Lecture 10: Geochronology VI: U-Th decay series dating
Lecture 10: Geochronology VI: U-Th decay series dating
Lecture 10: Geochronology VI: U-Th decay series dating
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
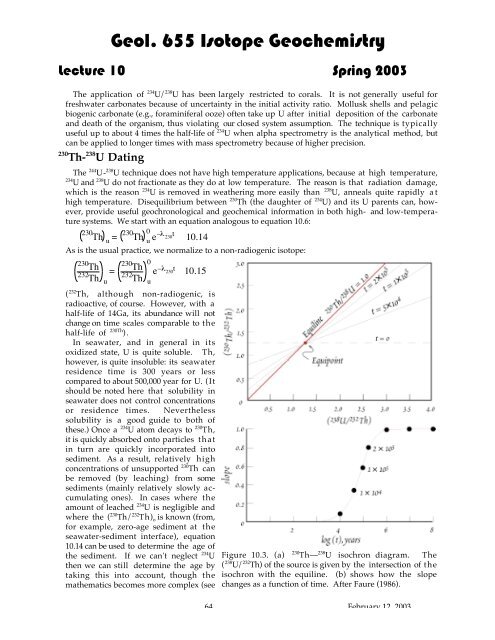
Geol. 655 Isotope Geochemistry<strong>Lecture</strong> <strong>10</strong> Spring 2003<strong>Th</strong>e application of 234 U/ 238 U has been largely restricted to corals. It is not generally useful forfreshwater carbonates because of uncertainty in the initial activity ratio. Mollusk shells and pelagicbiogenic carbonate (e.g., foraminiferal ooze) often take up U after initial deposition of the carbonateand death of the organism, thus violating our closed system assumption. <strong>Th</strong>e technique is typicallyuseful up to about 4 times the half-life of 234 U when alpha spectrometry is the analytical method, butcan be applied to longer times with mass spectrometry because of higher precision.230 <strong>Th</strong>- 238 U Dating<strong>Th</strong>e 244 U- 238 U technique does not have high temperature applications, because at high temperature,234 U and 238 U do not fractionate as they do at low temperature. <strong>Th</strong>e reason is that radiation damage,which is the reason 234 U is removed in weathering more easily than 238 U, anneals quite rapidly athigh temperature. Disequilibrium between 230 <strong>Th</strong> (the daughter of 234 U) and its U parents can, however,provide useful geochronological and geochemical information in both high- and low-temperaturesystems. We start with an equation analogous to equation <strong>10</strong>.6:230<strong>Th</strong>u = 230<strong>Th</strong>0u e–l 230t<strong>10</strong>.14As is the usual practice, we normalize to a non-radiogenic isotope:230<strong>Th</strong>232<strong>Th</strong>=u230<strong>Th</strong>232<strong>Th</strong>0e–l 230t<strong>10</strong>.15( 232 <strong>Th</strong>, although non-radiogenic, isradioactive, of course. However, with ahalf-life of 14Ga, its abundance will notchange on time scales comparable to thehalf-life of 230<strong>Th</strong> ).In seawater, and in general in itsoxidized state, U is quite soluble. <strong>Th</strong>,however, is quite insoluble: its seawaterresidence time is 300 years or lesscompared to about 500,000 year for U. (Itshould be noted here that solubility inseawater does not control concentrationsor residence times. Neverthelesssolubility is a good guide to both ofthese.) Once a 234 U atom <strong>decay</strong>s to 230 <strong>Th</strong>,it is quickly absorbed onto particles thatin turn are quickly incorporated intosediment. As a result, relatively highconcentrations of unsupported 230 <strong>Th</strong> canbe removed (by leaching) from somesediments (mainly relatively slowly accumulatingones). In cases where theamount of leached 234 U is negligible andwhere the ( 230 <strong>Th</strong>/ 232 <strong>Th</strong>) o is known (from,for example, zero-age sediment at theseawater-sediment interface), equation<strong>10</strong>.14 can be used to determine the age ofthe sediment. If we can't neglect 234 Uthen we can still determine the age bytaking this into account, though themathematics becomes more complex (seeuFigure <strong>10</strong>.3. (a) 230 <strong>Th</strong>— 238 U isochron diagram. <strong>Th</strong>e( 238 U/ 232 <strong>Th</strong>) of the source is given by the intersection of theisochron with the equiline. (b) shows how the slopechanges as a function of time. After Faure (1986).64 February 12, 2003