Calmet® Brochure - Tessenderlo Kerley
Calmet® Brochure - Tessenderlo Kerley
Calmet® Brochure - Tessenderlo Kerley
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
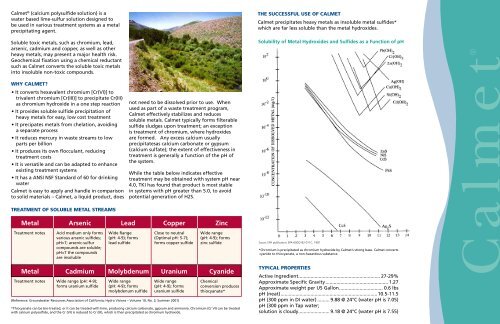
Calmet®Calmet ® (calcium polysulfide solution) is awater based lime-sulfur solution designed tobe used in various treatment systems as a metalprecipitating agent.Soluble toxic metals, such as chromium, lead,arsenic, cadmium and copper, as well as otherheavy metals, may present a major health risk.Geochemical fixation using a chemical reductantsuch as Calmet converts the soluble toxic metalsinto insoluble non-toxic compounds.Why Calmet?• It converts hexavalent chromium [Cr(VI)] totrivalent chromium [Cr(III)] to precipitate Cr(III)as chromium hydroxide in a one step reaction• It provides soluble sulfide precipitation ofheavy metals for easy, low cost treatment• It precipates metals from chelation, avoidinga separate process• It reduces mercury in waste streams to lowparts per billion• It produces its own flocculant, reducingtreatment costs• It is versatile and can be adapted to enhanceexisting treatment systems• It has a ANSI NSF Standard of 60 for drinkingwaterCalmet is easy to apply and handle in comparisonto solid materials – Calmet, a liquid product, doesTREATMENT OF SOLUBLE METAL STREAMSnot need to be dissolved prior to use. Whenused as part of a waste treatment program,Calmet effectively stabilizes and reducessoluble metals. Calmet typically forms filterablesulfide sludges upon treatment; an exceptionis treatment of chromium, where hydroxidesare formed. Any excess calcium usuallyprecipitatesas calcium carbonate or gypsum(calcium sulfate); the extent of effectiveness intreatment is generally a function of the pH ofthe system.While the table below indicates effectivetreatment may be obtained with system pH near4.0, TKI has found that product is most stablein systems with pH greater than 5.0, to avoidpotential generation of H2S.Metal Arsenic Lead Copper ZincTreatment notesMetal Cadmium Molybdenum Uranium CyanideTreatment notesAcid medium only formsvarious arsenic sulfides;pH>7; arsenic-sulfurcompounds are soluble;pH