Notes: Organic Chemistry - yourhomework.com Home Page
Notes: Organic Chemistry - yourhomework.com Home Page
Notes: Organic Chemistry - yourhomework.com Home Page
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
ex. Name the following Cycloalkanes1–ethyl–2–methylcyclobutane1,2–dimethylcyclohexaneHere, there are two correct ways to number the carbonssince either way, the methyl groups are placed at carbons 1and 2. A 1 is written for methyl group from carbon 1 and a2 is written for the methyl group at carbon 2. There are twomethyl groups, so the prefix “di” is used. The main chainhas forms a “circle” with six carbon atoms, so the name ofthe main chain is cyclohexane.The main chain is numbered starting at the bottom right (since theethyl group is attached at that carbon and ethyl is the longer ofthe two alkyl groups) and then continued counter-clockwise(since the next carbon would have the methyl group). A 1 iswritten for ethyl group at carbon 1 and a 2 is written for themethyl group at carbon 2. The main chain has forms a “circle”with four carbon atoms, so the name of the main chain iscyclobutane.ex. Draw the following Cycloalkanesdrawmainchain(connectthe carbonatoms withlines)1,3–Diethyl–4–methylcyclopentane1,2,3–Trimethylcyclopropaneaddalkylgroups(on theoutside ofthe“circle”)addH’sCarbon 2 and 5: add two H’s because each carbon isattached to two other carbons.Carbon 1, 3, and 4: add one H because each carbon isattached to three other carbons (including the alkylgroup).Carbon 1, 2, and 3: add one H because each carbon is attached tothree other carbons (including the alkyl group).
(3) Alkenes and CycloalkenesContain double bonds between carbon atoms.General Formula for Alkenes: C n H 2nex. An alkene has 15 carbon atoms, how many hydrogen atoms does it have? What is the chemical formula?n = 152n = 2(15) = 30formula: C 15 H 30Alkenes are called “unsaturated” hydrocarbons since they contain fewer hydrogen atoms for each atom of carbon.(i.e. an alkane with 15 carbon atoms has 32 hydrogen atoms and an alkene with 15 carbon atoms has 30 hydrogen atoms)Naming Alkenes(1) Identify the main chain containing the double bond.(2) Number each carbon on the main chain so that the carbon number of the double bond is the lowest. The double bond takespriority over alkyl groups. For cycloalkenes, the double bond is always numbered such that it is placed between carbons 1 and 2.(3) Assign a number to each alkyl group and name the alkyl groups in alphabetical order. If there is more than one of an alkylgroup, use prefixes (i.e. di, tri, tetra, etc.) to indicate the number.(4) Name the main chain based on the number of carbons it contains and change the ending to “ene”. Indicate the position of thedouble bond with the carbon number the double bond <strong>com</strong>es after. (For cycloalkenes, the number 1 is not written in the name)ex. Name the following alkenes1–buteneThe carbons are numbered from the left because that is the endclosest to the double bond. The double bond <strong>com</strong>es aftercarbon one, so the 1 is written before the name of the mainchain. There are four carbons in the main chain containing thedouble bond so it is called butene.2–buteneThe carbons can be numbered from either the left or the rightbecause both ends are equally close to the double bond. Thedouble bond <strong>com</strong>es after carbon two, so the 2 is written beforethe name of the main chain. There are four carbons in themain chain containing the double bond so it is called butene.2–methyl–3–hexene3–methylcyclohexeneThe carbons are numbered from the left because even thougheither end is equally close to the double bond, this places thealkyl group at the lowest number. A 2 is written for methylgroup at carbon 2. The double bond <strong>com</strong>es after carbon three,so the 3 is written before the name of the main chain. Thereare six carbons in the main chain containing the double bondso it is called hexene.For cycloalkenes, the double bond is always numbered suchthat it is placed between carbons 1 and 2 and in the direction sothat the lowest number is placed on alkyl groups. This number1 is not written in the name.Here the carbons are numbered counter clockwise so that themethyl is at carbon 3. The main chain forms a “circle” withsix carbon atoms and a double bond, so the name of the mainchain is cyclohexene.
Drawing Alkenes(1) Draw the main chain and number the carbons. Add the double bond at the indicated carbon of the main chain. Forcycloalkenes, the double bond is always between carbons one and two.(2) Add the alkyl groups at the correct number on the main chain.(3) Fill in the missing hydrogen atoms (so that each carbon has four bonds). The double bond accounts for two bonds.ex. Draw the following alkenes4–Methyl–2–pentene 4–Ethyl–3,5–dimethyl–2–hexene 3–methylcyclopentenedrawmainchain(withthedoublebond)note: the double bond is always placedbetween carbons 1 and 2 in a cycloalkeneaddalkylgroupsaddH’sCarbon 1 and 5: add three H’sbecause each carbon is onlyattached to one other carbonCarbon 2 and 3: add one Hbecause each carbon is attachedto two other carbons- one by asingle bond and another by adouble bond.Carbon 4: add one H becausethis carbon is attached to threeother carbons (including thealkyl group).Carbon 1 and 6: add three H’s because eachcarbon is only attached to one other carbon.Carbon 2: add one H because this carbon isattached to two other carbons- one by a singlebond and another by a double bond.Carbon 3: does not need any H’s because thiscarbon is attached to three other carbons(including the alkyl group)- two with singlebonds and one with a double bond. Theadjacent carbon 4 is drawn closer to carbon 3.Carbon 4 and 5: add one H because each carbonis attached to three other carbons (including thealkyl group).Carbon 1 and 2: add one H because eachcarbon is attached to two other carbons- oneby a single bond and another by a doublebond.Carbon 3: add one H because each carbon isattached to three other carbons (including thealkyl group).Carbon 4 and 5: add two H’s because eachcarbon is attached to two other carbons.
BenzeneThere is an important cycloalkene called 1,3,5-cyclohexatriene with six carbon atoms in a ring with alternating single and doublebonds. This molecule is <strong>com</strong>monly known as “benzene”.Benzene can be drawn as shown:Benzene can also be shown in several simplified forms:ororor sometimes as(Each vertex represents a carbon atom withthe correct number of hydrogen atoms)Benzene is a colourless, sweet smelling liquid that is highly flammable. Benzene is used in many industrial processes includingthe production of plastics, rubbers, lubricants, dyes, detergents, drugs, explosives, and pesticides. Benzene and <strong>com</strong>pounds likebenzene (with alternating single and double bonds) are collectively called “aromatics” (historically named for the observationthat many had distinct fragrances). Aromatic <strong>com</strong>pounds are very stable. Many aromatics are highly carcinogenic and havebeen linked to various forms of cancer.Examples of Aromatic MoleculesName Drawing Name DrawingNaphthaleneChryseneAnthraceneTetracenePhenanthreneCoronenePyrenePentaceneCorannuleneOvaleneCompounds containing aromatic regions are found in many biomolecules (for example vitamins, proteins, and pharmaceuticals)Vitamin B 2(Rioflavin)Tryptophan(an amino acid)Acetylsalicylic acid(Apsirin)
(4) AlkynesContain triple bonds between carbon atoms.General Formula for Alkynes: C n H 2n–2ex. An alkyne has 15 carbon atoms, how many hydrogen atoms does it have? What is the chemical formula?n = 152n – 2 = 2(15) – 2 = 28formula: C 15 H 28Naming Alkynes(1) Identify the main chain containing the triple bond.(2) Number each carbon on the main chain so that the carbon number of the triple bond is the lowest. The triple bond takespriority over alkyl groups.(3) Assign a number to each alkyl group and name the alkyl groups in alphabetical order. If there is more than one of an alkylgroup, use prefixes (i.e. di, tri, tetra, etc) to indicate the number.(4) Name the main chain based on the number of carbons it contains and change the ending to “yne”. Indicate the position ofthe triple bond with the carbon number the triple bond <strong>com</strong>es after.ex. Name the following alkynes1–butyneThe carbons are numbered from the left because that is the endclosest to the triple bond. The triple bond <strong>com</strong>es after carbonone, so the 1 is written before the name of the main chain.There are four carbons in the main chain containing the triplebond so it is called butyne.2,5–dimethyl–3–heptyneThe carbons are numbered from the left because that is the endclosest to the triple bond. A 2 is written for methyl group fromcarbon 2 and a 5 is written for the methyl group at carbon 5.There are two methyl groups, so the prefix “di” is used. Thetriple bond <strong>com</strong>es after carbon three, so the 3 is written beforethe name of the main chain. There are seven carbons in themain chain containing the triple bond so it is called heptyne.
Drawing Alkynes(1) Draw the main chain and number the carbons. Add the triple bond at the indicated carbon of the main chain.(2) Add the alkyl groups at the correct number on the main chain.(3) Fill in the missing hydrogen atoms (so that each carbon has four bonds). The triple bond accounts for three bonds.ex. Draw the following alkynes3,5–dimethyl–1–hexynedrawmainchain(with thetriple bond)4–ethyl–2–octyneaddalkylgroupsaddH’sCarbon 1: add one H because this carbon is attached toone other carbon with a triple bond.Carbon 2: does not need any H’s because this carbon isattached to two other carbons- one with a single bond andone with a triple bond.Carbon 3 and 5: add one H because each carbon isattached to three other carbons (including the alkylgroup).Carbon 4: add two H’s because each carbon is attached totwo other carbons.Carbon 6: add three H’s because this carbon is onlyattached to one other carbon.Carbon 1 and 8: add three H’s because each carbon is onlyattached to one other carbon.Carbon 2 and 3: do not need any H’s because each carbon isattached to two other carbons- one with a single bond and one witha triple bond.Carbon 4: add one H because this carbon is attached to three othercarbons (including the alkyl group).Carbon 5, 6, and 7: add two H’s because each carbon is attached totwo other carbons.Structural IsomersStructural Isomers are different molecules having the same molecular formula, but with a different arrangement of atoms.ex. C 4 H 10 has two structural isomers:butane2–methylpropaneCH 3 CH 2 CH 2 CH 3ex. C 5 H 12 has three structural isomers:pentane2–methylbutane2,2–dimethylpropaneCH 3 CH 2 CH 2 CH 2 CH 3
Structural isomers can draw for all types of hydrocarbons (alkanes, alkenes, and alkynes) as well as other types of organic<strong>com</strong>pounds.ex. C 4 H 8 has five structural isomerscyclobutane 2-methyl-1-propene 1-butene 2-butene 1-methylcyclopropaneStereoisomersThe carbon atoms connected by a double bond in an alkene are fixed meaning that the atoms surrounding them are stationary.Different orientations of alkyl groups relative to a double bond are called stereoisomers. Stereoisomers are only displayed forALKENES.ex. Consider 2-butene:cis-2-buteneor (Z)-2-buteneHere the hydrogen atoms are on the same side of the molecule.The molecule is named with a “cis” or a “Z”Can also be drawn as shown below:trans-2-buteneor (E)-2-buteneHere the hydrogen atoms are on opposites sides of the molecule.The molecule is named with a “trans” or an “E”Can also be drawn as shown below:ex. Draw and name the two stereoisomers of 3-noneneII. Functional GroupsNaming <strong>Organic</strong> Compounds with Functional Groups(1) Identify the functional group.(2) Number each carbon on the main chain containing the functional group so that the carbon number of the functional group isthe lowest.(3) Assign a number to each alkyl group and name the alkyl groups in alphabetical order. If there is more than one of an alkylgroup, use prefixes (i.e. di, tri, tetra, etc.) to indicate the number.(4) Name the functional group with the appropriate ending.
FunctionalGroupAlkaneAlkeneAlkyneStructure Ending Example <strong>Notes</strong>: ApplicationsR-RR= any alkyl groupR=R“ane”“ene”“yne”CH 3 CH 2 CH 2 CH 2 CH 3pentaneCH 3 CH=CHCH 2 CH 2 CH 32–hexene2–butyneContain only single bonds.Contain double bonds.The position of the doublebond is indicated with anumber.Contain triple bonds. Theposition of the triple bondis indicated with a number.- used as fuels: methane(natural gas), propane(BBQ), butane (lighterfluid), octane (gasoline)- styrofoam- plastics- ethyne (also calledacetylene) is used inwelding torchesAlkylHalideAlcoholR-XX= F, Cl, Br, Ifluoro, chloro,bromo, iodoR-OHdependson mainchain“ol”5–bromo–2–chloro–4–fluoro–3–iodoheptane2–propanolHalogen groups are namedin alphabetical order.The position of the OHgroup is indicated with anumber.- Teflon (anti-stickcoating)- pollutants calledCFC’s (chloro-fluorocarbons)lead to thedepletion of the ozonelayer- ethanol is drinkingalcohol and can also beused as fuel- isopropyl alcohol isrubbing alcoholEtherAldehydeKetoneCarboxylicAcidEsterAmineAmideR-NH 2NH 2 = amino“ether”“al”“one”“oicacid”“oate”dependson mainchain“amide”CH 3 CH 2 –O–CH 2 CH 2 CH 3ethyl propyl etherpropanal2–pentanoneethanoic acidmethyl propanoate2–aminobutanebutanamideThe alkyl groups arenamed in alphabeticalorder.The double bonded oxygenis always at thebeginning/carbon one ofthe main chain so nonumber (i.e. no “1”) isused in the name.The position of the doublebonded oxygen is indicatedwith a number.The double bonded oxygenwith the OH group isalways at thebeginning/carbon one ofthe main chain so nonumber (i.e. no “1”) isused in the name.The alkyl group attached tothe single bonded oxygenis named first and the alkylgroup attached to thedouble bonded oxygen isnamed second and takesthe ending change.The position of the aminogroup is indicated with anumber.The double bonded oxygenwith the NH 2 group isalways at thebeginning/carbon one ofthe main chain so nonumber (i.e. no “1”) isused in the name.- adhesives (i.e. glue)- diethyl ether is an outof-dateanesthetic- formaldeyde is apreservative forbiological specimens- acetone (ethanone) isfound in nail polishremover and used inmany industrialprocesses- acetic acid (ethanoicacid) is found invinegar- ascorbic acid isVitamin C- citric acid is found incitrus fruits- used in artificialfragrances- many stimulants/drugsare amines: caffeine,nicotine, morphine,cocaine, etc.- used for dyes- amide bonds (i.e.peptide bonds) connectamino acids in proteins
ex. Classify the functional group(s) for each molecule. Name/Draw the organic <strong>com</strong>pound.(1)OH(9) 3-Ethyl-4-methylpentanoic acidCH 3 CHCH 2 CH 3(2)CH 3 –O–CH 2 CH 2 CH 3(10) 3-Methylheptanal(3)(11) 1-Fluoro-3-methyl-2-pentanol(4)(12) 2-Amino-1-iodo-3-methyloctane(5)(13) Dipropyl ether(6)(14) 4-Methyl-3-hexanone(7)NH 2CH 3 CHCHCH 2 CH 3(15) 2-bromopropanamideCH 3(8)(16) Propyl pentanoate
Ester Condensation Reactions (Esterification or Ester Synthesis Reactions)Esters can be produced from the reaction of a carboxylic acid with an alcohol.carboxylic acid + alcohol ester + waterGenerally, this reaction is catalyzed with the addition of a strong acid.The “OH” from the carboxylic acid and the “H” from the alcohol <strong>com</strong>bine together to make water. The remaining parts of eachmolecule join to produce an ester.ex. butanoic acid is reacted with methanol in the presence of a strong acid.+H+ 2 Obutanoic acid + methanolmethyl butanoate+ waterex. <strong>com</strong>plete the following ester synthesis reaction:+HO-CH 2 CH 2 CH 2 CH 2 CH 3+H 2 O++ water
Formation of Peptide/Amide Bonds between Amino AcidsAmino Acids:Amino acids are the building blocks of proteins.Amino acids have one end that is an amino group and the other end is a carboxylic acid group. The carbon in the middle has a“side chain” shown as an “R” group. There are twenty different amino acids with different side chains. The names andstructures of the amino acids are shown below:Amino acids join together in order to make proteins. This occurs by the formation of a peptide/amide bond. The “OH” from thecarboxylic acid and the “H” from the amine <strong>com</strong>bine together to make water. The remaining parts of each amino acid join toproduce an amide, also called a polypeptide.+ +H 2 OThe molecule continues to build until all the amino acids required for the protein have been <strong>com</strong>bined. This can vary from afew hundred to tens of thousands of amino acids depending on the protein. The structure of the protein is determined by thesequence of amino acids. Proteins have a variety of functions including as acting as enzymes, providing structure, acting assignalling molecules, providing energy, and participating in transport.
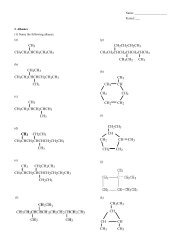
Review: Alkanes(1) Name the <strong>com</strong>pound.(a)(b)(c)CH 2 CH 3(d)CH 3 CHCH 2 CH 2 CHCH 3CH 2 CH 3(2) Draw the <strong>com</strong>pound.(a) 3,5-diethylheptane(b) 3-ethyl-2-methylnonane(c) 1,2-dimethylcyclopropane
Review: Alkanes/Alkenes/Alkynes(1) Name the <strong>com</strong>pound.(a)(b)(c)(d)(2) Draw the <strong>com</strong>pound.(a) 2-methyl-3-heptyne(b) 2-methyl-1-butene(c) 3,4-dimethylcyclopentene(d) 1,3-diethylcyclooctane
Review: <strong>Organic</strong> Compounds(1) Name the <strong>com</strong>pound.(a)(b)(c)(d)OHClCH 3 CHCH 2 CHCH 2 CH 3(e)NH 2(f)CH 3 CHCH 2 CH 3(2) Draw the <strong>com</strong>pound.(a) 3-methylhexanamide(b) 3-bromo-1-butyne(c) 3-methyl pentanoic acid(d) ethyl methyl ether(e) propyl butanoate