimprove outcomes in clinical trials, it is clear that not all patients been identified as a logical target in cancer treatment, and <strong>the</strong>rapiesbenefit from <strong>the</strong> <strong>the</strong>rapies. The current research challenge, <strong>the</strong>refore, have been developed to inhibit this signalling pathway.is to identify indicators that can predict response to treatment (3).The activity of EGFR can be inhibited by ei<strong>the</strong>r small molecule“Biomarker” is defined as “a characteristic that is objectivelyinhibitors or by monoclonal antibodies. Small molecule inhibitors,measured and evaluated as an indicator of normal biologicalsuch as erlotinib (Tarceva ® ), selectively inhibit <strong>the</strong> enzyme (tyrosineprocesses, pathogenic processes, or pharmacologic responses to kinase) activity of EGFR inside <strong>the</strong> cell; thus, <strong>the</strong>y are referred to asa <strong>the</strong>rapeutic intervention (4).” Whereas prognostic biomarkers tyrosine kinase inhibitors or TKIs (12). No TKI has been approvedindicate clinical outcomes independent of treatment, predictive for use in CRC to date. Monoclonal antibodies, such as cetuximabbiomarkers determine <strong>the</strong> response of a tumour to a specific(Erbitux ® ) and panitumumab (Vectibix ® ), target EGFR outside <strong>the</strong><strong>the</strong>rapy. Consequently, predictive biomarkers are used to identify cell by blocking ligand binding and subsequent activation of EGFR<strong>the</strong> treatment option that will result in <strong>the</strong> best patient outcomes. By signalling (8). In randomised clinical trials, both of <strong>the</strong>se anti-EGFRalso identifying patients who are not likely to benefit from <strong>the</strong>rapy, antibodies have been shown to improve patient outcomes andpredictive biomarkers will prevent exposure to and toxicity from provide alternate treatment options for patients with metastatic CRCineffective treatments while preventing treatment delays with o<strong>the</strong>r (mCRC) (13-19).potentially effective regimens and reducing healthcare costs.Despite promising results in clinical trials, not all patients respondThis article explains how personalised medicine may become a reality to cetuximab and panitumumab; in clinical trials, mono<strong>the</strong>rapy withfor patients who have colorectal cancer (CRC) by allowing healthcare <strong>the</strong>se agents yielded response rates of approximately 10% andproviders to select appropriate treatments for patients on <strong>the</strong> basis disease stabilisation rates of approximately 30% (13-15, 20). Theseof <strong>the</strong>ir specific genetic profile. Scientists have recently identified a results led to a search for a biomarker that would help identifydifferential response to antibodies that target <strong>the</strong> epidermal growth patients who were likely to respond to anti-EGFR <strong>the</strong>rapy.factor receptor (EGFR) based on <strong>the</strong> presence or absence of amutation of a specific gene called Kirsten RAS or KRAS. KRAS is a Biomarkers for Anti-EGFR Therapypart of <strong>the</strong> rat sarcoma oncogene virus (ras) family, which encodes EGFR overexpression as a biomarker<strong>the</strong> KRAS protein (5, 6). The biology of EGFR and KRAS, <strong>the</strong> effect of Preclinical data had suggested that sensitivity to anti-EGFR agents<strong>the</strong> KRAS mutation, and <strong>the</strong> studies that have led to <strong>the</strong> identification was linked to levels of expression of EGFR, and patients participatingof KRAS as a clinically relevant biomarker will be discussed.in <strong>the</strong> initial clinical trials were required to have detectable EGFRprotein expression as determined by immunohistochemistry (IHC)Figure 1: Relationship between EGFR pathway and KRAS in colorectal testing. However, objective responses were seen in patientscancerregardless of EGFR expression status, and <strong>the</strong> use of EGFR IHC asa predictive marker of response has been questioned despite <strong>the</strong>product labels’ requirement for testing (21-23).Several studies are also evaluating whe<strong>the</strong>r <strong>the</strong> presence of a largenumber of copies of <strong>the</strong> EGFR gene in <strong>the</strong> tumour cells (comparedto 2 copies in normal cells) are predictive of response; to date <strong>the</strong>sestudies have yielded conflicting results (22, 24-27). Thus, levels ofEGFR protein or gene in tumour cells are not considered predictivefor response based on currently available measurement methods.28 - newsletter fall 2008
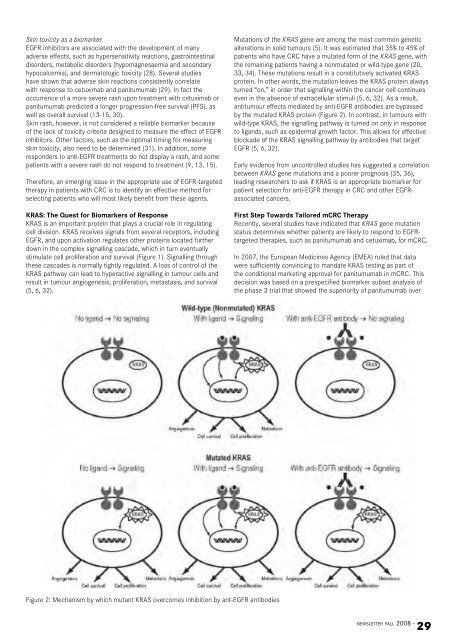
Skin toxicity as a biomarkerEGFR inhibitors are associated with <strong>the</strong> development of manyadverse effects, such as hypersensitivity reactions, gastrointestinaldisorders, metabolic disorders (hypomagnesaemia and secondaryhypocalcemia), and dermatologic toxicity (28). Several studieshave shown that adverse skin reactions consistently correlatewith response to cetuximab and panitumumab (29). In fact <strong>the</strong>occurrence of a more severe rash upon treatment with cetuximab orpanitumumab predicted a longer progression-free survival (PFS), aswell as overall survival (13-15, 30).Skin rash, however, is not considered a reliable biomarker becauseof <strong>the</strong> lack of toxicity criteria designed to measure <strong>the</strong> effect of EGFRinhibitors. O<strong>the</strong>r factors, such as <strong>the</strong> optimal timing for measuringskin toxicity, also need to be determined (31). In addition, someresponders to anti-EGFR treatments do not display a rash, and somepatients with a severe rash do not respond to treatment (9, 13, 15).Therefore, an emerging issue in <strong>the</strong> appropriate use of EGFR-targeted<strong>the</strong>rapy in patients with CRC is to identify an effective method forselecting patients who will most likely benefit from <strong>the</strong>se agents.KRAS: The Quest for Biomarkers of ResponseKRAS is an important protein that plays a crucial role in regulatingcell division. KRAS receives signals from several receptors, includingEGFR, and upon activation regulates o<strong>the</strong>r proteins located fur<strong>the</strong>rdown in <strong>the</strong> complex signalling cascade, which in turn eventuallystimulate cell proliferation and survival (Figure 1). Signalling through<strong>the</strong>se cascades is normally tightly regulated. A loss of control of <strong>the</strong>KRAS pathway can lead to hyperactive signalling in tumour cells andresult in tumour angiogenesis, proliferation, metastasis, and survival(5, 6, 32).Mutations of <strong>the</strong> KRAS gene are among <strong>the</strong> most common geneticalterations in solid tumours (5). It was estimated that 35% to 45% ofpatients who have CRC have a mutated form of <strong>the</strong> KRAS gene, with<strong>the</strong> remaining patients having a nonmutated or wild-type gene (20,33, 34). These mutations result in a constitutively activated KRASprotein. In o<strong>the</strong>r words, <strong>the</strong> mutation leaves <strong>the</strong> KRAS protein alwaysturned “on,” in order that signalling within <strong>the</strong> cancer cell continueseven in <strong>the</strong> absence of extracellular stimuli (5, 6, 32). As a result,antitumour effects mediated by anti-EGFR antibodies are bypassedby <strong>the</strong> mutated KRAS protein (Figure 2). In contrast, in tumours withwild-type KRAS, <strong>the</strong> signalling pathway is turned on only in responseto ligands, such as epidermal growth factor. This allows for effectiveblockade of <strong>the</strong> KRAS signalling pathway by antibodies that targetEGFR (5, 6, 32).Early evidence from uncontrolled studies has suggested a correlationbetween KRAS gene mutations and a poorer prognosis (35, 36),leading researchers to ask if KRAS is an appropriate biomarker forpatient selection for anti-EGFR <strong>the</strong>rapy in CRC and o<strong>the</strong>r EGFRassociatedcancers.First Step Towards Tailored mCRC TherapyRecently, several studies have indicated that KRAS gene mutationstatus determines whe<strong>the</strong>r patients are likely to respond to EGFRtargeted<strong>the</strong>rapies, such as panitumumab and cetuximab, for mCRC.In 2007, <strong>the</strong> <strong>European</strong> Medicines Agency (EMEA) ruled that datawere sufficiently convincing to mandate KRAS testing as part of<strong>the</strong> conditional marketing approval for panitumumab in mCRC. Thisdecision was based on a prespecified biomarker subset analysis of<strong>the</strong> phase 3 trial that showed <strong>the</strong> superiority of panitumumab overFigure 2: Mechanism by which mutant KRAS overcomes inhibition by ant-EGFR antibodiesnewsletter fall 2008 -29