Final Review Semester 2 - yourhomework.com Home Page
Final Review Semester 2 - yourhomework.com Home Page
Final Review Semester 2 - yourhomework.com Home Page
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Chemistry HP<strong>Final</strong> <strong>Review</strong>(1) Write the nuclide symbol for each isotope. State the number of protons, electrons, and neutrons for each isotope.(a) Tellurium–120 (b) Lanthanum–139 (c) Vanadium–50(2) For each of the following statements, state which type(s) or radiation they describe.(a) has the highest penetrating power(e) can be stopped by aluminum foil(b) has the same structure as an electron(f) can result in a transmutation(c) has the same structure as a helium nucleus(g) is energy released from an excited atom(d) can be stopped by a piece of paper(h) is a type of particle(3) Complete each of the following nuclear reactions.66 66209 209(a) Cu → Zn + ____ (c) Po*→ Po + ____29 30 84 84(b) 18579181235Au → Ir + ____ (d)77 92 U + 1 0 n → 9436 Kr + ____ + 3 1 0 n(e) 3917 Cl → 0100e + ____ (f)141 Nb → 4 He + ____2(4) The half–life of radium–224 is 3.660 days.(a) What mass of a 10.0 g sample will remain after 7.32 days?(b) How long will it take for a 60.00 g sample to decay to 3.750 g?(5) The half–life of radium–226 is 1599 years.(a) What mass of a 15.00 g sample will remain after 6396 years?(b) How long will it take for an 80.00 g sample to decay to 1.250 g?(6) Sulphur–35 has a half–life of 87.10 days.(a) What mass of a 64 g sample will remain after 348.4 days?(b) How long will it take for a 1024 g sample to decay to 4.000 g?(c) How long will it take for a sample to decay to 12.50% of the original amount?(d) After 522.6 days, there are 2.00 g of a sample remaining. What was the mass of the original sample?(7) A sample of helium has a volume of 400 mL at 1.20 atm. What will the volume be at 1.50 atm?(8) A balloon has a volume of 8.00 L at 47 °C. At what temperature will the balloon have a volume of 7.60 L?(9) A gas cylinder has a pressure reading of 7.0x10 4 Pa at 350 K. What will the pressure read at 400 K?
(10) A sample of gas has a volume of 500 mL at 3.00 atm and 200 K. What will the pressure be if the sample is expanded to 600mL at 300 K?(11) How many moles of carbon dioxide are found in 5.6 L at STP? What is the mass of the carbon dioxide?(12) How many moles of helium occupy 400 mL at 3.4 atm and 60 ºC? What is the mass of helium?(13) Methane (CH 4 ) gas reacts with oxygen to form carbon dioxide gas and water vapour.(a) Write a balanced chemical equation for this reaction.(b) If 448 mL of methane are present at STP, what mass and volume of oxygen are required in the reaction?(c) What is the volume and mass of each of the products?(14) Determine the concentration of a solution containing 0.500 mol hydrochloric acid in 800 mL.(15) What is the volume if a 0.15 M solution contains 0.24 mol of sodium chloride?(16) Determine the final concentration if 40 mL of water are added to 60 mL of 0.25 M silver nitrate solution.((17) Write a dissociation equation and determine the concentration of each ion in the solution.(a) 0.036 M Na 2 SO 4 (b) 0.40 M AlCl 3(18) Determine if the following <strong>com</strong>pounds are soluble or insoluble in water.(a) AgI (b) SrS (c) Ca(OH) 2 (e) Na 2 SO 3(19) Write the formula equation, <strong>com</strong>plete ionic equation, and net ionic equation for the reaction between NaI and Pb(NO 3 ) 2 .
(20) 150 mL of 0.200 M strontium chloride solution are reacted with 200 mL of silver nitrate solution.(a) Write a balanced chemical equation for this reaction.(b) What is the concentration of the silver nitrate solution?(c) What is the mass of each of the products?(21) List three properties of acids and three properties of bases.(22) Complete the following table.[H + ] pH pOH [OH – ] acidic or basic?3.153.2x10 –4 M2.8x10 –6 M5.05(23) Write a balanced equation for each of the following reactions. Classify the reactions.(a) nitric acid + barium hydroxide →(b) sulphuric acid + potassium hydroxide.(24) Calculate the amount of energy released when 15.0 g of acetic acid freezes.(25) Calculate the mass of ethanol that can be boiled with 2.93x10 3 J of energy.(26) Calculate the heat energy required to increase the temperature of 100 g of air from 20.0 C to 80.0 C.(27) Calculate the final temperature if 65 J of heat energy is added to 25 g of lead at 45 C.(28) How much energy is required to turn 22 g of ice at –12 C into steam at 114 C? How many kJ is this?
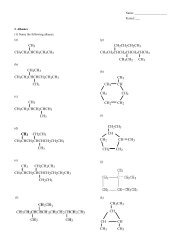
(29) Classify each reaction as endothermic or exothermic and determine ∆H.____ (a) CO (g) + SiO 2 (s) + 590.2 kJ/mol → SiO (g) + CO 2 (g); ∆H = ________ kJ/mol____ (b) 2ZnS (s) + 3O 2 (g) → 2ZnO (s) + 2SO 2 (g) + 878.3 kJ/mol; ∆H = ________ kJ/mol(30) (a) Use the heats of formation to calculate the heat of the following reaction:C 3 H 8 (g) + 5O 2 (g) → 3CO 2 (g) + 4H 2 O (g)(b) How much heat would be released by the <strong>com</strong>bustion of 10 mol of propane (C 3 H 8 )?(31) Name the following <strong>com</strong>pounds.(a) (b) (c)CH 2CH 2CH 2CHCH 2 CH 3CHCH 3(d)(32) Draw the following <strong>com</strong>pounds(a) 2,3–Dimethyloctane(c) 3–methyl–2–heptene(b) 3,4–Diethylcyclohexene(d) 5–Ethyl–2–nonyne(33) Classify each of the <strong>com</strong>pounds and match them with the correct name.________________________ (a) ________________________ (e)(1) propanoic acid#____________________________ (b)#____________________________ (c)#____________________________ (d)#____________________________ (f)#____________________________ (g)#____________________________ (h)(2) 3–chlorobutanal(3) 1–aminopropane(4) Ethyl propyl ether(5) 4–Methyl–2–pentanone(6) Methyl ethanoate(7) 4–Methyl–2–pentanol(8) 4–Ethylhexanamide#____#____
(34) Use SSR to explain what would happen to [C 5 H 11 N] for each of the following stresses.C 5 H 5 N (g) + 3H 2 (g) + 1.4 kJ/mol C 5 H 11 N (g)(a) increase pressure (b) decrease temperature (c) increase volume (d) increase [H 2 ](35) Write a Keq expression for each of the following equilibria.(a) 2CO (g) + O 2 (g) 2CO 2 (g)(b) 4HCl (g) + O 2 (g) 2H 2 O (l) + 2Cl 2 (g)(36) Write a Keq expression for each of the following equilbria. Determine the value of Keq. Does the equilibrium favour theproducts or the reactants?(a) 2H 2 O (l) + 2F 2 (g) 4HF (g) + O 2 (g)(b) 2CH 4 (g) C 2 H 2 (g) + 3H 2 (g)At equilibrium,At equilibrium, a 10.0 L container holds[F 2 ] = 0.160 M, [HF] = 1.20 M, and [O 2 ] = 0.200 M 2.00 mol CH 4 , 0.400 mol C 2 H 2 , and 6.00 mol H 2
Answers:120(1) (a)5213950Te 52 p, 52 e, 68 n (b) La 57 p, 57 e, 82 n (c)57 23(2) (a) gamma (b) beta (c) alpha (d) alpha(e) beta (f) alpha/beta (g) gamma (h) alpha/beta(3) (a) 01e (b) 4 2He (c) 0 0γ (d) 13956Ba (e) 3918Ar (f) 96 Y39(4) (a) 2.50 g (b) 14.64 days (5) (a) 0.9375 g (b) 9594 years(6) (a) 4.0 g (b) 696.8 days (c) 261.3 days (d) 128 gV 23 p, 23 e, 27 n(7) 320 mL (8) 304 K (9) 8.0x10 4 Pa (10) 3.75 atm (11) 0.25 mol and 11 g (12) 0.050 mol and 0.20 g(13) (a) CH 4 + 2O 2 → CO 2 + 2H 2 O (b) 0.896 L and 1.28 g O 2 (c) 0.448 L and 0.882 g CO 2 , 0.896 L and 0.721 g H 2 O(14) 0.625 M (15) 1.6 L (16) 0.15 M(17) (a) Na 2 SO 4 → 2Na + + SO 4 2– , [Na + ] = 0.072 M, [SO 4 2– ] = 0.036 M(b) AlCl 3 → Al 3+ + 3Cl – , [Al 3+ ] = 0.40 M, [Cl – ] = 1.2M(18) (a) insoluble (b) soluble (c) insoluble (d) soluble(19) formula equation: 2NaI (aq) + Pb(NO 3 ) 2 (aq) → 2NaNO 3 (aq) + PbI 2 (s)<strong>com</strong>plete ionic equation: 2Na + (aq) + 2I – (aq) + Pb 2+ (aq) + 2NO 3 – (aq) → 2Na + (aq) + 2NO 3 – (aq) + PbI 2 (s)net ionic equation: Pb 2+ (aq) + 2I – (aq) →PbI 2 (s)(20) (a) (a) SrCl 2 + 2AgNO 3 → Sr(NO 3 ) 2 + 2AgCl (b) [AgNO 3 ] = 0.300 M (c) 6.35 g Sr(NO 3 ) 2 and 8.60 g AgCl(21)Aciddissociate to give H + ionspH7.0taste bitterfeel slipperypH paper turns blue/greenphenolphthalein → pinkbromothymol blue → bluecabbage juice → blue(22)[H + ] pH pOH [OH – ]acidic, basic,or neutral?1.4x10 –11 M 10.85 3.15 7.1x10 –4 M basic3.2x10 –4 M 3.49 10.51 3.1x10 –11 M acidic3.5x10 –9 M 8.45 5.55 2.8x10 –6 M basic8.9x10 –6 M 5.05 8.95 1.1x10 –9 M acidic(23) neutralization (a) 2HNO 3 + Ba(OH) 2 → Ba(NO 3 ) 2 + 2H 2 O (b) H 2 SO 4 + 2KOH → K 2 SO 4 + 2H 2 O(24) 2.88x10 3 J (25) 5.00 g (26) 5.97x10 3 J (27) 65 C (28) 6.7x10 4 J, 67 kJ(29) (a) endo; ΔH= +590.2 kJ/mol (b) exo, ΔH=–878.3 kJ/mol (30) (a) –2043.8 kJ/mol (b) –20438 kJ
(31) (a) 3,5–Diethylheptane (b) 4–Methyl–2–pentene (c) 1–Ethyl–2–methylcyclopentane (d) 4–Ethyl–5–methyl–2–hexyne(32)(a) (b) (c) (d)(33) (a) #7, alcohol (b) #3, amine (c) #1, carboxylic acid (d) #5, ketone(e) #2, aldehyde and alkyl halide (f) #8, amide (g) #4, ether (h) #6, ester(34)(a) S: increase pressure S: right: R: increase [C 5 H 11 N] (b) S: decrease temperature S: left R: decrease [C 5 H 11 N](c) S: increase volume S: left: R: decrease [C 5 H 11 N] (d) S: increase [H 2 ] S: right R: increase [C 5 H 11 N]2[ CO2](35) (a) Keq 2[ CO] [ O ](b)2[ Cl ]Keq [ HCl] [ ]224O2(36) (a)4[ HF] [ O2]2[ F2]Keq , Keq = 16.2, products (b)3[ CH][ H]Keq Keq= 0.216, reactants2 2 22[ CH4]
Chemistry <strong>Final</strong> Exam Practice Multiple Choice(1) What is the nuclide symbol for Palladium–148?(a) 14846 Pd (b) 46102148Pd (c) Pd (d)148 46 102 Pd(2) Give the nuclide symbol that would <strong>com</strong>plete the given reaction: 21483 Bi → 01e + ____(a) 21482Pb (b)21482214213Bi (c) Po (d)84 84 Po(3) Give the nuclide symbol that would <strong>com</strong>plete the given reaction: 23592 U → 4 He + ____2(a) 23190Th (b)23590239235Th (c) Pu (d)94 94 Pu(4) The half–life of Thorium–234 is 24 days. What mass would remain of a 200 g sample after 120 days?(a) 12.5 g (b) 5.0 g (c) 6.25 g (d) 3.125 g(5) The half–life of Silver–102 is 70 minutes. How long would it take for a 64 g sample to decay to 4.0 g?(a) 70 minutes (b) 350 minutes (c) 210 minutes (d) 280 minutes(6) Which of the following is equivalent to 836 mm Hg?(a) 1.19 atm (b) 0.909 atm (c) 1.10 atm (d) 1.97 atm(7) A sample of nitrogen gas occupies a volume of 720 mL at a pressure of 1.40 atm. What volume will the gas occupy at apressure of 2.10 atm?(a) 480 mL (b) 1.08x10 3 mL (c) 245 mL (d) 2.12x10 3 mL(8) A sample of oxygen has a volume of 0.59 L at a temperature of 22 ºC. What will be the volume of the gas at a temperature of52 ºC?(a) 1.4 L (b) 0.54 L (c) 0.65 L (d) 0.25 L(9) A gas cylinder has a pressure of 3.00 atm at 400 K. At what temperature will the gas reach a pressure of 1.50 atm?(a) 800 K (b) 200 K (c) 100 K (d) 600 K(10) A sample of hydrogen gas has a volume of 60.0 mL at a pressure of 0.500 atm and a temperature of 200 K. At whattemperature will the volume be 80.0 mL at a pressure of 0.750 atm?(a) 150 K (b) 200 K (c) 300 K (d) 400 K(11) Which of the following states the conditions of standard temperature and pressure (STP)?(a) 0 K and 1.00 atm(c) 273 ºC and 1.00 atm(b) 273 K and 0 atm(d) 0 ºC and 1.00 atm(12) Which of the following is the volume of a sample containing 0.500 mol of butane gas at STP?(a) 11.2 L (b) 44.8 L (c) 22.4 L (d) 89.6 L
(13) How many molecules of helium gas are contained in 28 L at STP?(a) 4.2x10 24 molecules(c) 3.0x10 24 molecules(b) 4.8x10 23 molecules(d) 7.5x10 23 molecules(14) What is the volume if 0.430 mol of argon gas have a pressure of 1.50x10 4 Pa at 300 K?(a) 19.1 L (b) 9.63 L (c) 71.5 L (d) 21.5 L(15) What is the mass if a 500 mL sample of chlorine gas has a pressure of 2.00 atm at 15 ºC?(a) 6.00 g (b) 1.50 g (c) 3.00 g (d) 0.750 g(16) Nitrogen monoxide reacts with oxygen to produce nitrogen dioxide according to the following balanced chemical equation:2NO + O 2 → 2NO 2 . What volume of oxygen, at STP, would be required to produce 56.0 L of nitrogen dioxide?(a) 56.0 L (b) 28.0 L (c) 112 L (d) 224 L(17) Which of the following is the volume of a 0.40 M solution containing 0.800 mol of potassium nitrate?(a) 0.32 L (b) 2.0 L (c) 0.50 L (d) 1.0 L(18) Which of the following is the concentration of a solution containing 8.3 g of KI dissolved in 0.20 L?(a) 0.25 M (b) 0.33 M (c) 0.80 M (d) 1.1 M(19) What would be the final concentration if 60 mL of water are added to 40 mL of 18.0 M sulphuric acid solution?(a) 12 M (b) 27 M (c) 7.2 M (d) 5.4 M(20) Which of the following gives the concentration of each ion in a 0.20 M solution of aluminum chloride?(a) [Al 3+ ] = 0.20 M and [Cl – ] = 0.20 M (c) [Al 3+ ] = 0.20 M and [Cl – ] = 0.60 M(b) [Al 3+ ] = 0.60 M and [Cl – ] = 0.20 M (d) [Al 3+ ] = 0.050 M and [Cl – ] = 0. 15 M(21) Which of the following is INSOLUBLE in water?(a) Li 2 CO 3 (b) (NH 4 ) 3 PO 4 (c) PbI 4 (d) CaF 2(22) Which of the following gives the net ionic equation for the reaction of sodium carbonate with calcium nitrate?(a) Ca 2+ (aq) + CO 2– 3 (aq) → CaCO 3 (s) (c) Na + (aq) + NO – 3 (aq) → NaNO 3 (s)(b) CaCO 3 (s) → Ca 2+ (aq) + CO 2– 3 (aq) (d) NaNO 3 (s) → Na + (aq) + NO – 3 (aq)(23) A solution of calcium chloride is reacted with a solution of silver nitrate according to the following balanced chemicalequation: CaCl 2 + 2AgNO 3 → Ca(NO 3 ) 2 + 2AgCl. If 50 mL of 0.16 M calcium chloride reacts with 40 mL of silver nitrate, whatis the concentration of the silver nitrate solution?(a) 0.0889 M (b) 0.200 M (c) 0.400 M (d) 0.100 M(24) Which of the following is the pH of a solution with [H + ] = 5.4x10 –3 M?(a) 11.73 (b) 0.73 (c) 5.40 (d) 2.27(25) Which of the following is the pH of a 0.040 M solution of Ba(OH) 2 ?(a) 1.40 (b) 1.10 (c) 12.60 (d) 12.90
(26) What is the balanced equation for the reaction of chloric acid with potassium hydroxide?(a) HCl + KOH → KCl + H 2 O(c) HClO 2 + KOH → KClO 2 + H 2 O(b) HClO 3 + KOH → KClO 3 + H 2 O(d) HCl 2 + KOH → KCl 2 + H 2 O(27) Calculate the amount of energy required to melt 0.400 g of sulphur.(a) 46.0 J (b) 21.4 J (c) 178 J (d) 560 J(28) Calculate the amount of energy required to increase the temperature of 5.00 g of iron from 20.0 ºC to 60.0 ºC.(a) 135 J (b) 78.0 J (c) 45.0 J (d) 90.0 J(29) The reaction for the de<strong>com</strong>position of carbonic acid is given below.H 2 CO 3 (aq) + 64.2 kJ/mol → H 2 O (g) + CO 2 (g)Which of the following statements describes the enthalpy change for the de<strong>com</strong>position of carbonic acid?(a) The reaction is exothermic and H = –64.2 kJ/mol (c) The reaction is exothermic and H = +64.2 kJ/mol(b) The reaction is endothermic and H = –64.2 kJ/mol (d) The reaction is endothermic and H = +64.2 kJ/mol(30) Use the heats of formation to calculate the heat of the following reaction:2SO 2 (g) + O 2 (g) → 2SO 3 (g)(a) –98.9 kJ/mol (b) –692.5 kJ/mol (c) –197.8 kJ/mol (d) 197.8 kJ/mol(31) Which of the following is the name of the <strong>com</strong>pound shown below?(a) 3–ethyl–4,5–dimethylhexane(b) 4–ethyl–2,3–dimethylhexane(c) 4,5–ethyl–3–methylhexane(d) 2,3–diethyl–4–methylhexane(d)(32) Which of the following is the correct structure for the <strong>com</strong>pound 1,2–dimethylcyclopentane?(a)(b) (c)CH 2 CH CH 3CH 3CH 2 CHCH 2CH 2(33) Which of the following is the name of the <strong>com</strong>pound shown below?(a) 2–methyl–3–septene(b) 2–methyl–3–septyne(c) 2–methyl–3–heptene(d) 2–methyl–3–heptyne(34) Which of the following is the correct structure for the <strong>com</strong>pound 4–methyl–2–hexyne?(a) (b) (c) (d)
(35) Which of the following is the correct name for the <strong>com</strong>pound shown below?Use the following equilibria to answer questions 36–38.I. 2N 2 O (g) + O 2 (g) + 196 kJ/mol 4NO (g)II. 2SO 2 (g) + O 2 (g) 2SO 3 (g) + 198 kJ/molIII. 2CO 2 (g) + 566 kJ/mol 2CO (g) + O 2 (g)(a) ethyl propanoate(b) ethyl ethanoate(c) propyl ethanoate(d) pentanoate(36) Which of the equilibria would shift right if temperature was increased?(a) I and III (b) I only (c) II and III (d) II only(37) Which of the equilibria would shift left if [O 2 ] was increased?(a) I only (b) III only (c) I, II and III (d) I and II(38) Which of the equilibria would shift right if volume is increased?(a) III only (b) I only (c) I and III (d) I and II(39) Which of the following gives the correct Keq expression for the equilibrium given below?CO (g) + 3H 2 (g) CH 4 (g) + H 2 O (l)(a)[ CH ][ H O]Keq [ CO][ H ]4 232(b)3[ CO][ H2]Keq [ CH ][ H O]4 2(c)[ CH4]Keq [ CO][ H ]2(d)[ CH4]Keq [ CO][ H ]23(40) Consider the following equilibrium: HCONH 2 (g) NH 3 (g) + CO (g). At equilibrium, [HCONH 2 ] = 0.0062 M, [NH 3 ] =0.20 M, and [CO] = 0.15 M. Determine Keq.(a) 1.9x10 –4 (b) 0.030 (c) 4.8 (d) 0.21Answers:(1) a(2) c(3) a(4) c(5) d(6) c(7) a(8) c(9) b(10) d(11) d(12) a(13) d(14) c(15) c(16) b(17) b(18) a(19) c(20) c(21) d(22) a(23) c(24) d(25) d(26) b(27) b(28) d(29) d(30) c(31) b(32) b(33) c(34) b(35) a(36) a(37) b(38) c(39) d(40) c